Wound healing is a physiological process occurring after the onset of a skin lesion aiming to reconstruct the dermal barrier between the external environment and the body. Depending on the nature and duration of the healing process, wounds are classified as acute (e.g., trauma, surgical wounds) and chronic (e.g., diabetic ulcers) wounds. The latter take several months to heal or do not heal (non-healing chronic wounds), are usually prone to microbial infection and represent an important source of morbidity since they affect millions of people worldwide. Typical wound treatments comprise surgical (e.g., debridement, skin grafts/flaps) and non-surgical (e.g., topical formulations, wound dressings) methods. Modern experimental approaches include among others three dimensional (3D)-(bio)printed wound dressings.
1. Introduction
Skin is considered the first line of the body’s defense against external invaders causing various diseases since it protects the internal organs/tissues from direct sunlight (i.e., UV radiation), extreme temperatures, injuries, infections, etc. Hence, it is a very vulnerable tissue highly prone to injury, resulting in disruption of epidermal and probably dermal integrity or otherwise in skin wounds
[1][2][3][4]. Wounds can be classified as acute and chronic depending on the healing process’s nature and duration. Acute wounds such as trauma and surgical wounds usually heal in a relatively short time frame (e.g., 2–3 months) depending on the epidermal/dermal damage, size and depth of the wound. On the other hand, chronic wounds such as pressure, venous and diabetic ulcers do not heal (non-healing chronic wounds) or take a very long time to heal (e.g., several months), resulting in the formation of scars, and can be infected by bacteria and other exogenous factors
[2][5]. Chronic wounds affect millions of people (≥20 million) and represent a significant source of morbidity and an important financial burden to healthcare systems and society in general, consisting of direct (e.g., medical, health care) and indirect (e.g., sick leave, loss in productivity, early retirement, etc.) costs which increase year after year. This is mainly due to the aging population (i.e., chronic wounds are mostly common in elderly people
[4][6]) and the modern lifestyle involving increased food consumption and lack of exercise
[1][5][7][8].
Wound dressings are sterile pads typically applied to wounds to stop bleeding, absorb exudate and protect the wounded area from being infected by bacteria and potentially other microorganisms, as well as from further trauma, thus promoting wound healing
[5]. Medical adhesives such as fibrin glue, polyethylene glycol (PEG)- and cyanoacrylate-based adhesives have been extensively used for wound management and have been found to promote wound healing. However, difficulties in removing these adhesives due to strong attachment to the newly grown granulation tissue resulting in further injury and infection, limited oxygen permeability, inability for sufficient drug loading and absence of biomimicry have led to the development and exploration of advanced multifunctional wound dressings (e.g., patches with micro-, nano-architecture). The latter are capable of providing protection, maintaining the moisture in the wound microenvironment and accelerating the healing rate. Appropriate dressings should be selected as medical treatment for specific wounds taking into account the type of wound, its depth, its anatomical location, etc.
[8][9]. The novel multifunctional wound dressings aim to address the great medical need to heal chronic wounds
[8].
Hydrogels have received a lot of attention as possible wound dressings owing to their potential to mimic characteristics of the extracellular matrix (ECM) environment present in native tissues
[10][11], their elasticity (which plays a critical role in engineering soft and elastic tissues such as skin) and their high water content, which creates a cooling effect and facilitates the dressing application and removal, as well as their ability to encapsulate various bioactives (e.g., growth factors, antimicrobials, etc.)
[2][5]. Recent advances in 3D (bio)printing technologies permit hydrogel customization according to wound size and depth
[12][13] and enable the formation of multi-component hydrogels exhibiting various microstructures and networks of interconnected pores, which facilitate the transport of oxygen, nutrients, metabolic wastes, etc.
[12][13].
2. Wound Healing
Wound healing is a natural process via which the body regenerates the dermal and epidermal tissue
[2]. It is a common, complex but ordered physiological process that follows the onset of a skin tissue lesion (caused by an external factor like physical trauma, surgery, thermal injury, etc., or an internal factor like disease such as vascular diseases, diabetes, tumor, etc.
[5][14]) in order to reconstruct the barrier between the human body and the external environment. It is a dynamic, interactive process that involves soluble mediators, various cell types (e.g., blood cells, parenchymal cells) and extracellular matrix (ECM) and comprises four sequential and overlapping regeneration phases: hemostasis (formation of fibrin plug), inflammation (swelling), proliferation (formation of new tissue and blood vessels) and remodeling of newly formed tissue (
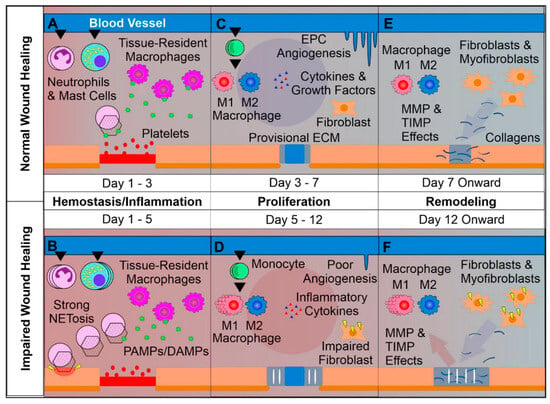
Figure 1)
[2][14][15][16], which can be affected by specific factors such as wound cause, wound size/depth, disease, age, nutrition, etc.
