
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberto Bava | -- | 4263 | 2024-02-26 14:24:36 | | | |
| 2 | Peter Tang | Meta information modification | 4263 | 2024-02-27 02:01:11 | | |
Video Upload Options
Plants are an untapped natural resource; their secondary metabolites take part in a variety of pharmacological activities, making them an essential ingredient in the synthesis of novel medications and the source of reserve resources in this process. Hepatitis and liver cancer are two conditions that can result from non-alcoholic fatty liver disease (NAFLD). NAFLD is a condition that now affects a significant section of the global population. There is a need for preventative action on predisposing factors. Due to their effectiveness and few side effects, herbal medications are frequently utilized for the prevention and treatment of NAFLD.
1. Introduction
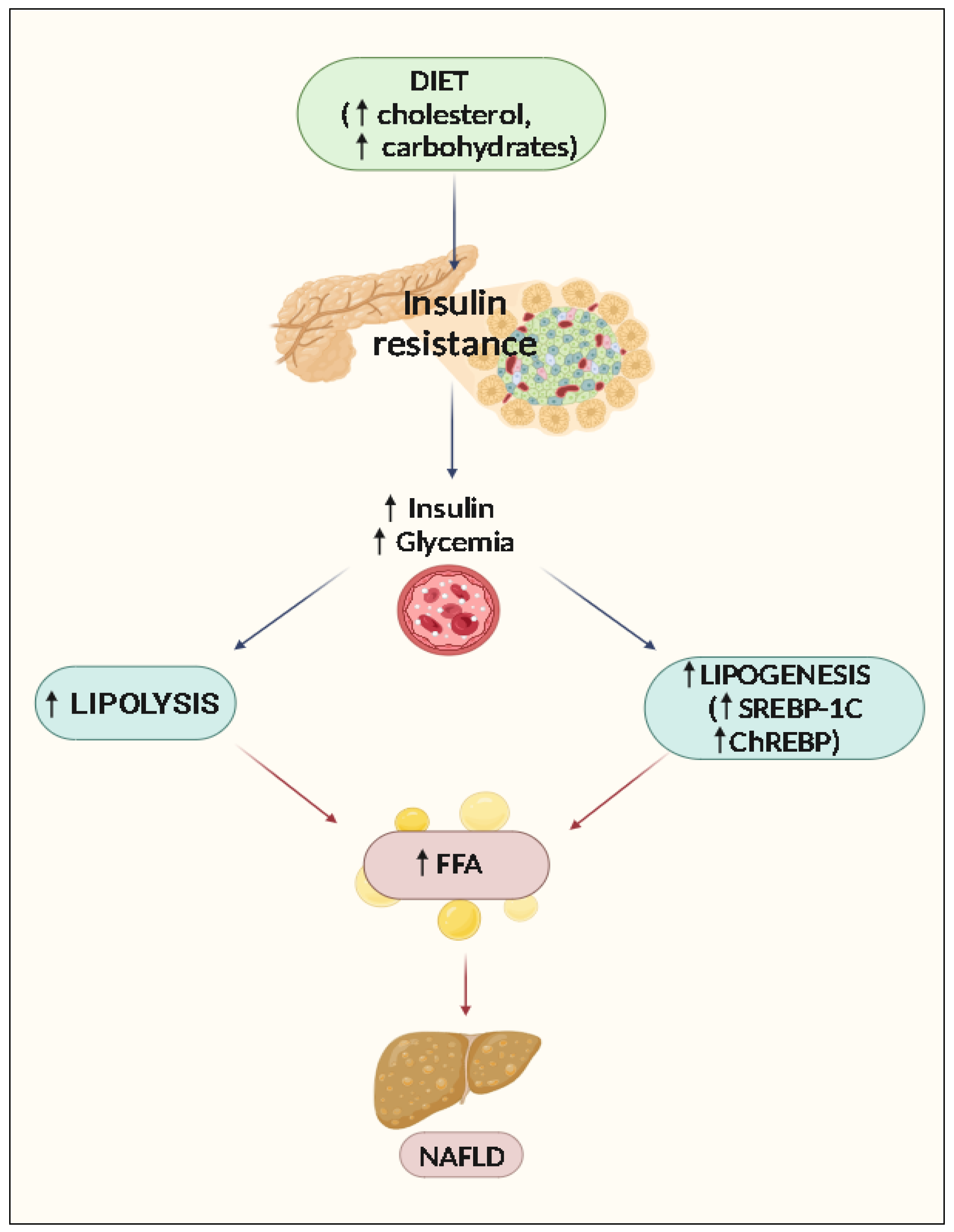
2. Pathophysiology of NAFLD
Accumulation of Lipids and Steatosis
3. Progression of Liver Damage: The Role of Oxidative Stress, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress
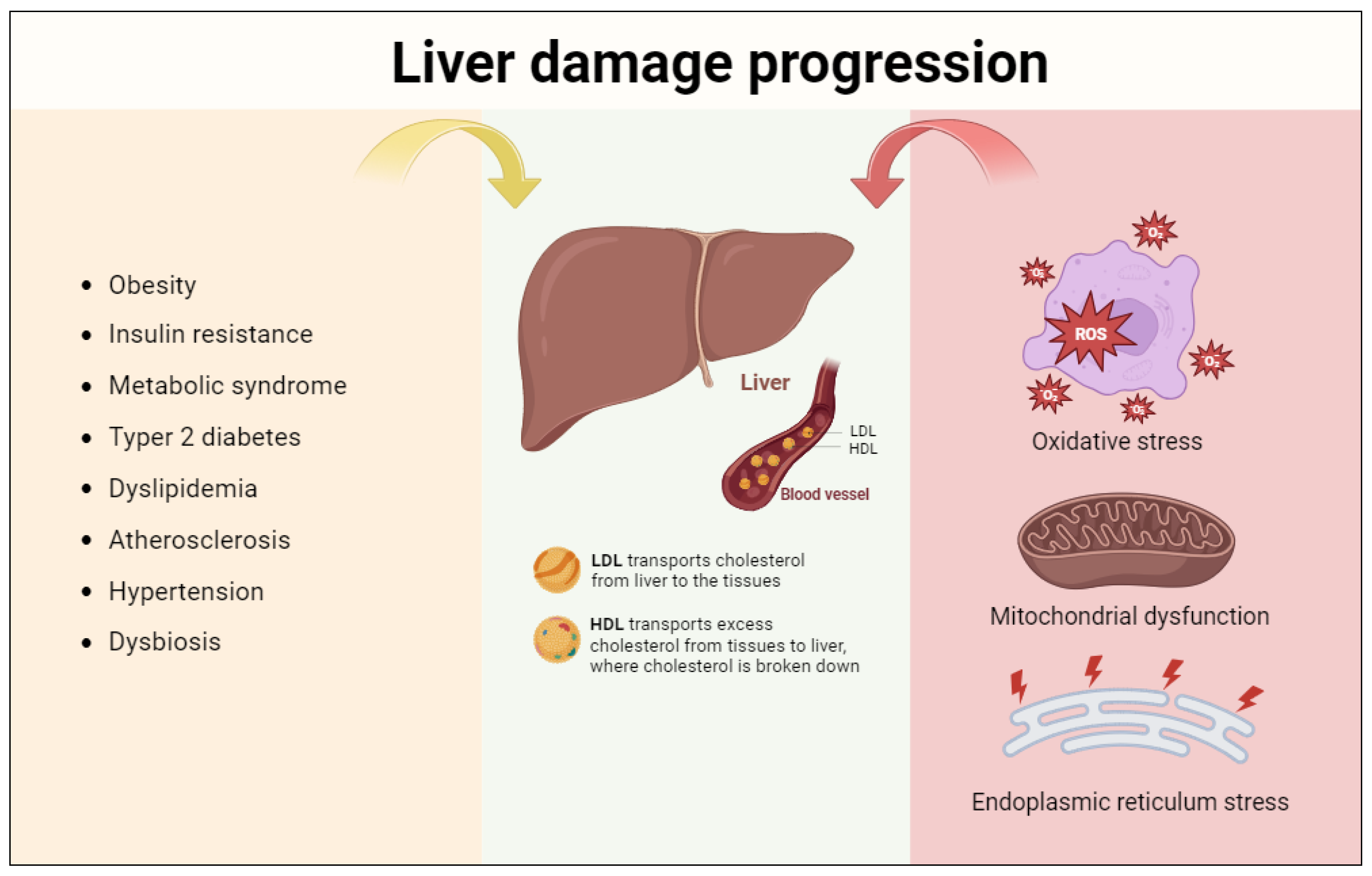
3.1. Oxidative Stress
3.2. Mitochondrial Dysfunction
3.3. Endoplasmic Reticulum Stress
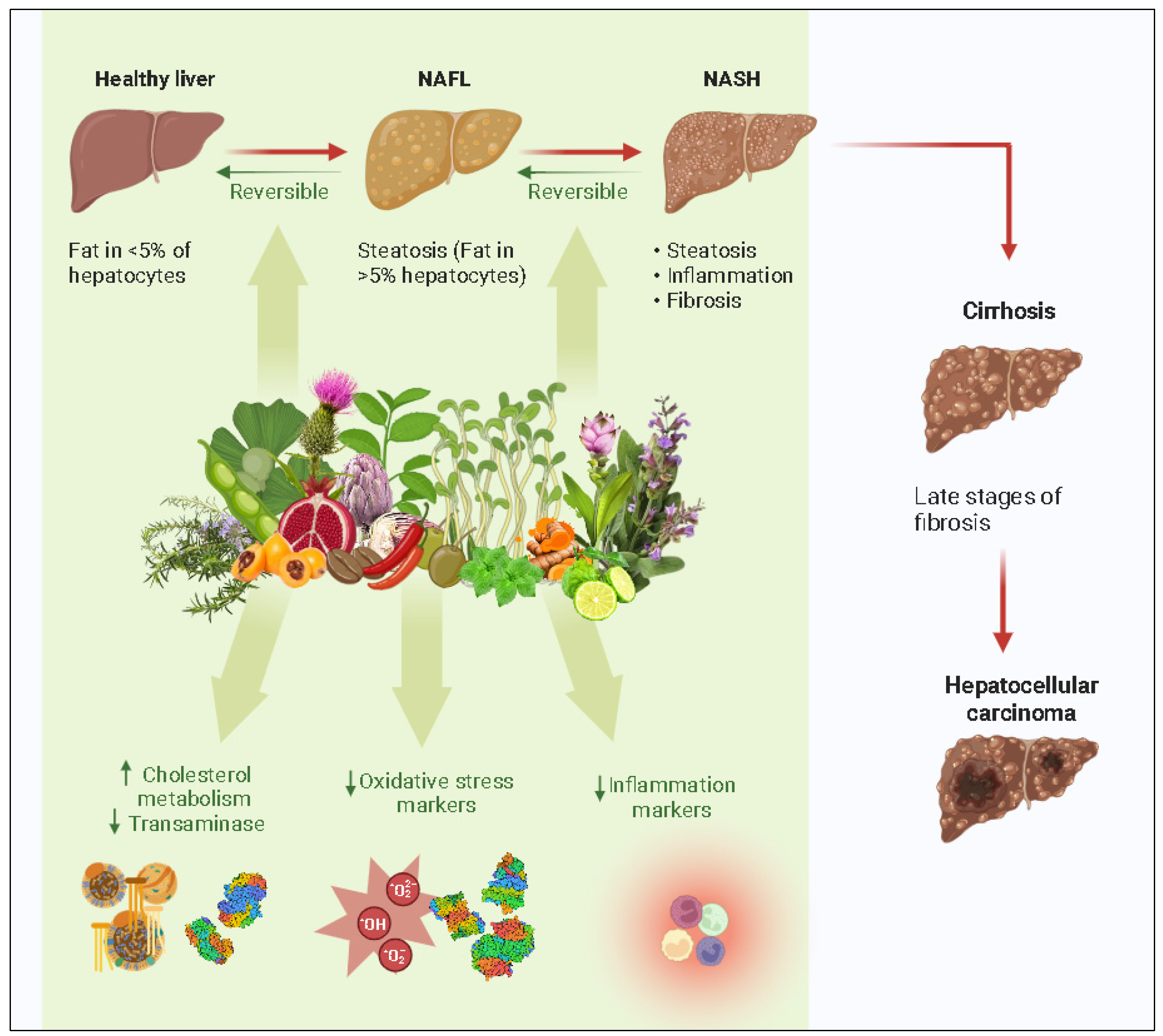
4. Natural Compounds Showing Activity towards NAFLD
|
Flavonoids Component |
Effects |
References |
|---|---|---|
|
Quercetin |
↓VLDL, ↓LDL, ↓TC, ↓TG, ↓FFA, ↓ALT, ↓AST, ↓PI3K/AKT pathway, ↑IRE1a/XBP1 pathway, ↓NAFLD activity score, ↓Lipid peroxidation, ↓TNFα, ↓IL-6, ↑SIRT1, ↓NF-kB, ↓iNOS, ↓TLR4 |
