Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariusz Winiecki | -- | 2206 | 2024-02-26 10:05:19 | | | |
| 2 | Catherine Yang | Meta information modification | 2206 | 2024-02-27 02:11:09 | | | | |
| 3 | Mariusz Winiecki | + 3 word(s) | 2209 | 2024-02-29 10:51:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Uklejewski, R.; Winiecki, M.; Dąbrowski, M.; Rogala, P. Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses. Encyclopedia. Available online: https://encyclopedia.pub/entry/55446 (accessed on 07 February 2026).
Uklejewski R, Winiecki M, Dąbrowski M, Rogala P. Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses. Encyclopedia. Available at: https://encyclopedia.pub/entry/55446. Accessed February 07, 2026.
Uklejewski, Ryszard, Mariusz Winiecki, Mikołaj Dąbrowski, Piotr Rogala. "Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses" Encyclopedia, https://encyclopedia.pub/entry/55446 (accessed February 07, 2026).
Uklejewski, R., Winiecki, M., Dąbrowski, M., & Rogala, P. (2024, February 26). Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses. In Encyclopedia. https://encyclopedia.pub/entry/55446
Uklejewski, Ryszard, et al. "Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses." Encyclopedia. Web. 26 February, 2024.
Copy Citation
The goals of hip resurfacing arthroplasty (HRA) are restoring joint anatomy, biomechanics, and function while prolonging the life of a patient with endoprosthesis by preserving bone stock for easy further possible revision arthroplasty in the form of traditional total hip arthroplasty. Over decades of its history, the designs of HRA endoprostheses have undergone a variety of technological innovations in terms of evolution in materials and fixation techniques, which often resulted in promising outcomes and the extension of implant survival.
resurfacing arthroplasty
resurfacing endoprostheses
1. Introduction
Hip resurfacing arthroplasty (HRA) involves replacing the femoral head acetabular articular cartilage, and subchondral bone with prosthetic components designed to replace the removed articular cartilage and subchondral periarticular bone to minimize the change in overall joint kinematics [1]. The important benefit of advocating for HRA over the long-stem total hip arthroplasty (THA) is the possibility of preserving at the initial operation a bone stock. Replacing diseased tissue with near anatomic-sized femoral component retains the potential for revision since the femoral canal was not violated [2][3]. Along with the bone tissue preservation, the HRA design solutions and applied fixation techniques are assumed, contrary to the long-stem THA systems, to allow near-physiological load transfer in periarticular bone allowing the recreation of closely native hip kinematics and bone biomechanics [4]. With its excellent functional outcome [5][6][7], HRA remains a reasonable alternative to THA in the appropriate patient cohort [8], and by many surgeons, it is considered an excellent option for hip reconstruction in young patients and/or high activity level patients diagnosed with osteonecrosis of the femoral head or acetabular dysplasia [9].
The contemporary generation of HRA endoprostheses has been used for over 20 years, while resurfacing arthroplasty has a hundred-year-long history [10][11][12][13]. From its beginning, the concept of resurfacing arthroplasty evolved through the variety of designs of endoprosthesis components, different material choices used and through the changes in fixation methods. Through the decades, its success has varied widely and was always limited by specific technological limitations of particular eras. The registered failures of resurfacing arthroplasty endoprostheses coupled with new technological capabilities are the foundations of occurring innovations and indications for the new designs. To achieve successful long-term results, apart from design features of resurfacing arthroplasty endoprostheses and applied manufacturing processes, the key importance also are patient selection and surgeon experience [9].
2. Characteristics of Contemporary Hip Resurfacing Arthroplasty Endoprostheses
Resurfacing arthroplasty has been experiencing a renaissance since the early 1990s. The first two designs to appear were introduced in the early 1990s by Wagner [14] and McMinn [15]. From this time on, all further hip resurfacing devices used exclusively CoCrMo metal-on-metal bearings. Both of these first systems were cementless. Wagner’s endoprosthesis had a threaded internal geometry of the femoral component and a grit-blasted Ti surface coating at the bone interfaces, while McMinn’s endoprosthesis had anti-rotation ridges and a short epiphyseal stem to assist with femoral component alignment and stability; the first was coated with hydroxyapatite (HA) and later press fit, while the acetabular component had HA coating and peripheral fins for rotational stability. The experience gained with these solutions has shown the enduring fixation of the acetabular components [16] and improved results in terms of loosening at the early stage [17] but has demonstrated poor outcomes in longer-term follow-up [18][19][20]. The Wagner system was discontinued, while subsequent modifications to the McMinn design involved cement fixation of both components, and then the hybrid configuration evolved into the development of the Cormet™ (Corin Group, Cirencester, UK) in 1997 and current Cormet 2000 (Corin Medical Ltd., Cirencester, UK) in 2007, as well as Birmingham Hip Resurfacing (BHR)™ (Smith & Nephew, Memphis, TN, USA) endoprostheses in 1997. Meanwhile, Amstutz began a series of innovations that culminated in the Conserve Plus™ (Wright Medical Technology Inc., Arlington, TN, USA) in 1993 and began implanting it at the end of 1996 [20]. It was a hybrid (i.e., porous fixation with sintered beads on the acetabular side and cemented on the femoral side) [18].
At the turn of the 21st century, most hip resurfacing systems were hybrid with a thin-walled one-piece cementless acetabular component and a cemented femoral component. The femoral components featured a short epiphyseal stem designed for alignment during insertion.
