Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhendong Tan | -- | 1628 | 2024-02-26 15:23:39 | | | |
| 2 | Wendy Huang | Meta information modification | 1628 | 2024-02-27 02:15:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tan, Z.; Jiang, H. Development and Growth of Intramuscular Fat in Cattle. Encyclopedia. Available online: https://encyclopedia.pub/entry/55472 (accessed on 07 February 2026).
Tan Z, Jiang H. Development and Growth of Intramuscular Fat in Cattle. Encyclopedia. Available at: https://encyclopedia.pub/entry/55472. Accessed February 07, 2026.
Tan, Zhendong, Honglin Jiang. "Development and Growth of Intramuscular Fat in Cattle" Encyclopedia, https://encyclopedia.pub/entry/55472 (accessed February 07, 2026).
Tan, Z., & Jiang, H. (2024, February 26). Development and Growth of Intramuscular Fat in Cattle. In Encyclopedia. https://encyclopedia.pub/entry/55472
Tan, Zhendong and Honglin Jiang. "Development and Growth of Intramuscular Fat in Cattle." Encyclopedia. Web. 26 February, 2024.
Copy Citation
Besides protein, beef contains a significant amount of fat. Intramuscular fat (IMF), also referred to as marbling fat, is the white fat deposited within skeletal muscle tissue. The content of intramuscular fat in the skeletal muscle, particularly the longissimus dorsi muscle, of cattle is a critical determinant of beef quality and value. Physiologically, IMF is believed to have similar functions to other fat depots in cattle, serving as an energy reserve and providing fuel during times of increased metabolic demand or inadequate nutrient supply. As food, IMF substantially enhances the texture and flavor of the meat and the overall satisfaction of consumers.
intramuscular fat
skeletal muscle
beef
development
growth
adipogenesis
1. Introduction
Meat products have historically served as a substantial source of protein, essential amino acids, vitamins, and trace minerals for the global population. While the sale of beef is restricted in certain countries due to religion and culture, it consistently remains one of the most consumed meat products among the general populace. Consumers generally perceive health benefits as derivable from adequate beef consumption compared to other protein sources [1][2]. A mere 55 g or 45 g of high-quality beef can satisfy the daily protein requirements of an adult male or female, respectively [3]. With the rise in global prosperity, the demand for high-quality beef has continuously increased.
The marketing of fresh beef relies significantly on IMF, especially in the longissimus dorsi muscle (LM), where higher grades command higher prices. The current dietary trend suggests that consumers are more concerned about saturated fatty acids (SFA) in the diet, as a high intake of SFA may increase the risk of cardiovascular disease [4]. Intramuscular fat contains more monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA) than other fat depots in cattle [3]. Beef with a higher percentage of IMF, or a higher degree of “marbling”, has a higher ratio of MUFA to SFA, and is therefore considered healthier meat [5].
2. Adipogenesis
Adipocytes are terminally differentiated cells of adipose tissue, and the change of adipose tissue mass depends on both the hyperplasia (cell number increase) and hypertrophy (cell size increase) of adipocytes [6]. Adipocytes originate from mesenchymal stem cells (MSCs) or adipose progenitor cells (APCs) [7]. Adipogenesis can be divided into two stages: commitment (or determination) and differentiation [8] (Figure 1). The transition from APCs or MSCs to preadipocytes, which is marked by the expression of the PPARG gene, is controlled at the transcriptional level by several transcription factors and extracellular signals (Figure 1). Bone morphogenetic proteins (BMPs) [9][10], platelet-derived growth factor receptor alpha (PDGFRA) [11], and zinc finger proteins 423 and 467 (ZFP423 and ZFP467) [12] are the major regulators of the commitment of MSCs or APCs to preadipocytes, while factors like RUNX1 partner transcriptional co-repressor 1 (RUNX1T1) [13] suppress this commitment [14].