|
|
Anthocyanins |
↓TC, ↓TG, ↓LDL, ↑HDL, ↓ALT, ↑PPARα, ↓TLR4, ↓MAPK pathway, ↑AMPK pathway, ↓MPO, ↓MCP, ↓Fibrosis score, ↓WAT, ↓TNFα, ↓IL-1β, ↓IL-6, ↑IL-10, ↑IL-22, ↓ROS, ↑SOD, ↓COX-2, ↓iNOS |
|
|
Kaempferol |
↓TC, ↓ALT, ↓AST, ↑GSH, ↓MDA, ↑SIRT1, ↑CPT-1a, ↓SREBP-1c, ↓FAS, ↓SCD-1, ↓PPAR, ↓C/EBP, ↑SOD, ↓NF-kB ↓PARP1, ↓FOXO-1 |
[70] |
|
Hesperidin |
↓TC, ↓ALT, ↓AST, ↓RBP4, ↓H-FABP, ↓ROS, ↑HO-1, ↑PI3K/AKT-Nrf2 pathway, ↑SIRT1-AMPK pathway, ↑SOD, ↑GPx, ↑GSH, ↓TNFα, ↓IL-1β, ↓IL-2, ↓IL-6, ↓NF-kB |
|
|
Baicalein |
↓TC, ↓ALT, ↓AST, ↓LDL, ↓TG, ↓FFA, ↓TNFα, ↓SREBP-1c, ↓SREBP-1c, ↓TG, ↓GLU, ↓FAS |
|
|
Troxerutin |
↑CYP1A1, ↑PGC-1α, ↑CPT-1 β, ↑GSH, ↑PPARα, ↓SREBP-1c, ↓ROS, ↓NF-kB, ↓FATP-1, ↓SCD1 |
|
|
Phenolic components |
||
|
Resveratrol |
↓TC, ↓TG, ↓ALT, ↓AST, ↑Nrf2, ↑HO-1, ↑SOD, ↑GPx, ↓ROS, ↓FAS, ↓SREBP-1c, ↓IL-1, ↓IL-6, ↓TNFα, ↑TNFβ, ↑SIRT1-AMPK pathway, ↓NF-kB |
|
|
Curcumin |
↑HDL-C, ↓ALT, ↓AST, ↓TG, ↑Leptin, ↑Nrf2/FXR/LXR pathway, ↓CYP3A, ↓CYP7A, ↓CD4+, ↓Leptin, ↓ACC, ↓FAS, ↓SREBP-1c, ↓C/EBP, ↓PPAR, ↓SCD, ↑HO-1, ↓ROS, ↑AMPK/mTOR pathway |
|
|
Alkaloid components |
||
|
Berberine |
↓TC, ↓TG, ↓ALT, ↓AST, ↑FXR, ↓SREBP-1c, ↓FAS, ↑SOD, ↑GSH, ↓MDA, ↓TNFα, ↓IL-1, ↓IL-6, ↓NF-kB, ↑Nrf2/ARE, ↓TLR4, ↓MyD88, ↑MTTP, ↑CPT-1a, ↑GCK |
|
|
Betaine |
↓TC, ↓TG, ↓FFA, ↓ALT, ↓AST, ↑GSH, ↑GPx, ↓NAFLD activity score, ↑SOD, ↑CAT, ↑MDA, ↓ROS, ↓TLR4, ↓NF-kB, ↓IL-1, ↓IL-6, ↓MAPK, ↓FOXO-1 |
|
|
Terpenes components |
||
|
Celastrol |
↓TG, ↓FFA, ↓ALT, ↓SREBP-1c, ↑AMPKα, ↑LKB1, ↓NF-kB, ↑SIRT1, ↓TNFα, ↓IL-1β, ↓IL-6, ↓ROS, ↑Nrf2/HO-1 |
|
|
Boswellic acid |