[2].
Figure 1. Overview of normal vs. impaired wound healing. (
A) Platelets form a clot at the site of injury and chemoattractants are released, recruiting key inflammatory cells. Next, inflammation takes charge, with infiltrating neutrophils and mast cells releasing pro-inflammatory cytokines and inducing strong sanitizing effects. This is accompanied by neutrophil extracellular trap (NETosis) induction, which assists in capturing and destroying invading pathogens. Tissue-resident macrophages react to pathogen- and damage-associated molecular patterns (PAMPs and DAMPs), activating. Later a provisional matrix comprised of fibronectin and other provisional extracellular matrix (ECM) components forms from the clot. (
B) Impaired wounds see an upregulated influx of neutrophils and mast cells, leading to an overactive inflammatory response, causing collateral damage and extending the inflammatory phase to the detriment of subsequent phases. (
C) Following the resolution of strong inflammation, the proliferative phase begins. Crucially, endothelial progenitor cells are stimulated by growth factors to induce angiogenesis. This angiogenesis allows for wound-resident cells to be supplied with oxygen and nutrients, facilitating their function. Infiltrating monocytes differentiate into M1 and M2 macrophage subsets. M1 macrophages maintain a strong inflammatory profile but are counterbalanced by pro-regenerative M2 macrophages, which release anti-inflammatory cytokines, growth factors and proteases, which replace the provisional ECM with collagens, assisted by properly functioning fibroblasts. This process results in thick granular tissue and full keratinocyte coverage. (
D) Impaired wounds result in poor angiogenesis and, in the case of T2DM, glycated proteins. This hypoxic environment induces oxidative stress, driving inflammatory M1 macrophage polarization and impairment of fibroblasts, resulting in poor ECM reorganization and a persistent inflammatory environment. (
E) Remodeling is carried out by macrophages, fibroblasts and myofibroblasts re-organizing the provisional ECM into a coherent scar structure primarily using matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), resulting in tissue with strong tensile strength and functionality. (
F) Impaired wound-resident cells remain ineffective and pro-inflammatory. Collagen reorganization resolves poorly, resulting in weak, non-functional skin that is apt to re-injure and potentially ulcerate, perpetually inflamed
[15].
Trauma leads to the immediate activation of a clotting cascade, which results in the formation of a fibrin plug ensuring hemostasis. Platelets trapped in the clot release pro-inflammatory mediators (e.g., growth factors, cytokines) into the local wound environment, thus ensuring the invasion and recruitment of inflammatory (neutrophils, monocytes) and other cells to the wound area. Hemostasis typically lasts 2–3 h and triggers inflammation, which begins when the injured blood vessels leak transudate resulting in swelling. Inflammation controls bleeding and at the same time prevents infection. During this phase, the duration of which can vary from hours to days, pathogens, bacteria and damaged cells are removed from the wound. In the proliferative phase, proliferation and migration of endothelial cells and fibroblasts take place promoting angiogenesis and formation of new ECM. In this way, the wound is rebuilt with new tissue made up of nascent ECM. Furthermore, a new network of blood vessels is created allowing the granulation tissue to receive sufficient oxygen and nutrients. Epithelial cells migrate from wound edges, initiating epithelialization and thus a new epithelial barrier appears. As new tissues are built, the wound contracts. Keratinocyte differentiation aids in restoring the function of the epidermis as a barrier. The remodeling or maturation stage (lasting for months), refers to the phase when the new ECM is constantly reorganized/reconstructed by myofibroblasts. The collagen network is densified by the microfilaments attached to the ECM and the wound is further contracted. Simultaneously, new components are secreted, increasing matrix density and stability. Finally, ECM is further strengthened by fibroblasts and the wound fully closes, usually with the production of disordered tissue
[2][4][15][16]. Disturbance of the healing process by microbial invasion or underlying pathological mechanism results in interruption and/or deregulation of one or more of the aforementioned phases and leads to non-healing (chronic) wounds such as diabetic, venous and pressure ulcers
[2][4][7][15][16]. The latter are characterized by the accumulation of reactive oxygen species (ROS) and proneness to infection as well as a prominent and prolonged inflammatory phase leading to destruction of ECM and sequential effect on resident fibroblasts. This includes the alteration of fibroblasts’ ability to proliferate and synthesize/remodel ECM since the chronic wound-associated fibroblasts develop a senescent phenotype. Finally, the chronic wound environment impairs angiogenesis and delays epithelialization leading to delayed and/or impaired wound healing
[14][17].
