The marketing of fresh beef relies significantly on IMF, especially in the
longissimus dorsi muscle (
LM), where higher grades command higher prices. The current dietary trend suggests that consumers are more concerned about saturated fatty acids (
SFA) in the diet, as a high intake of SFA may increase the risk of cardiovascular disease [
6]. Intramuscular fat contains more monounsaturated (
MUFA) and polyunsaturated fatty acids (
PUFA) than other fat depots in cattle [
3]. Beef with a higher percentage of IMF, or a higher degree of “marbling”, has a higher ratio of MUFA to SFA, and is therefore considered healthier meat [
7].
2. Adipogenesis
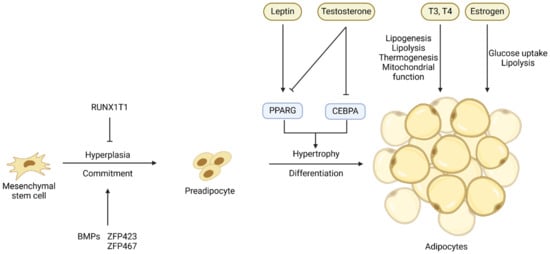
Adipocytes are terminally differentiated cells of adipose tissue, and the change of adipose tissue mass depends on both the hyperplasia (cell number increase) and hypertrophy (cell size increase) of adipocytes [
23]. Adipocytes originate from mesenchymal stem cells (
MSCs) or adipose progenitor cells (
APCs) [
24]. Adipogenesis can be divided into two stages: commitment (or determination) and differentiation [
25] (
Figure 1). The transition from APCs or MSCs to preadipocytes, which is marked by the expression of the
PPARG gene, is controlled at the transcriptional level by several transcription factors and extracellular signals (
Figure 1). Bone morphogenetic proteins (
BMPs) [
16,
26], platelet-derived growth factor receptor alpha (
PDGFRA) [
27], and zinc finger proteins 423 and 467 (
ZFP423 and
ZFP467) [
28] are the major regulators of the commitment of MSCs or APCs to preadipocytes, while factors like RUNX1 partner transcriptional co-repressor 1 (
RUNX1T1) [
29] suppress this commitment [
30].
Figure 1. Key transcriptional and hormonal regulators of the commitment of mesenchymal stem cells or adipose progenitor cells to preadipocytes and the differentiation of preadipocytes into adipocytes. Arrows denote stimulation and T-shaped lines denote inhibition.
The process of adipogenic differentiation has been extensively studied, with PPARG and CEBPs identified as the core regulators of this process [
31]. The
CEBPB and
CEBPD genes are expressed early in adipogenic differentiation, and they induce the co-expression of
PPARG and
CEBPA, two crucial transcription factors in the later stages of adipogenesis [
32,
33,
34]. These transcription factors ultimately activate the expression of genes specific for adipocytes and the deposition of lipids, resulting in the formation of mature adipocytes [
35]. The process of differentiation of preadipocytes into adipocytes is also controlled by hormones and other extracellular signals such as leptin and testosterone (
Figure 1), and these extracellular signals will be discussed in detail in the sections below.
3. Adipogenesis within Skeletal Msucle
The majority of research on the developmental biology of IMF has been conducted in the mouse model, although mice are not known for their ability to accumulate IMF. In mice, IMF formation starts in the late stages of pregnancy [
36]. In the process of embryonic skeletal muscle development, primary myofibers are first formed followed by secondary myofibers [
37]. The formation of adipocytes, i.e., adipogenesis, in skeletal muscle overlaps that of secondary myofibers in the middle or late stages of pregnancy [
38]. It is highly likely that mesenchymal progenitor cells differentiate into either myogenic or fibro/adipogenic lineage [
20]. Cells from the myogenic lineage further develop into muscle fibers, intramuscular brown adipocytes, and satellite cells, while cells from the fibro/adipogenic lineage develop into white adipocytes and fibroblasts [
39]. Since myogenic and adipogenic lineage cells originate from the same pool of stem cells, the commitment of stem cells to myogenesis or adipogenesis is a competing process. More myogenesis implies suppressed adipogenesis, and vice versa. The formation of initial adipocytes has a dominant effect on the number of total intramuscular adipocytes. As a result of the paracrine effects and their close proximity to adipocytes, skeletal muscle insulin resistance develops, causing a shift in the commitment of stem cells from myogenesis to adipogenesis [
40,
41].
During embryonic development, the Wnt signaling pathway activates myogenic and osteogenic differentiation while inhibiting the adipogenic differentiation of mesenchymal multipotent cells [
38,
42]. This primarily involves Wnt signaling suppressing the key adipogenic regulators CEBPA and PPARG [
43]. Farmer and colleagues observed a functional interaction between β-catenin (a component of the Wnt signaling pathway) and PPARG, wherein these two proteins negatively regulate the activity of each other [
44]. The Wnt signaling pathway is thought to be involved in maintaining a balance between adipogenesis and myogenesis. Specifically, the loss of WNT10B, either due to aging or targeted gene deletion, leads to an increased adipogenic potential of myoblasts and the acquisition of adipocyte characteristics during the process of muscle regeneration [
45]. Bromodomain-containing protein 4 (
BRD4) is a member of the bromodomain and extra-terminal domain (
BET) family of proteins, which are epigenetic readers that recognize acetylated histones and facilitate the recruitment of transcriptional regulators that promote the expression of
PPARG.