↓TC, ↓ALT, ↓AST, ↓TNFα, ↓IL-6, ↓iNOS, ↑UCP1, ↑CPT1, ↓NF-kB, ↓TGFβ, ↓COX-2 |
|
↑: Increase, ↓: decrease of expression, concentrations, or activity. ACC: acetyl-CoA carboxylase; AKT: serine/threonine kinase 1; ALT: alanine aminotransferase; AMPK: adenosine monophosphate-activated protein kinase; ARE: antioxidant responsive element; AST: aspartate aminotransferase; CAT: catalase; C/EBP: CCAAT/enhancer binding protein; CPT-1a: carnitine palmitoyl transferase-1; FAS: fatty acid synthase; SCD-1: stearoyl-CoA desaturase 1; FFA: free fatty acids; FOXO-1: forkhead box protein O1; GCK: germinal center kinase; GPx: glutathione peroxidase; GSH: glutathione; HO-1: heme oxygenase-1; iNOS: inducible nitric oxide synthase; IL: interleukin; IRE1a: inositol-requiring enzyme 1a; LDL: low-density lipoprotein; LKB1: liver kinase B1 (STK11); MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; MPO: myeloperoxidase; MTTP: microsomal triglyceride transfer protein; MyD88: myeloid differentiation primary response gene 88; Nrf2: Nuclear factor erythroid 2-related factor 2; PI3K: phosphoinositide-3-kinase; PPARα: peroxisome proliferator-activated receptor α; RBP4: retinol-binding protein-4; ROS: reactive oxygen species; SIRT1: silent mating type information regulation 2 homolog 1; SOD: superoxide dismutase; SREBP-1c: sterol regulatory element binding protein-1c; TC: total cholesterol; TLR4: Toll-like receptor 4; TG: triglycerides; TNF: tumor necrosis factor; UCP1: uncoupling protein-1; VLDL: very low density lipoprotein; WAT: white adipose tissue; and XBP1: X-box-binding protein 1.