In the specific case of diabetic ulcers, hyperglycemia and mitochondrial dysfunction lead to permeation of the wound microenvironment by reactive oxygen species (ROS) resulting in persistent and prolonged inflammation and vascular endothelial dysfunction as well as tissue necrosis
[18]. More specifically, hypoxia is considered a major reason for wound damage caused by the limited supply of oxygen due to neuropathy and vascular dysfunction, as well as enhanced oxygen consumption by cells at the wound site. Moreover, the disproportion between angiogenic and angiostatic factors can result in angiogenic imbalance and exacerbate wound hypoxia. Hypoxia can also intensify inflammatory responses, thus prolonging injury by enhancing oxygen radicals’ levels
[16].
To date, all types of antibiotics have been administered for the treatment of bacterial infections. However, chronic use of antibiotics should be avoided since it leads to drug resistance. Recently, different biomaterials exhibiting superior antibacterial activity have been developed based on metallic ions (e.g., silver and copper ions, zinc oxide, etc.), which, however, could cause serious toxicity. Therefore, the design/development of biomaterials that, apart from hindering mtROS generation and enhancing the supplementation of energy during hyperglycemia, could also sterilize wounds in an efficient and biosafe way, is urgently needed
[18].
3. Wound Dressings
Typical wound treatments comprise surgical (e.g., debridement, skin grafts/flaps), and non-surgical (e.g., topical formulations, skin replacement, wound dressings incorporating or not growth factors, bioactive agents, nanoparticles) methods. Modern approaches include growth factors/cytokine therapy, stem cells (SCs) therapy, vacuum-aided wound closure, and three-dimensional (bio)printed wound dressings. Another approach involves the bioengineering of skin substitutes based on combinations of biomaterials, growth factors and cells
[19].
Wound dressings are typically compresses or sterile pads that are applied directly onto the surface of wounds in order to protect them from further injury and promote their healing process. The required characteristics of wound dressings are presented in Table 1.
Table 1. Required characteristics of wound dressings
[2][20].
| Required Characteristics of Wound Dressings |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dressings can be categorized into traditional and modern. Traditional dressings such as gauzes (woven and nonwoven), plasters, cotton wool and bandages (natural and synthetic) are economical and are typically used either as primary or secondary dressing products to protect wounds from being infected
[2][20]. Due to their fibril-based structure, gauze pads are capable of absorbing wound exudates. However, since they are dry, they tend to stick to granulation tissue and their removal becomes very painful.
The latter have been designed to ease wound healing apart from simply protecting the injured area. Modern dressings can be classified into biological and artificial. Biological dressings comprise donated human, animal or cadaver skin. These “auto-grafting” dressings are most appropriate for the complete healing of chronic deep wounds and/or burns. However, the insufficiency of donor skin is a serious drawback
[2]. Artificial dressings are made of natural or synthetic polymers, composites, etc., and are characterized by the presence of a highly absorbing layer and semi-permeability
[2][21].
The selection of the ideal dressing for chronic wounds depends on the physiological conditions of the wound (e.g., location, wound depth and extent, wound adhesion, secreted exudate volume and viscosity, infection, etc.
[6][20]). Consequently, it is crucial to select an appropriate dressing to stimulate the healing process
[6]. For example, highly exuding wounds should be covered with dressings exhibiting adequate liquid absorption to avoid leakage around or through them. Apart from proper absorption capacity, the dressing should be capable of retaining the absorbed fluid. A balanced moisture wound environment would thus prevent maceration or overhydration and promote healing. At this point, it should be mentioned that the exudate composition varies with wound type. While in acute wounds it promotes wound remodeling and repair, in chronic wounds it slows down wound reconstructing cells’ proliferation because of the increased level of denaturing proteins (e.g., proteases, proinflammatory cytokines)
[20].
Modern dressings are considered the top choice for curing various wound types due to their exceptional biocompatibility/biodegradability, ability to maintain a moist environment and temperature, pain relief, and improvement of a hypoxic environment. Those most commonly used in clinical practice are films, foams, hydrocolloids, alginates and hydrogels
[6].
4. Three-Dimensional Printing
Three-dimensional (3D) printing is a well-known method employed to fabricate accurately designed 3D architectures with high resolution based on computer-aided design (CAD) and with the use of biocompatible inks (i.e., biomaterials that can be 3D printed). To be sure, 3D printing enables the fabrication of scaffolds of specific shapes, exhibiting controlled porous structure, permeability, mechanical properties, etc., for tissue engineering (TE) applications (e.g., tissue regeneration, engineering of artificial organs, etc.)