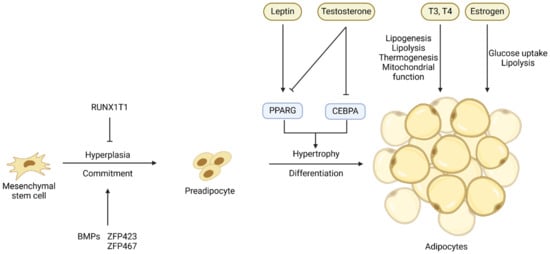
Figure 1. Key transcriptional and hormonal regulators of the commitment of mesenchymal stem cells or adipose progenitor cells to preadipocytes and the differentiation of preadipocytes into adipocytes. Arrows denote stimulation and T-shaped lines denote inhibition.
The process of adipogenic differentiation has been extensively studied, with PPARG and CEBPs identified as the core regulators of this process [15]. The CEBPB and CEBPD genes are expressed early in adipogenic differentiation, and they induce the co-expression of PPARG and CEBPA, two crucial transcription factors in the later stages of adipogenesis [16][17][18]. These transcription factors ultimately activate the expression of genes specific for adipocytes and the deposition of lipids, resulting in the formation of mature adipocytes [19]. The process of differentiation of preadipocytes into adipocytes is also controlled by hormones and other extracellular signals such as leptin and testosterone (Figure 1), and these extracellular signals will be discussed in detail in the sections below.
3. Adipogenesis within Skeletal Msucle
The majority of research on the developmental biology of IMF has been conducted in the mouse model, although mice are not known for their ability to accumulate IMF. In mice, IMF formation starts in the late stages of pregnancy [20]. In the process of embryonic skeletal muscle development, primary myofibers are first formed followed by secondary myofibers [21]. The formation of adipocytes, i.e., adipogenesis, in skeletal muscle overlaps that of secondary myofibers in the middle or late stages of pregnancy [22]. It is highly likely that mesenchymal progenitor cells differentiate into either myogenic or fibro/adipogenic lineage [23]. Cells from the myogenic lineage further develop into muscle fibers, intramuscular brown adipocytes, and satellite cells, while cells from the fibro/adipogenic lineage develop into white adipocytes and fibroblasts [24]. Since myogenic and adipogenic lineage cells originate from the same pool of stem cells, the commitment of stem cells to myogenesis or adipogenesis is a competing process. More myogenesis implies suppressed adipogenesis, and vice versa. The formation of initial adipocytes has a dominant effect on the number of total intramuscular adipocytes. As a result of the paracrine effects and their close proximity to adipocytes, skeletal muscle insulin resistance develops, causing a shift in the commitment of stem cells from myogenesis to adipogenesis [25][26].
During embryonic development, the Wnt signaling pathway activates myogenic and osteogenic differentiation while inhibiting the adipogenic differentiation of mesenchymal multipotent cells [22][27]. This primarily involves Wnt signaling suppressing the key adipogenic regulators CEBPA and PPARG [28]. Farmer and colleagues observed a functional interaction between β-catenin (a component of the Wnt signaling pathway) and PPARG, wherein these two proteins negatively regulate the activity of each other [29]. The Wnt signaling pathway is thought to be involved in maintaining a balance between adipogenesis and myogenesis. Specifically, the loss of WNT10B, either due to aging or targeted gene deletion, leads to an increased adipogenic potential of myoblasts and the acquisition of adipocyte characteristics during the process of muscle regeneration [30]. Bromodomain-containing protein 4 (BRD4) is a member of the bromodomain and extra-terminal domain (BET) family of proteins, which are epigenetic readers that recognize acetylated histones and facilitate the recruitment of transcriptional regulators that promote the expression of PPARG. BRD4 knockout inhibits the expression of PPARG and suppresses adipogenesis [31]. Cooperating with lineage-determining transcription factors (LDTFs), BRD4 recruits regulators such as MLL3/MLL4 and CBP/p300 to the enhancers [32]. BRD4 knockout mice showed reduced brown fat and muscle mass, indicating that BRD4 is an important factor for both adipogenesis and myogenesis [32]. Myostatin (MSTN) is a major negative regulator of skeletal muscle development and growth [33]. Myostatin also inhibits adipose tissue growth by reducing lipid accumulation through the ERK1/2 and PKA signaling pathways [34]. In addition to multipotent stem cells, highly committed cells like myoblasts [35], fibroblasts [36], and pericytes [37] in muscle tissue can also be induced to differentiate into mature adipocytes at least in vitro, as depicted in Figure 2.