|
Plants |
Effects |
References |
|---|---|---|
|
Tea (Camellia sinensis) |
↓ALT, ↓AST, ↓NADPH, ↓MPO, ↓RO, ↓Lipid peroxidation |
|
|
Curcuma (Curcuma longa) |
↓TC, ↓TG, ↓LDL, ↑HDL, ↓ALT, ↓AST, ↓ROS, ↓Lipid peroxidation, ↓Cyt-c, ↓CASP 3, ↓CASP 8, ↓NF-kB, ↑AMPK, ↓SREBP-1c, ↓FAS, |
|
|
Loquat (Eriobotrya japonica) |
↓ALT, ↓AST, ↓ROS, ↓TGF, ↓Collagen |
|
|
Ginkgo (Ginkgo biloba) |
↓TC, ↓LDL, ↓TG, ↑HDL, ↓ALT, ↓AST, ↑CPT-1a, ↓TNFα |
|
|
Olive tree (Olea europaea) |
↓TRX-1, ↓4-HNE, ↓ROS, ↑Nrf2, ↓SOD, ↓CAT, ↓GPx, ↓IL-6, ↓IL-8, ↓TNFα |
|
|
Pomegranate (Punica granatum) |
↓TC, ↓TG, ↓LDL, ↑PPAR, ↑CPT-1a, ↑ACO, ↓SCD-1, ↓ALT, ↓AST |
|
|
Milk thistle (Silybum marianum) |
↑AMPK activity, ↓PPAR-γ, ↓ACC, ↓ROS, ↓FAS, ↓SIRT1, ↑CAT, ↑NAD+ homeostasis, ↑IRS-1/PI3K/Akt pathway, ↓FXR, ↓NF-kB, ↓4-HNE, ↓glutathione depletion, ↓mitochondrial hydrogen peroxide formation |
|
|
Coffea spp. |
↑SIRT3, ↑AMPK, ↓PPAR-α, ↓SREBP-1c, ↓ROS, ↑Bcl-2, ↓Bax, ↑STAT-3, ↓TLR4 |
|
|
Red rice (Oryza sativa) |
↓TC, ↓TG, ↓HMG-CoA reductase, ↓ROS, |
|
|
Artichoke (Cynara scolymus) |
↓ALT, ↓AST, ↓ROS, ↓MDA, ↓8-deoxyguanosine, ↓CoQ9, ↓GSH |
|
|
Soy (Glycine max) |
↓TG, ↓ALT, ↓AST, ↓ROS, ↑SOD, ↑CAT, ↓FFA, ↑UCP2 |
|
|
Alfalfa (Medicago sativa) |
↓LDL, ↑2-deoxy-glucose, ↑glycogen, ↓HMG-CoA reductase, ↓ACAT2 |
|
|
Bergamot (Citrus bergamia) |
↓TC, ↓TG, ↓LDL, ↓ROS, ↑HDL, ↓Cardiomyocyte death, ↑autophagy, ↑SOD, ↑GPx, ↓MDA, ↓TNF |
[149][150][151][152][153][154][155][156][157][158][159][160][161][162][163] |
|
Rosemary (Rosmarinus officinalis) |
↓ALT, ↓AST, ↓TAG, ↓FFA ↑AMPK, ↑PPAR, ↓SIRT1/p66shc pathway |
|
|
Peppermint (Mentha piperita) |
↓TC, ↓LDL, ↓TAG ↓CYP2B9, ↓Leptin, ↓ROS, ↓lipogenesis, ↑PPAR, ↓SREBP1 |
|
|
Sage (Salvia officinalis) |
↓TC, ↓LDL, ↑HDL, ↓ALT, ↓AST, ↑PPAR, ↓Lipase |
|
|
Hot pepper (Capsicum annuum) |
↑TRPV1, ↑PPAR ↓IL-6, ↓TNF-α, ↓MCP-1, ↓COX-2 |
↑: Increase, ↓: decrease of expression, concentrations, or activity. 4-HNE: 4-hydroxynonenal; ACAT2: acetyl-CoA acetyltransferase 2; ACC: acetyl-CoA carboxylase; ACO: acyl-CoA oxidase; AKT: serine/threonine kinase 1; ALT: alanine aminotransferase; AMPK: adenosine monophosphate-activated protein kinase; ARE: antioxidant responsive element; AST: aspartate aminotransferase; CAT: catalase; CASP: caspase; CoQ9: coenzyme Q9; CPT-1a: carnitine palmitoyl transferase-1; CYT: cytochrome; FAS: fatty acid synthase; SCD-1: stearoyl-CoA desaturase 1; FFA: free fatty acids; FOXO-1: forkhead box protein O1; GCK: germinal center kinase; GPx: glutathione peroxidase; GSH: glutathione; HO-1: heme oxygenase-1; IL: interleukin; IRE1a: inositol-requiring enzyme 1a; LDL: low-density lipoprotein; MDA: malondialdehyde; MPO: myeloperoxidase; MTTP: microsomal triglyceride transfer protein; Nrf2: Nuclear factor erythroid 2-related factor 2; PI3K: phosphoinositide-3-kinase; PPARα: peroxisome proliferator-activated receptor α; ROS: reactive oxygen species; SIRT1: silent mating type information regulation 2 homolog 1; SOD: superoxide dismutase; SREBP-1c: sterol regulatory element binding protein-1c; TC: total cholesterol; STAT3: Signal transducer and activator of transcription 3; TAG: tryacylglicerol; TLR4: toll-like receptor 4; TG: triglycerides; TGF: transforming growth factor; TNF: tumor necrosis factor; TRPV1: Transient receptor potential vanilloid 1; TRX-1: thioredoxin-1; UCP1: uncoupling protein-1; VLDL: very low density lipoprotein; WAT: white adipose tissue; and XBP1: X-box-binding protein 1.
References
- Hassan, K.; Bhalla, V.; El Regal, M.E.; A-Kader, H.H. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J. Gastroenterol. 2014, 20, 12082.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945.
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis—New insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636.
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185.
- Propst, A. Prognosis in nonalcoholic steatohepatitis. Gastroenterology 1995, 108, 1607.
- Nseir, W.; Hellou, E.; Assy, N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 9338.
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of art. Int. J. Mol. Sci. 2021, 22, 2350.