[19][22][23][24][25]. The 3D-printed porous constructs promote oxygen exchange and ease the removal of metabolites, improving this way of cell proliferation
[22]. The introduction of 3D printing to wound dressings has revealed promising results as a consequence of the method’s capability for controlled design and fabrication of dressings exhibiting tuned microstructure
[8].
3D-Printed Hydrogels
Hydrogels are a popular class of biomaterial inks owing to their biomimetic properties and their benign processing conditions entitling them as suitable candidates for TE applications. They are usually printed in the form of their precursor materials and their final structure is obtained via crosslinking during or post 3D printing
[24]. Shape fidelity and collapsing are typical challenges in 3D hydrogel printing related to the viscoelastic properties of the ink and its solid content, respectively. Ideally, the ink should be able to flow through the nozzle throughout the printing process and preserve its shape post printing
[26]. Hydrogel precursors need to have a suitable viscosity to preserve their structural integrity until crosslinking. This can be facilitated by the increase in the polymer concentration, the addition of composites and the use of (near) gel-phase inks such as gelatin solutions which can be printed at a temperature close to their sol–gel transition (near gel-phase inks) and partially crosslinked hydrogels like alginate solutions containing low concentrations of calcium chloride (gel-phase inks)
[24], as well as via the use of rheology modifiers such as cellulose nanofibrils, which could improve ink printability and achieve shape fidelity post printing
[26].
Recent advances in 3D printing technologies (e.g., extrusion-based 3D printing) permit hydrogel customization according to wound size and depth
[12][13] and enable the formation of multi-component hydrogels exhibiting various microstructures and networks of interconnected pores which facilitate the transport of oxygen, nutrients, metabolic wastes, etc.
[12][13], as well as the temporal and spatial control of bioactive’s release thus promoting bacteria reduction, favoring tissue proliferation and decreasing healing time
[13].
Various types of polymers, both natural (e.g., sodium alginate (SA), chitosan, gelatin, carboxymethyl cellulose (CMC-Na), etc.
[25][27]) and synthetic (e.g., 4-arm PEG
[24], 2-hydroxyethyl methacrylate (HEMA), polyethylene glycol dimethacrylate (PEGDA), poly(acrylic acid) (PAA)
[28], etc.), as well as combinations thereof, have been used for the formation of hydrogel inks. From a chemical point of view, the selected materials should be easily modified with various chemical groups in order to be crosslinked via different mechanisms (e.g., free radical, ionic, etc.) and functionalized with appropriate molecules (e.g., functional polymers, adhesion peptides, peptides cleavable by proteases). They should also undergo hydrolysis and/or enzymatic degradation, potentially exhibiting inherent antibacterial properties, stimuli responsiveness, etc. Finally, they could permit the formation of a reversible 3D network via dynamic chemical bonding to enable selfhealing [
5].
Sodium alginate (SA), a cost-effective marine-derived polysaccharide, characterized by excellent biocompatibility, enhanced aqueous solubility and minimal toxicity, has been widely utilized in 3D printing of wound dressings since it can rapidly crosslink with divalent cations (e.g., Ca
2+, Mn
2+, Ba
2+, Cu
2+) and absorbs excess wound fluid while it maintains a physiological moist wound environment
[12][27][29][30]. On the other hand, shape infidelity, excessive swelling, rapid degradation rate, low mechanical properties, etc., could hinder its use in the 3D printing of wound dressings
[27][29][30].
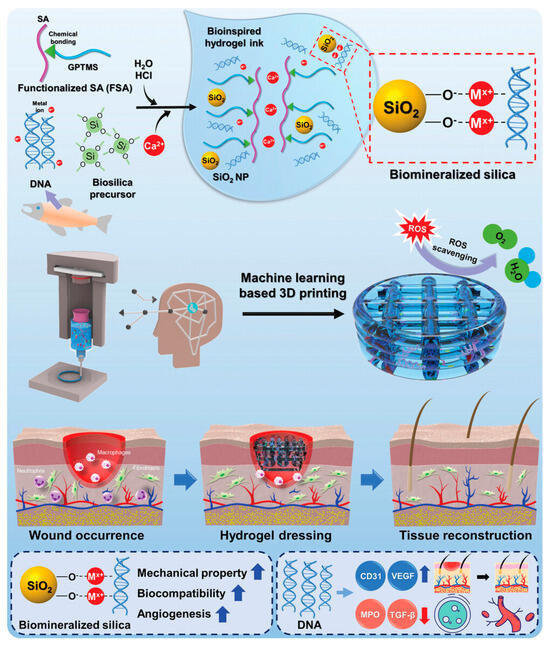
In a very recent work, Kim and coworkers (2023) developed a bioactive alginate hydrogel ink incorporating salmon sperm-derived DNA and DNA-induced biomineralized silica and exhibiting biocompatibility, printability, mechanical stability as well as a reactive oxygen species (ROS) scavenging effect for machine learning-assisted 3D printing of hydrogel wound dressings (Figure 2).