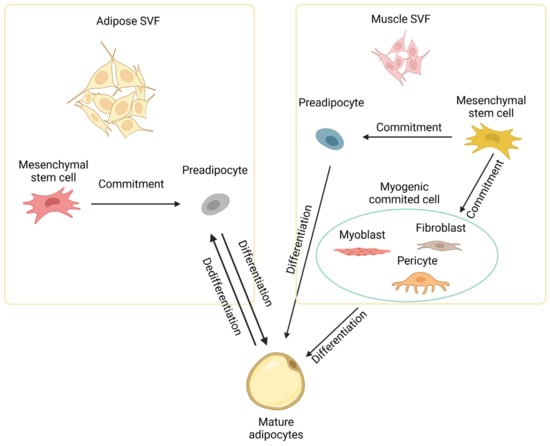
Figure 2. Development of intramuscular adipocytes. Intramuscular adipocytes can be formed from mesenchymal stem cells in the stromal vascular fraction (SVF) of both adipose and muscle tissues through commitment and differentiation. Intramuscular adipocytes might also be formed from committed cells like myoblasts, fibroblasts, and pericytes in muscle. Diagram has been drawn based mainly on mouse studies. See text for details.
In cattle, embryonic stem cells begin to form adipocytes at three months of gestation. Visceral adipocytes are formed first, followed by subcutaneous adipocytes, intermuscular adipocytes, and lastly intramuscular adipocytes [38]. The first intramuscular adipocytes are generated at around 180 days of gestation [39]. Since intramuscular fat matures later than subcutaneous fat, pursuing a high level of marbling often increases the overall carcass fat, particularly subcutaneous fat [40]. Fortunately, the hyperplasia phase of intramuscular adipocytes continues until 250 days of age, far surpassing other fat cell populations, which stop hyperplasia during the weaning stage (intermuscular and subcutaneous adipocytes) or the neonatal stage (visceral adipocytes) [41]. The differential timing of adipocyte hyperplasia creates the concept of a “marbling window” (from 150 to 250 days of age), which provides an opportunity for stimulating intramuscular adipocyte formation without increasing overall fat accumulation. During this period of marbling window, active intramuscular preadipocyte proliferation is a major mechanism of IMF deposition [42]. From 250 days of age to slaughter, the hypertrophy of adipocytes plays a more important role than hyperplasia in IMF growth [38][43].
The primary driving force in current research on IMF is to find a way to improve IMF growth while avoiding an overall increase in body fat. A number of genes that potentially regulate IMF development and growth in cattle have been identified (Table 1). These genes are often the focus of research aimed at evaluating how various factors, such as dietary supplements, influence adipose tissue development and growth in cattle.
Table 1. Genes associated with intramuscular fat development in cattle.