BRD4 knockout inhibits the expression of
PPARG and suppresses adipogenesis [
46]. Cooperating with lineage-determining transcription factors (
LDTFs), BRD4 recruits regulators such as MLL3/MLL4 and CBP/p300 to the enhancers [
47].
BRD4 knockout mice showed reduced brown fat and muscle mass, indicating that BRD4 is an important factor for both adipogenesis and myogenesis [
47]. Myostatin (
MSTN) is a major negative regulator of skeletal muscle development and growth [
48]. Myostatin also inhibits adipose tissue growth by reducing lipid accumulation through the ERK1/2 and PKA signaling pathways [
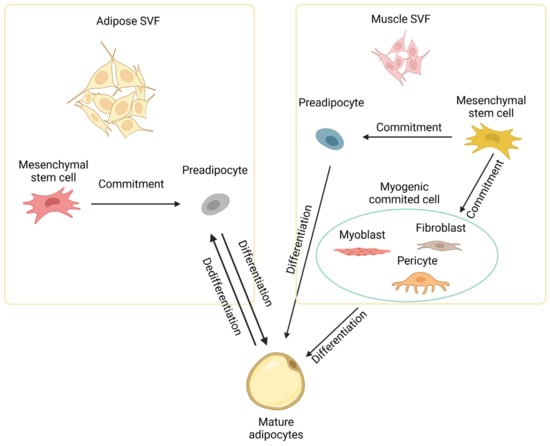
49]. In addition to multipotent stem cells, highly committed cells like myoblasts [
50], fibroblasts [
51], and pericytes [
52] in muscle tissue can also be induced to differentiate into mature adipocytes at least in vitro, as depicted in
Figure 2.
Figure 2. Development of intramuscular adipocytes. Intramuscular adipocytes can be formed from mesenchymal stem cells in the stromal vascular fraction (SVF) of both adipose and muscle tissues through commitment and differentiation. Intramuscular adipocytes might also be formed from committed cells like myoblasts, fibroblasts, and pericytes in muscle. Diagram has been drawn based mainly on mouse studies. See text for details.
In cattle, embryonic stem cells begin to form adipocytes at three months of gestation. Visceral adipocytes are formed first, followed by subcutaneous adipocytes, intermuscular adipocytes, and lastly intramuscular adipocytes [
53]. The first intramuscular adipocytes are generated at around 180 days of gestation [
54]. Since intramuscular fat matures later than subcutaneous fat, pursuing a high level of marbling often increases the overall carcass fat, particularly subcutaneous fat [
55]. Fortunately, the hyperplasia phase of intramuscular adipocytes continues until 250 days of age, far surpassing other fat cell populations, which stop hyperplasia during the weaning stage (intermuscular and subcutaneous adipocytes) or the neonatal stage (visceral adipocytes) [
56]. The differential timing of adipocyte hyperplasia creates the concept of a “marbling window” (from 150 to 250 days of age), which provides an opportunity for stimulating intramuscular adipocyte formation without increasing overall fat accumulation. During this period of marbling window, active intramuscular preadipocyte proliferation is a major mechanism of IMF deposition [
57]. From 250 days of age to slaughter, the hypertrophy of adipocytes plays a more important role than hyperplasia in IMF growth [
53,
58].
The primary driving force in current research on IMF is to find a way to improve IMF growth while avoiding an overall increase in body fat. A number of genes that potentially regulate IMF development and growth in cattle have been identified (Table 1). These genes are often the focus of research aimed at evaluating how various factors, such as dietary supplements, influence adipose tissue development and growth in cattle.
Table 1. Genes associated with intramuscular fat development in cattle.
| Genes |
Species |
Association with IMF |
Impact |
References |
| ICER |
Hanwoo |
Highly expressed in the late fattening stage and during preadipocyte differentiation into adipocytes |
+ |
[59] |
| WISP2 |
Wagyu |
Highly expressed in adipose precursor cells |
+ |
[60] |
| PPARG |
|
A master transcriptional regulator of adipose differentiation |
+ |
[61] |
| PDGFRA |
Angus |
Abundance is correlated with IMF content |
+ |
[62] |
| CEBPA |
|
A core transcription factor of fat differentiation |
+ |
[63] |
| SCD1 |
Wagyu, Simmental |
Increases IMF deposition and unsaturated fatty acid content |
+ |
[64] |
| KLF |
Yak |
Inhibitory factor of IMF differentiation |
− |
[65] |
| DGAT1 |
Holstein, Charolais, |
Involved in fatty acid esterification and correlated with IMF content |
+ |
[66,67,68] |
| ADIPOQ, THRSP |
Wagyu × Hereford |
Abundance is highly correlated with IMF content |
+ |
[69] |
| ZFP423 |
Angus |
Promotes adipogenic differentiation of muscle stromal vascular cells |
+ |
[70] |
| ACSL1 |
Charolais × Holstein |
Regulates lipid composition and polyunsaturated fatty acids synthesis in adipocytes |
+ |
[71,72] |
| CD36 |
Qinchuan |
Cattle with the combined genotype WWCCAA in the CD36 gene had higher IMF contents |
+ |
[73] |
| ABHD5 |
Qinchuan |
An accelerator for adipose triglyceride lipase |
− |
[74] |
| WNT gene family |
Korean |
Inhibits the differentiation of fat cells |
− |
[75] |
| FOXO1 |
Luxi |
Affects the expression of genes associated with apoptosis of intramuscular adipocytes |
− |
[76] |
| KLF3 |
Qinchuan |
The polymorphism of the KLF3 gene has an impact on the intramuscular fat content |
− |
[77] |