Figure 2. Schematic diagram of the fabrication of bioinspired 3D-printed hydrogels using DNA-induced biomineralization
[29].
Gelatin, a low-cost, collagen-derived biocompatible/biodegradable material containing an RGD (i.e., arginine−glycine−aspartic acid) adhesive peptide sequence supporting cell adhesion, and matrix metalloproteinase (MMP) cleavage sites enabling its degradation in the presence of MMPs, has been also used for the development of inks but combined with other polymers due to its insufficient mechanical properties
[31][32]. Gelatin methacrylate (GelMA) is also considered a promising option for the development of 3D-printed wound dressings because of its tunable properties controlled by the degree of methacrylation, as well as its ability to be photocrosslinked
[23]. On the other hand, its poor mechanical properties could restrict its clinical application
[33]. Chondroitin sulfate (CHS) is a glycosaminoglycan that has an essential role in ECM and cartilage, as well as in wound healing, growth factor signaling and inflammation. Nonetheless, it is difficult to form CHS-based materials for tissue regeneration through physical/chemical crosslinking
[30].
Low-cost 3D-printed chitosan hydrogels have also attracted a lot of interest owing to their significant biocompatibility, biodegradability, absence of toxicity and antibacterial activity. On the other hand, its potential relation with toxic organic solvents and its poor mechanical properties (i.e., chitosan is very soft and thus deforms or collapses post printing) could limit its application in TE
[34]. Therefore, it should be reinforced with other materials such as (bio)polymers in order to be successfully printed and preserve the scaffold shape
[35].
Cellulose-derived materials have been found to be promising for the fabrication of scaffolds mimicking ECM due to their biocompatibility, non-cytotoxicity, porous microstructure, favorite mechanical properties and tunable architecture, despite their challenging degradability in vivo as a result of the absence of relevant enzymes
[25]. Bacterial cellulose has been widely studied and used in various commercial applications. Yet, because of the nature of its cultivation, post-printing shaping remains challenging. The more shapeable wood-derived nanocellulose has been shown to be biocompatible and to support important cellular processes during the culture of various cell lines. Nanocellulose could be used for the development of inks because of its mechanical strength and mimicry of ECM structure provided that the stability issues of the printed scaffold can be surpassed (e.g., via double crosslinking
[25] and/or the use of an auxiliary polymer
[36])
[25]. Carboxymethyl cellulose (CMC), a cellulose derivative has been used in wound healing applications as hydrogel dressing because of its biocompatibility, good mechanical properties and increased stability. CMC has been also combined with ε-Polylysine (ε-PL), a sort of cationic polypeptide exhibiting antibacterial properties but poor mechanical properties, for the 3D printing of hydrogel dressings having an ordered porous structure and antibacterial/antioxidant properties, suitable for large irregular wounds
[37].
Temperature-stimulated polymers such as poly(N-isopropylacrylamide) (PNIPAAm), capable of gelling at body temperature due to their low critical solution temperature (LCST) have been widely studied as potential materials for wound dressings. Furthermore, PNIPAAm-based hybrid networks have exhibited potential as injectable materials for wound closure
[38]. Interpenetrating polymer networks, also known as superporous hydrogels, formed via the reaction of ethylene monomers such as N-isopropylacrylamide, acrylic acid, acrylamide, vinylpyrrolidone, etc., with natural polysaccharides and/or polymers containing hydroxyl or carboxyl groups are mostly attractive for the management of chronic wounds due to their increased porosity and cell adhesion. Nonetheless, typical superporous hydrogels lack antibacterial properties and enhanced swelling following the uptake of liquids
[39].
The autologous platelet-rich plasma (PRP) gel, rich in growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), etc., is considered to be a promising approach for the treatment of diabetic foot ulcers due to absence of immunological reaction, acceleration of cell proliferation and wound tissues’ migration. Still, PRP gel undergoes rapid growth factor (GF) release, thus requiring repeated administration resulting in retarded wound healing, costly treatment and increased patient suffering
[40]. To resolve this problem, Huang and coworkers (2023) developed multi-layered, fibrous, core–shell, bioactive hydrogels loaded with PRP via coaxial microfluidic 3D bioprinting
[40]. Integration of microfluidics with 3D printing technology allows the precise control of the structure and composition of engineered constructs (e.g., biomimetic, vascular-like channel structures) promoting vascular network formation and tissue regeneration
[41]. The formed hydrogels were revealed to be biocompatible, possess remarkable water absorption/retention properties and antibacterial activity, as well as enable the sustained release of the growth factors and promote cell adhesion, orientation, growth and proliferation
[41].