- Talebi, S.; Bagherniya, M.; Atkin, S.L.; Askari, G.; Orafai, H.M.; Sahebkar, A. The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: A clinical review. Lipids Health Dis. 2020, 19, 1–21.
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural bioactive compounds useful in clinical management of metabolic syndrome. Nutrients 2021, 13, 630.
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109.
- Jo, H.K.; Kim, G.W.; Jeong, K.J.; Do, Y.K.; Chung, S.H. Eugenol ameliorates hepatic steatosis and fibrosis by down-regulating SREBP1 gene expression via AMPK-mTOR-p70S6K signaling pathway. Biol. Pharm. Bull. 2014, 37, 1341–1351.
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919.
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019, 11, 677.
- Mills, S.J.; Harrison, S.A. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 2005, 7, 32–36.
- Moore, J.B. Non-alcoholic fatty liver disease: The hepatic consequence of obesity and the metabolic syndrome. Proc. Nutr. Soc. 2010, 69, 211–220.
- Masarone, M.; Federico, A.; Abenavoli, L.; Loguercio, C.; Persico, M. Non alcoholic fatty liver: Epidemiology and natural history. Rev. Recent Clin. Trials 2014, 9, 126–133.
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Zenari, L.; Cigolini, M.; Falezza, G.; Arcaro, G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006, 29, 1325–1330.
- Kasper, P.; Martin, A.; Lang, S.; Kuetting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937.
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.
- Nucera, S.; Ruga, S.; Cardamone, A.; Coppoletta, A.R.; Guarnieri, L.; Zito, M.C.; Bosco, F.; Macrì, R.; Scarano, F.; Scicchitano, M. MAFLD progression contributes to altered thalamus metabolism and brain structure. Sci. Rep. 2022, 12, 1207.
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: Evidence from a randomized clinical trial. J. Gastrointest. Liver Dis. 2018, 27, 41–49.
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302.
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9.
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 32002.
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062.
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775.
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023.
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845.
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42.
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564.
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728.
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846.
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351.
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441.
- Goldberg, I.J. Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971.
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742.
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949.
- Kovacs, P.; Stumvoll, M. Fatty acids and insulin resistance in muscle and liver. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 625–635.
- Pan, M.; Lai, C.; Tsai, M.; Ho, C. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol. Nutr. Food Res. 2014, 58, 147–171.
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 2013, 8, e54059.
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115.
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038.
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Khorzoughi, R.B.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667.
- Robertson, G.; Leclercq, I.; Farrell, G.C., II. Cytochrome P-450 enzymes and oxidative stress. Am. J. Physiol. Liver Physiol. 2001, 281, G1135–G1139.
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26.
- Mansouri, A.; Demeilliers, C.; Amsellem, S.; Pessayre, D.; Fromenty, B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J. Pharmacol. Exp. Ther. 2001, 298, 737–743.
- Weltman, M.D.; Farrell, G.C.; Hall, P.; Ingelman-Sundberg, M.; Liddle, C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998, 27, 128–133.
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer cells in non-alcoholic fatty liver disease: Friend or foe? Int. J. Biol. Sci. 2020, 16, 2367.
- Zisser, A.; Ipsen, D.H.; Tveden-Nyborg, P. Hepatic stellate cell activation and inactivation in NASH-fibrosis—Roles as putative treatment targets? Biomedicines 2021, 9, 365.
- Oktia, R.T.; Okita, J.R. Cytochrome P450 4A fatty acid omega hydroxylases. Curr. Drug Metab. 2001, 2, 265–281.
- Hardwick, J.P.; Osei-Hyiaman, D.; Wiland, H.; Abdelmegeed, M.A.; Song, B.-J. PPAR/RXR regulation of fatty acid metabolism and fatty acid ω-hydroxylase (CYP4) isozymes: Implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009, 2009, 952734.
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.-H.; Aguilar, M.-I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457.
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388.
- Reddy, J.K.; Sambasiva Rao, M. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Liver Physiol. 2006, 290, G852–G858.
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358.
- Durand, M.; Coué, M.; Croyal, M.; Moyon, T.; Tesse, A.; Atger, F.; Ouguerram, K.; Jacobi, D. Changes in key mitochondrial lipids accompany mitochondrial dysfunction and oxidative stress in NAFLD. Oxid. Med. Cell. Longev. 2021, 2021, 9986299.
- Ron, D. Proteotoxicity in the endoplasmic reticulum: Lessons from the Akita diabetic mouse. J. Clin. Investig. 2002, 109, 443–445.
- Demirtas, L.; Guclu, A.; Erdur, F.M.; Akbas, E.M.; Ozcicek, A.; Onk, D.; Turkmen, K. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J. Med. Res. 2016, 144, 515–524.
- Zhong, Q.; Wu, Y.; Xiong, F.; Liu, M.; Liu, Y.; Wang, C.; Chen, Y. Higher flavonoid intake is associated with a lower progression risk of non-alcoholic fatty liver disease in adults: A prospective study. Br. J. Nutr. 2021, 125, 460–470.
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60.
- Ji, G.; Wang, Y.; Deng, Y.; Li, X.; Jiang, Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015, 14, 134.
- Suenaga, F.; Hatsushika, K.; Takano, S.; Ando, T.; Ohnuma, Y.; Ogawa, H.; Nakao, A. A possible link between resveratrol and TGF-β: Resveratrol induction of TGF-β expression and signaling. FEBS Lett. 2008, 582, 586–590.
- Zhang, P.-W.; Chen, F.-X.; Li, D.; Ling, W.-H.; Guo, H.-H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758.
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327.
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315.
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e0154817.
- Zhang, Y.; Meng, Q.; Yin, J.; Zhang, Z.; Bao, H.; Wang, X. Anthocyanins attenuate neuroinflammation through the suppression of MLK3 activation in a mouse model of perioperative neurocognitive disorders. Brain Res. 2020, 1726, 146504.
- Karunarathne, W.A.H.M.; Lee, K.T.; Choi, Y.H.; Jin, C.-Y.; Kim, G.-Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine 2020, 76, 153237.
- Zang, Y.; Zhang, D.; Yu, C.; Jin, C.; Igarashi, K. Antioxidant and hepatoprotective activity of kaempferol 3-O-β-D-(2, 6-di-O-α-L-rhamnopyranosyl) galactopyronoside against carbon tetrachloride-induced liver injury in mice. Food Sci. Biotechnol. 2017, 26, 1071–1076.