| Genes | Species | Association with IMF | Impact | References |
|---|---|---|---|---|
| ICER | Hanwoo | Highly expressed in the late fattening stage and during preadipocyte differentiation into adipocytes | + | [44] |
| WISP2 | Wagyu | Highly expressed in adipose precursor cells | + | [45] |
| PPARG | A master transcriptional regulator of adipose differentiation | + | [46] | |
| PDGFRA | Angus | Abundance is correlated with IMF content | + | [47] |
| CEBPA | A core transcription factor of fat differentiation | + | [48] | |
| SCD1 | Wagyu, Simmental | Increases IMF deposition and unsaturated fatty acid content | + | [49] |
| KLF | Yak | Inhibitory factor of IMF differentiation | − | [50] |
| DGAT1 | Holstein, Charolais, | Involved in fatty acid esterification and correlated with IMF content | + | [51][52][53] |
| ADIPOQ, THRSP | Wagyu × Hereford | Abundance is highly correlated with IMF content | + | [54] |
| ZFP423 | Angus | Promotes adipogenic differentiation of muscle stromal vascular cells | + | [55] |
| ACSL1 | Charolais × Holstein | Regulates lipid composition and polyunsaturated fatty acids synthesis in adipocytes | + | [56][57] |
| CD36 | Qinchuan | Cattle with the combined genotype WWCCAA in the CD36 gene had higher IMF contents | + | [58] |
| ABHD5 | Qinchuan | An accelerator for adipose triglyceride lipase | − | [59] |
| WNT gene family | Korean | Inhibits the differentiation of fat cells | − | [60] |
| FOXO1 | Luxi | Affects the expression of genes associated with apoptosis of intramuscular adipocytes | − | [61] |
| KLF3 | Qinchuan | The polymorphism of the KLF3 gene has an impact on the intramuscular fat content | − | [62] |
ICER: Inducible cAMP early repressor. WISP2: WNT1 inducible signaling pathway protein 2. PPARG: Peroxisome proliferator-activated receptor gamma. PDGFRA: Platelet-derived growth factor receptor alpha. CEBPA: CCAAT/enhancer-binding protein alpha. SCD1: Stearoyl-CoA desaturase 1. KLF: Krüppel-like factor. DGAT1: Diacylglycerol O-acyltransferase 1. ADIPOQ: Adiponectin. THRSP: Thyroid hormone responsive. ZFP423: Zinc finger protein 423. ACSL1: Acyl-CoA synthetase long-chain family member 1. CD36: Cluster of differentiation 36. FOXO1: Forkhead box O1. ABHD5: Abhydrolase domain containing 5. The “+” and “−“ signs indicate positive and negative effects of the genes on IMF deposition, respectively.
References
- Magalhaes, D.R.; Çakmakçı, C.; Campo, M.d.M.; Çakmakçı, Y.; Makishi, F.; Silva, V.L.d.S.; Trindade, M.A. Changes in the Current Pat-terns of Beef Consumption and Consumer Behavior Trends—Cross-Cultural Study Brazil-Spain-Turkey. Foods 2023, 12, 475.
- Predanócyová, K.; Pindešová, D.; Kubicová, Ľ. Consumer attitudes towards beef consumption and future perspectives. Acad. J. 2023, 51, 1–62.
- Troy, D.J.; Tiwari, B.K.; Joo, S.T. Health Implications of Beef Intramuscular Fat Consumption. Korean J. Food Sci. Anim. Resour. 2016, 36, 577–582.
- Valsta, L.M.; Tapanainen, H.; Männistö, S. Meat fats in nutrition. Meat Sci. 2005, 70, 525–530.
- Smith, S.; Johnson, B. Marbling: Management of cattle to maximize the deposition of intramuscular adipose tissue. J. Anim. Sci. 2016, 94, 382.
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324.
- Liu, S.; Huang, J.; Wang, X.; Ma, Y. Transcription factors regulate adipocyte differentiation in beef cattle. Anim. Genet. 2020, 51, 351–357.
- MacDougald, O.A.; Mandrup, S. Adipogenesis: Forces that tip the scales. Trends Endocrinol. Metab. TEM 2002, 13, 5–11.
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611.
- Huang, H.; Song, T.J.; Li, X.; Hu, L.; He, Q.; Liu, M.; Lane, M.D.; Tang, Q.Q. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2009, 106, 12670–12675.
- Lau, A.M.; Tseng, Y.H.; Schulz, T.J. Adipogenic fate commitment of muscle-derived progenitor cells: Isolation, culture, and dif-ferentiation. Methods Mol. Biol. 2014, 1213, 229–243.
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010, 464, 619–623.
- Deng, K.; Ren, C.; Liu, Z.; Gao, X.; Fan, Y.; Zhang, G.; Zhang, Y.; MA, E.-S.; Wang, F.; You, P. Characterization of RUNX1T1, an Adi-pogenesis Regulator in Ovine Preadipocyte Differentiation. Int. J. Mol. Sci. 2018, 19, 1300.
- Vernochet, C.; Milstone, D.S.; Iehlé, C.; Belmonte, N.; Phillips, B.; Wdziekonski, B.; Villageois, P.; Amri, E.Z.; O’Donnell, P.E.; Mortensen, R.M.; et al. PPARγ-dependent and PPARγ-independent effects on the development of adipose cells from embryonic stem cells. FEBS Lett. 2002, 510, 94–98.