5. Three-Dimensional Bioprinting
Three-dimensional bioprinting, a subfield of 3D printing, is an emergent adaptive bio-manufacturing technology for the accurate fabrication of complex topological constructs based on computer-aided design (CAD), which has been broadly applied to TE, modeling of organoids, etc. It involves the development of bioinks (i.e., biomaterial formulations that contain cells and bioactive agents such as growth factors and can be readily processed by an automated biofabrication technology
[42][43][44]) to engineer biologically appropriate constructs mimicking and restoring natural ECM
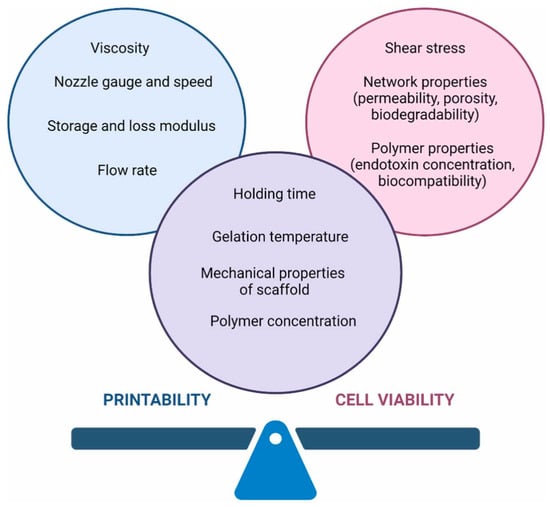
[45]. Bioinks have a fundamental role in the successful fabrication of a skin tissue construct. Their physicochemical/mechanical properties and their composition need to be carefully selected in order to achieve high printability and high cell viability (
Figure 3) and to assist cellular functions
[44][46]. Bioinks need to be biocompatible to ease cell growth, exhibit controllable rheological properties (e.g., shear thinning) in order to flow effortlessly through the nozzle and possess enhanced shape fidelity after printing (i.e., redeem sufficient storage modulus after printing), biofunctionality to meet biomimicry requirements (i.e., they should mimic ECM regarding biochemical/biomechanical properties
[45]) and mechanical stability
[22][43][47][48][49], which can be difficult to accomplish with a single-component bioink
[43]. Other important parameters that should be taken into consideration for the design and development of bioinks are their compatibility with various printing techniques, printing resolution, bioprintability, biodegradability, maintenance of cell viability and process scalability
[46]. There exist various types of bioinks, like cell-laden hydrogels, cell aggregates, etc. Those based on polymers usually comprise natural polymers such as alginate, gelatin, collagen, hyaluronic acid, silk fibroin, fibrin, etc., and/or synthetic polymers like polylactic acid, polyethylene glycol, etc.
[42][48]. They are crucial for the printing of constructs with high resolution, good mechanical properties and increased structure stability, as well as the regulation of cell adhesion, proliferation, migration and differentiation
[48].
Figure 3. The main challenge in the bioprinting process is to obtain a material with the highest printability, providing good cell viability. Both parameters may be influenced by many factors
[44].
The 3D bioprinting process applied for the manufacture of a wound dressing is similar to the traditional 3D printing process. More specifically, the methods of computed tomography (CT) and/or magnetic resonance imaging (MRI) are utilized to scan the wound site and the images are converted to a CAD model
[8]. This part of the bioprinting process is followed by the selection of the appropriate bioink, which is the principal component for the fabrication of functional tissues
[8][50], the bioprinting method (e.g., inkjet, micro-extrusion, laser assisted, etc.), the stabilization of the dressing shape via crosslinking (e.g., photopolymerization, etc.) and the direct application to the wound
[8]. Traditionally, 3D bioprinting is performed based on plane layering (i.e., on flat substrates). Nonetheless, in real clinical practice, wounds are commonly irregular and uneven
[43]. Curvilinear bioprinting enables bioink printing on curved surfaces, resulting in the manufacturing of more irregular/complicated constructs in comparison with typical planar bioprinting. These constructs are characterized by enhanced structural integrity due to the continuity of filaments and the presence of inter-filament bonds in shell-like curvilinear structures. It has been reported that skin, engineered via curvilinear bioprinting with an appropriate bioink, exhibits increased structural integrity for the treatment of irregular cutaneous wounds
[51]. In situ bioprinting allows for the direct deposition of biomaterials and cells at the wound site following wound debridement and scanning of its morphological features, thus permitting individualized wound treatment
[43].
5.1. 3D-Bioprinted Hydrogels
Hydrogels are the preferred category of bioinks for the in vitro development of various tissues such as skin, cartilage, bone, cardiac, etc., as well as vascularized tissues, due to their biocompatibility/biodegradability, superb bio-adaptability, mimicry of ECM, enhanced water content, and effortlessly tunable 3D structure
[22][52]. To date, various hydrogels based on natural and/or synthetic polymers (e.g., hyaluronic acid, chitosan, alginic acid, agarose, PEG, etc.
[53]) have been used as bioinks with the majority of them being based on natural polymers.