- Wang, X.; Hasegawa, J.; Kitamura, Y.; Wang, Z.; Matsuda, A.; Shinoda, W.; Miura, N.; Kimura, K. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J. Pharmacol. Sci. 2011, 117, 129–138.
- Kumar, R.; Khan, M.I.; Ashfaq, F.; Alsayegh, A.A.; Khatoon, F.; Altamimi, T.N.; Rizvi, S.I. Hesperidin Supplementation Improves Altered PON-1, LDL Oxidation, Inflammatory Response and Hepatic Function in an Experimental Rat Model of Hyperlipidemia. Indian J. Clin. Biochem. 2023, 1–7.
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331.
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial. Phyther. Res. 2019, 33, 2118–2125.
- Guo, H.; Liu, D.; Ma, Y.; Liu, J.; Wang, Y.; Du, Z.; Wang, X.; Shen, J.; Peng, H. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol. Sin. 2009, 30, 1505–1512.
- Xi, Y.; Wu, M.; Li, H.; Dong, S.; Luo, E.; Gu, M.; Shen, X.; Jiang, Y.; Liu, Y.; Liu, H. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: Dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell. Physiol. Biochem. 2015, 35, 2349–2359.
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666.
- Malinska, H.; Hüttl, M.; Oliyarnyk, O.; Markova, I.; Poruba, M.; Racova, Z.; Kazdova, L.; Vecera, R. Beneficial effects of troxerutin on metabolic disorders in non-obese model of metabolic syndrome. PLoS ONE 2019, 14, e0220377.
- Geetha, R.; Yogalakshmi, B.; Sreeja, S.; Bhavani, K.; Anuradha, C.V. Troxerutin suppresses lipid abnormalities in the heart of high-fat–high-fructose diet-fed mice. Mol. Cell. Biochem. 2014, 387, 123–134.
- Zhang, Z.; Wang, X.; Zheng, G.; Shan, Q.; Lu, J.; Fan, S.; Sun, C.; Wu, D.; Zhang, C.; Su, W. Troxerutin attenuates enhancement of hepatic gluconeogenesis by inhibiting NOD activation-mediated inflammation in high-fat diet-treated mice. Int. J. Mol. Sci. 2016, 18, 31.
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843.
- Cheng, K.; Song, Z.; Zhang, H.; Li, S.; Wang, C.; Zhang, L.; Wang, T. The therapeutic effects of resveratrol on hepatic steatosis in high-fat diet-induced obese mice by improving oxidative stress, inflammation and lipid-related gene transcriptional expression. Med. Mol. Morphol. 2019, 52, 187–197.
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84.
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281.
- Inzaugarat, M.E.; De Matteo, E.; Baz, P.; Lucero, D.; García, C.C.; Gonzalez Ballerga, E.; Daruich, J.; Sorda, J.A.; Wald, M.R.; Cherñavsky, A.C. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE 2017, 12, e0172900.
- Alappat, L.; Awad, A.B. Curcumin and obesity: Evidence and mechanisms. Nutr. Rev. 2010, 68, 729–738.
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649.
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8.
- Liu, Y.N.; Zhang, Z.X. Effect of berberine on a cellular model of non-alcoholic fatty liver disease. Int J Clin Exp Med 2017, 10, 16360–16366.
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098.
- Wang, L.; Jia, Z.; Wang, B.; Zhang, B. Berberine inhibits liver damage in rats with non-alcoholic fatty liver disease by regulating TLR4/MyD88/NF-κB pathway. Turkish J. Gastroenterol. 2020, 31, 902.
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS ONE 2015, 10, e0134172.
- Chen, W.; Zhang, X.; Xu, M.; Jiang, L.; Zhou, M.; Liu, W.; Chen, Z.; Wang, Y.; Zou, Q.; Wang, L. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE−/− mice. Eur. J. Nutr. 2021, 60, 1655–1668.
- Zhang, W.; Wang, L.; Wang, L.; Li, X.; Zhang, H.; Luo, L.-P.; Song, J.-C.; Gong, Z. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig. Dis. Sci. 2013, 58, 3198–3206.
- Chen, Y.; Liu, Y.; Zhou, R.; Chen, X.; Wang, C.; Tan, X.; Wang, L.; Zheng, R.; Zhang, H.; Ling, W. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076.
- Zhang, Y.; Geng, C.; Liu, X.; Li, M.; Gao, M.; Liu, X.; Fang, F.; Chang, Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol. Metab. 2017, 6, 138–147.
- Sun, J.; Wang, H.; Yu, J.; Li, T.; Han, Y. Protective effect of celastrol on type 2 diabetes mellitus with nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2020, 8, 6207–6216.
- Bakar, M.H.A.; Tan, J.S. Improvement of mitochondrial function by celastrol in palmitate-treated C2C12 myotubes via activation of PI3K-Akt signaling pathway. Biomed. Pharmacother. 2017, 93, 903–912.
- Yu, X.; Meng, X.; Xu, M.; Zhang, X.; Zhang, Y.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 2018, 36, 266–280.
- Zaitone, S.A.; Barakat, B.M.; Bilasy, S.E.; Fawzy, M.S.; Abdelaziz, E.Z.; Farag, N.E. Protective effect of boswellic acids versus pioglitazone in a rat model of diet-induced non-alcoholic fatty liver disease: Influence on insulin resistance and energy expenditure. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 587–600.
- Varma, K.; Haponiuk, J.T.; Gopi, S. Antiinflammatory Activity of Boswellia. In Inflammation and Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–159.
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol,(-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683.
- Kuzu, N.; Bahcecioglu, I.H.; Dagli, A.F.; Ozercan, I.H.; Ustündag, B.; Sahin, K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2008, 23, e465–e470.
- Chung, M.-Y.; Park, H.J.; Manautou, J.E.; Koo, S.I.; Bruno, R.S. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 2012, 23, 361–367.
- Nakamoto, K.; Takayama, F.; Mankura, M.; Hidaka, Y.; Egashira, T.; Ogino, T.; Kawasaki, H.; Mori, A. Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 2009, 44, 239–246.
- Elahi, R.K. Preventive effects of turmeric (Curcuma longa Linn.) powder on hepatic steatosis in the rats fed with high fat diet. Life Sci. J. 2012, 9, 5462–5468.