- Avram, M.M.; Avram, A.S.; James, W.D. Subcutaneous fat in normal and diseased states 3. Adipogenesis: From stem cell to fat cell. J. Am. Acad. Dermatol. 2007, 56, 472–492.
- Fajas, L.; Debril, M.B.; Auwerx, J. Peroxisome proliferator-activated receptor-gamma: From adipogenesis to carcinogenesis. J. Mol. Endocrinol. 2001, 27, 1–9.
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389.
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896.
- Brun, R.P.; Spiegelman, B.M. PPAR gamma and the molecular control of adipogenesis. J. Endocrinol. 1997, 155, 217–218.
- Du, M.; Yin, J.; Zhu, M.J. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010, 86, 103–109.
- Du, M.; Yan, X.; Tong, J.F.; Zhao, J.; Zhu, M.J. Maternal Obesity, Inflammation, and Fetal Skeletal Muscle Development. Biol. Reprod. 2010, 82, 4–12.
- Du, M.; Zhao, J.X.; Yan, X.; Huang, Y.; Nicodemus, L.V.; Yue, W.; McCormick, R.J.; Zhu, M.J. Fetal muscle development, mesenchymal multipotent cell differentiation, and associated signaling pathways. J. Anim. Sci. 2011, 89, 583–590.
- Gerrard, D.E.; Grant, A.L. Principles of Animal Growth and Development; Kendall Hunt: Dubuque, IA, USA, 2003.
- Taga, H.; Chilliard, Y.; Picard, B.; Zingaretti, M.C.; Bonnet, M. Foetal bovine intermuscular adipose tissue exhibits histological and metabolic features of brown and white adipocytes during the last third of pregnancy. Anim. Int. J. Anim. Biosci. 2012, 6, 641–649.
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11–18.
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced mi-tochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593.
- Cossu, G.; Borello, U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999, 18, 6867–6872.
- Prestwich, T.C.; Macdougald, O.A. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617.
- Liu, J.; Farmer, S.R. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin sig-naling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J. Biol. Chem. 2004, 279, 45020–45027.
- Taylor-Jones, J.M.; McGehee, R.E.; Rando, T.A.; Lecka-Czernik, B.; Lipschitz, D.A.; Peterson, C.A. Activation of an adipogenic program in adult myoblasts with age. Mech. Ageing Dev. 2002, 123, 649–661.
- Brown, J.D.; Feldman, Z.B.; Doherty, S.P.; Reyes, J.M.; Rahl, P.B.; Lin, C.Y.; Sheng, Q.; Duan, Q.; Federation, A.J.; Kung, A.L.; et al. BET bromodomain proteins regulate enhancer function during adipogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 2144–2149.
- Lee, J.-E.; Park, Y.-K.; Park, S.; Jang, Y.; Waring, N.; Dey, A.; Ozato, K.; Lai, B.; Peng, W.; Ge, K. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat. Commun. 2017, 8, 2217.
- Gao, F.; Kishida, T.; Ejima, A.; Gojo, S.; Mazda, O. Myostatin acts as an autocrine/paracrine negative regulator in myoblast dif-ferentiation from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2013, 431, 309–314.
- Pan, S.; Zhang, L.; Liu, Z.; Xing, H. Myostatin suppresses adipogenic differentiation and li-pid accumulation by activating crosstalk between ERK1/2 and PKA signaling pathways in porcine subcutaneous preadipocytes. J. Anim. Sci. 2021, 99, skab287.
- Huangfu, Y.; Zan, L.; Wang, H.; Cheng, G.; Liu, Y.; Gao, J.; Li, Y.; Yang, N. Insulin induced adipogenic differentiation of bovine myoblasts. J. Northwest A F Univ. Nat. Sci. Ed. 2014, 42, 16.
- Wang, H.; Cheng, G.; Fu, C.; Wang, H.; Yang, W.; Wang, H.; Zan, L. Sequence analysis of bovine C/EBPδ gene and its adipogenic effects on fibroblasts. Mol. Biol. Rep. 2014, 41, 251–257.