An important advantage of hydrogel bioprinting, in comparison with traditional fabrication methods, is that it enables accurate control over spatial patterning and construct architecture of individual bioinks containing cellular and biochemical components, thus allowing the replication of biological tissues’ native spatial organization
[14]. The method of 3D bioprinting has been applied for wound healing and skin tissue repair since 2012. Initially, collagen bioinks were used followed by various natural-based, individual or composite bioinks, aiming to accelerate wound healing and restore skin integrity due to exceptional biocompatibility, the resemblance of skin ECM and great printability
[47]. Conductive polymers (e.g., polyaniline, etc.) have also emerged as an innovative strategy for the development of bioinks that could promote wound healing via electrical stimulation of the wound bed. The latter has been found to facilitate cell migration, proliferation, and differentiation, increase angiogenesis and modulate inflammatory responses, thus speeding up wound healing. Additionally, conductive hydrogels (CHs) could act as platforms for stimuli-responsive delivery of bioactive agents like growth factors, therapeutic molecules, cytokines, etc.
[54].
The healing of acute wounds is frequently marked by scarring; that is, primarily caused by excessive deposition of pro-fibrotic collagen throughout the proliferative stage. The resultant scar tissue is slightly functional in comparison with healthy skin tissue and exhibits poorer mechanical properties. On the other hand, chronic wounds are characterized by a lack of dermal regeneration as a result of persistent inflammation, diabetic conditions, oxidative stress, accumulation of necrotic tissue and other factors inhibiting the typical process of wound healing. Therefore, the microenvironments of acute and chronic wounds are very different and require the unique designs of bioprinted hydrogels
[14].
5.1.1. Bioinks
Naturally derived biomaterials like collagen, gelatin, chitosan, etc., are generally used in TE applications since they are biocompatible/biodegradable and stimulate cell interactions. On the other hand, uncontrolled degradation, low mechanical strength and possible immunogenicity limit their broad application. To address these challenges, chemical modifications of these polymers have been performed in various cases
[55]. Typically, an ideal bioink should contain cell anchoring ligands and exhibit appropriate stability and elasticity for the in vitro maturation period
[45]. Among the abundant biomaterials, sodium alginate (SA) and gelatin (Gel) have received broad attention for the development of bioinks
[43].
Gelatin (Gel), a natural biopolymer produced by collagen hydrolysis, has received a lot of interest as a bioink component due to its hygroscopic nature, cytocompatibility, plentiful cell recognition sites and thermosensitivity, allowing viscosity adjustment to favor printability. On the other hand, its low printing resolution, poor mechanical properties and fast biodegradation, in the presence or not of enzymes, drastically limits its broad application
[48][51]. The methacrylation of gelatin gives an easily photocrosslinkable, non-immunogenic derivative (methacrylated gelatin, GelMA) with tunable biodegradation that has been widely used for the fabrication of constructs
[48].
Photocrosslinkable gelatin-methacryloyl (GelMa), produced via the reaction of gelatin with methacrylic anhydride and the introduction of substituent methacryloyl groups onto the reactive amine has also received a lot of attention for TE applications, owing to its biocompatibility and tunable mechanical properties. Lee and coworkers (2022) developed a GelMA-based bioink containing various agents for skin TE. The bioink was shown to exhibit exceptional biocompatibility and bioactivity because of increased cell adhesion and proliferation, and enhanced expression of biomarkers related to ECM remodeling (e.g., MMP9, Ki67 and decorin)
[55].
Alginate, an algae-extracted linear polysaccharide, is characterized by hydrophilicity, biocompatibility/biodegradability, porosity, nontoxicity and facile functionalization, as well as lack of adequate biological cues for adhesion and proliferation of cells
[48][52][56]. Its combination with gelatin leads to the formation of semi-interpenetrating networks mimicking ECM structure, which provide sufficient cell adhesion sites facilitating hydrogel–cell interactions
[56].
Silk fibroin (SF), the main protein of silk, a natural biomimetic fibrous polymer is characterized by biocompatibility (high cell proliferation and adherence, low inflammation) and biodegradability as well as increased tensile strength. It can be chemically modified by glycidyl methacrylate (Silk-GMA) and bioprinted into different structures (e.g., hydrogel, membrane, porous sponges, etc.) via photopolymerization and aqueous processing. SF bioprinting has been found to result in stable porous structures favoring cell adhesion and growth
[57].
Biodegradable polyurethane (PU) is a synthetic polymer with sufficient printability and elasticity, as well as adjustable mechanical properties
[51]. Hydrogels comprising gelatin and PU have been found to exhibit adequate printability and modifiable mechanical strength to support MSCs’ proliferation and differentiation
[51].