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855.
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202.
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 23.
- Perri, M.R.; Marrelli, M.; Statti, G.; Conforti, F. Olea europaea bud extracts: Inhibitory effects on pancreatic lipase and α-amylase activities of different cultivars from Calabria region (Italy). Plant Biosyst. 2020.
- Jafri, M.A.; Aslam, M.; Javed, K.; Singh, S. Effect of Punica granatum Linn.(flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2000, 70, 309–314.
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54.
- Li, Y.; Wen, S.; Kota, B.P.; Peng, G.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005, 99, 239–244.
- Xu, K.Z.-Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287.
- Hajiaghamohammadi, A.A.; Ziaee, A.; Oveisi, S.; Masroor, H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic fatty liver disease: A randomized controlled pilot study. Hepat. Mon. 2012, 12, e6099.
- Salomone, F.; Barbagallo, I.; Godos, J.; Lembo, V.; Currenti, W.; Cinà, D.; Avola, R.; D’Orazio, N.; Morisco, F.; Galvano, F. Silibinin restores NAD+ levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients 2017, 9, 1086.
- Cui, C.-X.; Deng, J.-N.; Yan, L.; Liu, Y.-Y.; Fan, J.-Y.; Mu, H.-N.; Sun, H.-Y.; Wang, Y.-H.; Han, J.-Y. Silibinin capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J. Ethnopharmacol. 2017, 208, 24–35.
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front. Pharmacol. 2016, 7, 345.
- Haddad, Y.; Vallerand, D.; Brault, A.; Haddad, P.S. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid.-Based Complement. Altern. Med. 2011, 2011, 647903.
- Serviddio, G.; Bellanti, F.; Giudetti, A.M.; Gnoni, G.V.; Petrella, A.; Tamborra, R.; Romano, A.D.; Rollo, T.; Vendemiale, G.; Altomare, E. A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J. Pharmacol. Exp. Ther. 2010, 332, 922–932.
- Kim, H.M.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet–induced obese mice by regulating autophagy. Nutrition 2018, 55, 63–70.
- Zhang, S.-J.; Li, Y.-F.; Wang, G.-E.; Tan, R.-R.; Tsoi, B.; Mao, G.-W.; Zhai, Y.-J.; Cao, L.-F.; Chen, M.; Kurihara, H. Caffeine ameliorates high energy diet-induced hepatic steatosis: Sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015, 6, 2578–2587.
- Watanabe, S.; Takahashi, T.; Ogawa, H.; Uehara, H.; Tsunematsu, T.; Baba, H.; Morimoto, Y.; Tsuneyama, K. Daily coffee intake inhibits pancreatic beta cell damage and nonalcoholic steatohepatitis in a mouse model of spontaneous metabolic syndrome, tsumura-suzuki obese diabetic mice. Metab. Syndr. Relat. Disord. 2017, 15, 170–177.
- Ontawong, A.; Boonphang, O.; Pasachan, T.; Duangjai, A.; Pongchaidecha, A.; Phatsara, M.; Jinakote, M.; Amornlerdpison, D.; Srimaroeng, C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct. Foods 2019, 54, 568–577.
- Vitaglione, P.; Mazzone, G.; Lembo, V.; D’Argenio, G.; Rossi, A.; Guido, M.; Savoia, M.; Salomone, F.; Mennella, I.; De Filippis, F. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut–liver axis. J. Nutr. Sci. 2019, 8, e15.
- Fang, C.; Cai, X.; Hayashi, S.; Hao, S.; Sakiyama, H.; Wang, X.; Yang, Q.; Akira, S.; Nishiguchi, S.; Fujiwara, N. Caffeine-stimulated muscle IL-6 mediates alleviation of non-alcoholic fatty liver disease. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 2019, 1864, 271–280.
- Brandt, A.; Nier, A.; Jin, C.J.; Baumann, A.; Jung, F.; Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C.; Bergheim, I. Consumption of decaffeinated coffee protects against the development of early non-alcoholic steatohepatitis: Role of intestinal barrier function. Redox Biol. 2019, 21, 101092.
- Chung, S.I.; Kim, T.H.; Rico, C.W.; Kang, M.Y. Effect of instant cooked giant embryonic rice on body fat weight and plasma lipid profile in high fat-fed mice. Nutrients 2014, 6, 2266–2278.
- Wang, Y.-X.; Li, Y.; Sun, A.-M.; Wang, F.-J.; Yu, G.-P. Hypolipidemic and antioxidative effects of aqueous enzymatic extract from rice bran in rats fed a high-fat and-cholesterol diet. Nutrients 2014, 6, 3696–3710.
- Choi, W.H.; Um, M.Y.; Ahn, J.; Jung, C.H.; Ha, T.Y. Long-term intake of rice improves insulin sensitivity in mice fed a high-fat diet. Nutrition 2014, 30, 920–927.
- Senadheera, S.P.A.S.; Ekanayake, S.; Wanigatunge, C. Anti-diabetic properties of rice-based herbal porridges in diabetic Wistar rats. Phyther. Res. 2014, 28, 1567–1572.
- Yang, C.W.; Mousa, S.A. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement. Ther. Med. 2012, 20, 466–474.
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009, 34, 313.
- Childress, L.; Gay, A.; Zargar, A.; Ito, M.K. Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 2013, 7, 117–122.
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423.
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277.
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830.
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.Y.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215.
- Küçükgergin, C.; Aydın, A.F.; Özdemirler-Erata, G.; Mehmetçik, G.; Koçak-Toker, N.; Uysal, M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol. Trace Elem. Res. 2010, 135, 264–274.
- Jacociunas, L.V.; de Andrade, H.H.R.; Lehmann, M.; de Abreu, B.R.R.; Ferraz, A.d.B.F.; da Silva, J.; Grivicich, I.; Dihl, R.R. Artichoke induces genetic toxicity in the cytokinesis-block micronucleus (CBMN) cytome assay. Food Chem. Toxicol. 2013, 55, 56–59.
- Pereira, C.; Barreira, J.C.M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. New insights into the effects of formulation type and compositional mixtures on the antioxidant and cytotoxic activities of dietary supplements based-on hepatoprotective plants. Food Funct. 2014, 5, 2052–2060.