- Kirton, J.P.; Crofts, N.J.; George, S.J.; Brennan, K.; Canfield, A.E. Wnt/β-Catenin Signaling Stimulates Chondrogenic and Inhibits Adipogenic Differentiation of Pericytes: Potential relevance to vascular disease? Circ. Res. 2007, 101, 581–589.
- Vernon, R. The Growth and Metabolism of Adipocytes; Butterworths: London, UK, 1986.
- Taga, H.; Bonnet, M.; Picard, B.; Zingaretti, M.C.; Cassar-Malek, I.; Cinti, S.; Chilliard, Y. Adipocyte metabolism and cellularity are related to differences in adipose tissue maturity between Holstein and Charolais or Blond d’Aquitaine fetuses. J. Anim. Sci. 2011, 89, 711–721.
- Font-i-Furnols, M.; Maltin, C.; Povše, M.P.; Karlsson, A.H.; Silva, S.; Teixeira, A.; Gispert, M.; Lebret, B.; Kragten, S.A.; Gil, M.; et al. A Handbook of Reference Methods for Meat Quality Assessment; European Cooperation in Science and Technology (COST): Brussels, Belgium, 2015.
- Keogh, K.; Kelly, A.K.; Kenny, D.A. Effect of plane of nutrition in early life on the transcriptome of visceral adipose tissue in Angus heifer calves. Sci. Rep. 2021, 11, 9716.
- Du, M.; Wang, B.; Fu, X.; Yang, Q.; Zhu, M.J. Fetal programming in meat production. Meat Sci. 2015, 109, 40–47.
- Du, M.; Ford, S.P.; Zhu, M.-J. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Anim. Front. 2017, 7, 5–11.
- Lee, S.H.; Park, E.W.; Cho, Y.M.; Kim, S.K.; Lee, J.H.; Jeon, J.T.; Lee, C.S.; Im, S.K.; Oh, S.J.; Thompson, J.M.; et al. Identification of differentially expressed genes related to intramuscular fat development in the early and late fattening stages of hanwoo steers. J. Biochem. Mol. Biol. 2007, 40, 757–764.
- Hudson, N.J.; Reverter, A.; Greenwood, P.L.; Guo, B.; Cafe, L.M.; Dalrymple, B.P. Longitudinal muscle gene expression patterns associated with differential intramuscular fat in cattle. Anim. Int. J. Anim. Biosci. 2015, 9, 650–659.
- Moisá, S.J.; Shike, D.W.; Faulkner, D.B.; Meteer, W.T.; Keisler, D.; Loor, J.J. Central Role of the PPARγ Gene Network in Coordinating Beef Cattle Intramuscular Adipogenesis in Response to Weaning Age and Nutrition. Gene Regul. Syst. Biol. 2014, 8, 17–32.
- Martins, T.S.; Sanglard, L.M.; Silva, W.; Chizzotti, M.L.; Rennó, L.N.; Serão, N.V.; Silva, F.F.; Guimarães, S.E.; Ladeira, M.M.; Dodson, M.V.; et al. Molecular Factors Underlying the Deposition of Intramuscular Fat and Collagen in Skeletal Muscle of Nellore and Angus Cattle. PLoS ONE 2015, 10, e0139943.
- Hongfang, G.; Khan, R.; Raza, S.H.A.; Nurgulsim, K.; Suhail, S.M.; Rahman, A.; Ahmed, I.; Ijaz, A.; Ahmad, I.; Linsen, Z. Transcriptional regulation of adipogenic marker genes for the improvement of intramuscular fat in Qinchuan beef cattle. Anim. Biotechnol. 2022, 33, 776–795.
- Jiang, Z.; Michal, J.J.; Tobey, D.J.; Daniels, T.F.; Rule, D.C.; Macneil, M.D. Significant associations of stearoyl-CoA desaturase (SCD1) gene with fat deposition and composition in skeletal muscle. Int. J. Biol. Sci. 2008, 4, 345–351.