To combine manufacturability with biomimicry, Hao and coworkers (2023) developed a thermo-responsive stepwise multi-crosslinking (i.e., pre-crosslinking via the Michael addition reaction of thiol–acrylate groups before bioprinting, hydrophobic interaction during bioprinting, and “thiol-ene” click reaction of the remaining thiol–acrylate groups under UV after bioprinting) bioink comprising thiolated Pluronic F127 (PF127-SH) and methacrylated hyaluronic acid (HAMA). This strategy was revealed to significantly improve the bioink regarding rheology, printability, mechanical properties, structural integrity, cytocompatibility, etc.
[58].
Adipose tissue decellularized extracellular matrix (dECM), which preserves the composition of glycosaminoglycans and proteins (e.g., (micro-)fibrillar collagens, glycoproteins and proteoglycans
[59]) in ECM, together with a ranked nanofibrous structure
[59], is a thermosensitive biomaterial which could form hydrogels via self-assembly, promote accumulation, proliferation and differentiation of cells, stimulate angiogenesis and endorse skin tissue repair
[60].
Platelet-rich plasma (PRP) obtained via centrifugation of whole blood could, at high concentrations, speed up hemostasis through the promotion of thrombosis and coagulation at the wound site. Furthermore, increased concentrations of bioactive agents like growth factors, chemokines, miRNA and immunoglobulins, which exhibit exceptional synergistic effects, could be released by α-particles of PLTs, thus avoiding the disadvantageous application of a single growth factor. Accordingly, PRP has been used in cutaneous wound healing and regeneration/rejuvenation of skin appendages. Additionally, owing to the slow release of bioactive agents, it is anticipated to act for a long time on the wound and achieve the initiation of regenerative signals. Finally, PRP is in alignment with personalized medicine since it is a source of autologous patient-specific growth factors
[43].
5.1.2. Cells
Fibroblasts and keratinocytes are the frequently used cells in skin TE. Nonetheless, in cases of deep or extensive skin defects, cell extraction from patients is difficult. In this respect, stem cells (SCs) have been favored for skin tissue repair
[60]. SCs exhibit desirable properties (e.g., differentiation into different types of tissues and enhanced proliferation)
[44]. Initially derived from mesoblasts, adipose tissue-derived stem cells (ADSCs) can effortlessly be obtained in large quantities via minimally invasive methods (e.g., liposuction) in high purity. ADSCs exhibit low immunogenicity and are capable of rapid proliferation in vitro as well as multidirectional differentiation. Additionally, they are characterized by substantial self-renewalability and have been found to endorse wound healing acceleration
[60][61].
5.1.3. Bioprinting Methods
Hydrogels can be (bio)printed utilizing various 3D (bio)printing techniques such as extrusion printing, inkjet printing, laser-assisted bioprinting, etc. In extrusion printing, the most commonly used method, the bioink is deposited via the application of pneumatic or mechanical force. This technique is similar to classic hydrogel extrusion from a syringe and is thus readily applied to different hydrogel bioinks. To minimize the imposed shear force on the hydrogel bioink, as well as the required pressure, shear-thinning biomaterials can be used for hydrogel formation. Additionally, monomer solutions of low viscosity can be deposited, which could be crosslinked after printing, or alternatively, materials’ viscosity could be reduced via temperature increase
[14]. Inkjet printing comprises the piezoelectric or thermal deposition of small bioink droplets onto a substrate where they spontaneously crosslink and/or fuse leading to the solidification of the printed construct. Laser-assisted bioprinting uses periodic laser excitation for the heating of a donor substrate and the subsequent spatial release of defined bioink droplets adsorbed to the substrate. Digital light processing (DLP) is another method that could aid in overcoming the drawbacks of extrusion or inkjet bioprinting but has not been adequately elucidated
[57].
5.1.4. Encapsulation of Bioactive Agents
The encapsulation of bioactive agents and/or drug-loaded nanocarriers (NCs) in hydrogel bioinks can have additional benefits for wound healing. Various NC types have been utilized to increase drug therapeutic efficiency including polymer or lipid-based NPs, liposomes, inorganic NPs, nanohydrogels and nanofibrous structures.
Particularly, polymer-based NPs have demonstrated enhanced encapsulation efficiency, thermodynamic stability, drug protection against degradation, topical sustained release, etc. Catechol (i.e., benzene derivative exhibiting anti-inflammatory and antioxidant activity, mitigating inflammation and assisting neovascularization) bearing polymer-based NPs have received a lot of attention due to sustained drug release and reduction of side effects of antimicrobials and various anticancer drugs. In a recent study, Puertas-Bartolome and coworkers (2021) incorporated catechol-bearing polymer NPs encapsulating a model hydrophobic drug in a hydrogel comprising carboxymethyl chitosan and HA for the development of a novel bioink. The bioprinted hydrogel construct was found to exhibit shape fidelity, mechanical stability and uniform distribution of NPs, and support the proliferation of the encapsulated fibroblasts for more than 14 days
[62].