- Imai, S. Soybean and processed soy foods ingredients, and their role in cardiometabolic risk prevention. Recent Pat. Food. Nutr. Agric. 2015, 7, 75–82.
- Yoon, G.-A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624.
- Oh, H.-G.; Kang, Y.-R.; Lee, H.-Y.; Kim, J.-H.; Shin, E.-H.; Lee, B.-G.; Park, S.-H.; Moon, D.-I.; Kim, O.-J.; Lee, I.-A. Ameliorative effects of Monascus pilosus-fermented black soybean (Glycine max L. Merrill) on high-fat diet-induced obesity. J. Med. Food 2014, 17, 972–978.
- Kim, Y.; Yoon, S.; Lee, S.B.; Han, H.W.; Oh, H.; Lee, W.J.; Lee, S.-M. Fermentation of soy milk via Lactobacillus plantarum improves dysregulated lipid metabolism in rats on a high cholesterol diet. PLoS ONE 2014, 9, e88231.
- Gray, A.M.; Flatt, P.R. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br. J. Nutr. 1997, 78, 325–334.
- Raeeszadeh, M.; Beheshtipour, J.; Jamali, R.; Akbari, A. The antioxidant properties of Alfalfa (Medicago sativa L.) and its biochemical, antioxidant, anti-inflammatory, and pathological effects on nicotine-induced oxidative stress in the rat liver. Oxid. Med. Cell. Longev. 2022, 2022, 2691577.
- Shi, Y.; Guo, R.; Wang, X.; Yuan, D.; Zhang, S.; Wang, J.; Yan, X.; Wang, C. The regulation of alfalfa saponin extract on key genes involved in hepatic cholesterol metabolism in hyperlipidemic rats. PLoS ONE 2014, 9, e88282.
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S. Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo-controlled study. Endocr. Metab. Immune Disord. Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 136–143.
- Quirino, A.; Giorgi, V.; Palma, E.; Marascio, N.; Morelli, P.; Maletta, A.; Divenuto, F.; De Angelis, G.; Tancrè, V.; Nucera, S. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics 2022, 11, 361.
- Maiuolo, J.; Bosco, F.; Guarnieri, L.; Nucera, S.; Ruga, S.; Oppedisano, F.; Tucci, L.; Muscoli, C.; Palma, E.; Giuffrè, A.M. Protective Role of an Extract Waste Product from Citrus bergamia in an In Vitro Model of Neurodegeneration. Plants 2023, 12, 2126.
- Miceli, N.; Mondello, M.R.; Monforte, M.T.; Sdrafkakis, V.; Dugo, P.; Crupi, M.L.; Taviano, M.F.; De Pasquale, R.; Trovato, A. Hypolipidemic effects of Citrus bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. J. Agric. Food Chem. 2007, 55, 10671–10677.
- Mare, R.; Mazza, E.; Ferro, Y.; Gliozzi, M.; Nucera, S.; Paone, S.; Aversa, I.; Pujia, R.; Marafioti, G.; Musolino, V. A new breakfast brioche containing bergamot fiber prevents insulin and glucose increase in healthy volunteers: A pilot study. Minerva Endocrinol. 2020, 46, 214–225.
- Pernice, R.; Borriello, G.; Ferracane, R.; Borrelli, R.C.; Cennamo, F.; Ritieni, A. Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009, 112, 545–550.
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18.
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus, and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158.
- Navarra, M.; Ursino, M.R.; Ferlazzo, N.; Russo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus bergamia juice on human neuroblastoma cells in vitro and in metastatic xenograft models. Fitoterapia 2014, 95, 83–92.
- Visalli, G.; Ferlazzo, N.; Cirmi, S.; Campiglia, P.; Gangemi, S.; Di Pietro, A.; Calapai, G.; Navarra, M. Bergamot juice extract inhibits proliferation by inducing apoptosis in human colon cancer cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2014, 14, 1402–1413.
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus bergamia juice. PLoS ONE 2013, 8, e61484.
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316.
- Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Scicchitano, M.; Maretta, A.; Palma, E. Lipid-lowering effect of bergamot polyphenolic fraction: Role of pancreatic cholesterol ester hydrolase. J. Biol. Regul. Homeost. Agents 2017, 31, 1087–1093.
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145.
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274.
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520.
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.T.; Rivelli, D.P.; Barros, S.B.M.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19.
- Labban, L.; Mustafa, U.E.-S.; Ibrahim, Y.M. The effects of rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid perodoxation. Int. J. Clin. Med. 2014, 5, 297–304.
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Béolor, J.-C.; Raab, T.; Macé, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 2010, 76, 566–571.
- Ibarra, A.; Cases, J.; Roller, M.; Chiralt-Boix, A.; Coussaert, A.; Ripoll, C. Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br. J. Nutr. 2011, 106, 1182–1189.
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006, 72, 881–887.
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92.
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2015, 63, 4843–4852.
- Shan, W.; Gao, L.; Zeng, W.; Hu, Y.; Wang, G.; Li, M.; Zhou, J.; Ma, X.; Tian, X.; Yao, J. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015, 6, e1833.
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510.
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 2015, 64, 1658–1669.
- Mesbahzadeh, B.; Akbari, M.; Zadeh, J.B. The effects of different levels of peppermint alcoholic extract on body-weight gain and blood biochemical parameters of adult male Wistar rats. Electron. Physician 2015, 7, 1376.
- Lima, C.F.; Valentao, P.C.R.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem. Biol. Interact. 2007, 167, 107–115.
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946.
- Sá, C.M.; Ramos, A.A.; Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950.
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Ren. Inj. Prev. 2013, 2, 51.
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62.
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Hear. 2015, 2, e000262.
- Hong, Q.; Xia, C.; Xiangying, H.; Quan, Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol. Med. Rep. 2015, 11, 1669–1674.
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflügers Arch. J. Physiol. 2013, 465, 1303–1316.
- Kang, J.-H.; Kim, C.-S.; Han, I.-S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396.
- Lee, G.; Shin, M.K.; Yoon, D.; Kim, A.; Yu, R.; Park, N.; Han, I. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 21, 115–122.
- Kang, J.; Tsuyoshi, G.; Han, I.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787.
- McCarty, M.F. High mitochondrial redox potential may promote induction and activation of UCP2 in hepatocytes during hepatothermic therapy. Med. Hypotheses 2005, 64, 1216–1219.