- Zhu, J.; Lin, Y.; Zuo, L.; Wang, Y.; Bai, X.; Jiang, M. Molecular cloning, tissue expression of KLF5, KLF6, KLF7 genes, and their correlations with intramuscular fat content in yak. Acta Vet. Zootech. Sin. 2017, 48, 416–424.
- Thaller, G.; Kühn, C.; Winter, A.; Ewald, G.; Bellmann, O.; Wegner, J.; Zühlke, H.; Fries, R. DGAT1, a new positional and functional candidate gene for intramuscular fat deposition in cattle. Anim. Genet. 2003, 34, 354–357.
- Kong, H.S.; Oh, J.D.; Lee, J.H.; Yoon, D.H.; Choi, Y.H.; Cho, B.W.; Lee, H.K.; Jeon, G.J. Association of Sequence Variations in DGAT 1 Gene with Economic Traits in Hanwoo (Korea Cattle). Asian-Australas. J. Anim. Sci. 2007, 20, 817–820.
- Middleton, C.K.; Kazala, E.C.; Lozeman, F.J.; Hurly, T.A.; Mir, P.S.; Bailey, D.R.C.; Jones, S.D.M.; Weselake, R.J. Evaluation of diacylglycerol acyltransferase as an indicator of intramuscular fat content in beef cattle. Can. J. Anim. Sci. 1998, 78, 265–270.
- Wang, Y.H.; Bower, N.I.; Reverter, A.; Tan, S.H.; De Jager, N.; Wang, R.; McWilliam, S.M.; Cafe, L.M.; Greenwood, P.L.; Lehnert, S.A. Gene expression patterns during intramuscular fat development in cattle. J. Anim. Sci. 2009, 87, 119–130.
- Huang, Y.; Das, A.K.; Yang, Q.Y.; Zhu, M.J.; Du, M. Zfp423 promotes adipogenic differentiation of bovine stromal vascular cells. PLoS ONE 2012, 7, e47496.
- Widmann, P.; Nuernberg, K.; Kuehn, C.; Weikard, R. Association of an ACSL1 gene variant with polyunsaturated fatty acids in bovine skeletal muscle. BMC Genet. 2011, 12, 96.
- Zhao, Z.; Abbas Raza, S.H.; Tian, H.; Shi, B.; Luo, Y.; Wang, J.; Liu, X.; Li, S.; Bai, Y.; Hu, J. Effects of overexpression of ACSL1 gene on the synthesis of unsaturated fatty acids in adipocytes of bovine. Arch. Biochem. Biophys. 2020, 695, 108648.
- Zhang, S.; Zan, L.; Wang, H.; Wang, G.; Fu, C.; Zhang, Y.; Mei, C.; Deng, G. Association of CD36 gene polymorphism with meat traits in Qinchuan cattle. Acta Vet. Zootech. Sin. 2014, 45, 1060–1067.
- Cui, H.; Meng, Q.; Qu, Y.; Liu, H.; Feng, C.; Wang, H.; Liu, Y.; Zan, L.; Li, N. Two novel missense mutations in bovine ATGL gene and their association with economic traits in Qinchuan cattle. Afr. J. Biotechnol. 2011, 10, 2353–2359.
- Jeong, J.Y.; Kim, J.S.; Nguyen, T.H.; Lee, H.J.; Baik, M. Wnt/β-catenin signaling and adipogenic genes are associated with intra-muscular fat content in the longissimus dorsi muscle of Korean cattle. Anim. Genet. 2013, 44, 627–635.
- Liu, X.; Zhao, H.; Jin, Q.; You, W.; Cheng, H.; Liu, Y.; Song, E.; Liu, G.; Tan, X.; Zhang, X.; et al. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signalling pathway in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2018, 439, 213–223.
- Guo, H.; Raza, S.H.A.; Schreurs, N.M.; Khan, R.; Wei, D.; Wang, L.; Zhang, S.; Zhang, L.; Wu, S.; Ullah, I.; et al. Genetic variants in the promoter region of the KLF3 gene associated with fat deposition in Qinchuan cattle. Gene 2018, 672, 50–55.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
987
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