These systems offered several benefits, such as enhanced durability in fixation, reduced wear, improved bone tissue protection, and a decreased rate of complications, particularly fractures and sprains. Numerous clinical studies and joint registry reports provided extensive evidence indicating positive outcomes and survival of surface implants. They proved to be a success, with 96%, 92%, and 88.5% survivorship at ten years for Cormet, BHR, and Converse, respectively [21][22][23][24][25].
Following these successes, in the early 2000s, numerous hybrid hip resurfacing systems emerged, e.g., the Durom™ (Zimmer Inc., Warsaw, IN, USA) introduced in 2001, the Articular Surface Replacement (ASR™) (DePuy Orthopaedics Inc., Warsaw, IN, USA), introduced in 2003, the Icon™ (IO International Orthopaedics Holding, Geisingen, Germany) and the ReCap™ (Biomet Inc., Warsaw, IN, USA), both introduced in 2004, the ADEPT™ hip resurfacing system (Finsbury Orthopaedics Ltd., Leatherhead, UK), introduced in 2005, the MITCH hip resurfacing system (Stryker, Kalamazoo, MI, USA), introduced in 2006, the ROMAX® Resurfacing System (Medacta, Castel San Pietro, Switzerland), introduced in 2008, the DynaMoM hip resurfacing prosthesis (Tornier, Saint-Ismier, France), introduced in 2008, and the Minimally Invasive Hip Resurfacing (MIHR) International® metal-on-metal (MoM) hip system (Comis Orthopaedics Ltd., Birmingham, UK), introduced in 2009 [26]. A list of contemporary generations of HRA endoprostheses is presented in Table 1.
Table 1. Contemporary HRA endoprostheses.
| System | Introduced | Femoral Component Material and Fixation |
Acetabular Component Bearing Material/Bone-Contacting Material |
References |
|---|---|---|---|---|
| Wagner’s | 1991 | CoCrMo cementless, press-fit |
CoCrMo/grit-blasted Ti coating, cementless |
[14] |
| McMinn’s | 1992 | CoCrMo cementless, initially HA coating, then cemented |
CoCrMo/HA coating, cementless |
[15] |
| Conserve Plus™ (Wright Medical Technology Inc., Arlington, TN, USA) | 1996 | CoCrMo cemented |
CoCrMo/CrCrMo beads + HA coating, cementless |
[27][28][29] |
| BHR™ (Smith & Nephew, Memphis, TN, USA) | 1997 | CoCrMo cemented |
CoCrMo/CrCrMo beads + HA coating, cementless |
[30][31] |
| Cormet™ (Corin Group, Cirencester, UK) | 1997 | CoCrMo cemented (cementless option with PS Ti, HA coating) |
CoCrMo/PS Ti + HA coating, cementless | [32][33] |
| Durom™ (Zimmer Inc., Warsaw, IN, USA) | 2001 | CoCrMo cemented |
CoCrMo/PS Ti, cementless | [34][35] |
| ASR™ (DePuy Orthopaedics Inc., Warsaw, IN, USA) | 2003 | CoCrMo cemented |
CoCrMo/CrCrMo beads + HA coating, cementless |
[36][37] |
| Icon™ (IO International Orthopaedics Holding, Geisingen, Germany) |
2004 | CoCrMo cemented |
CoCrMo/CrCrMo beads + HA coating |
[38][39] |
| ReCap™ (Biomet Inc., Warsaw, IN, USA) |
2004 | CoCrMo cemented (cementless option PS Ti-6Al-4V) |
CoCrMo/PS Ti-6Al-4V + HA coating |
[40] |
| ACCIS™ (Van Straten Medical, The Netherlands; Implantcast, Buxtehude, Germany) |
2004 | TiNbN-coated CoCrMo cemented fixation, cementless from 2009 |
TiNbN-coated CoCrMo/ PS Ti |
[41] |
| ADEPT® (Finsbury Orthopaedics Ltd., Leatherhead, UK) |
2005 | CoCrMo cemented |
CoCrMo/CrCrMo beads + HA coating |
[42][43] |
| MITCH (Stryker, Kalamazoo, MI, USA) |
2006 | CoCrMo cemented |
CoCrMo/PS Ti + HA coating | [44] |
| ESKA-BIONIK® (ESKA Implants GmbH & Co., Lübeck, Germany) |
2006 | CoCrMo; Spongiosa Metal® (cemented option) |
CoCrMo + CoCrMo insert/Spongiosa Metal® | [45][46][47] |
| ESKA-CERAM® (ESKA Implants GmbH & Co., Lübeck, Germany) |
2007 | CoCrMo; Spongiosa Metal® (CoCrMo with TiNb coating) |
polyurethane/Al2O3 + polyurethane/Al2O3 insert/ Spongiosa Metal® (CoCrMo with TiNb coating) |
[47][48][49] |
| Cormet 2000 (Corin Medical Ltd., Cirencester, UK) |
2007 | CoCrMo cemented |
CoCrMo/PS Ti + HA coating | [33][50] |
| ROMAX® (Medacta, Castel San Pietro, Switzerland) |
2008 | CoCrMo cemented |
CoCrMo/PS Ti + HA coating | [51][52] |
| DynaMoM (Tornier, Saint-Ismier, France) |
2008 | CoCrMo cemented |
CoCrMo/PS Ti + HA coating | [53] |
| MIHR International® (Comis Orthopaedics Ltd., Birmingham, UK) |
2009 | CoCrMo cemented |
CoCrMo/HA coating, cementless |
[54] |
Instead of all implants having a CoCrMo-on-CoCrMo bearing, the Advanced Ceramic Coated Implant Systems (ACCIS™) (Van Straten Medical, The Netherlands; Implantcast, Buxtehude, Germany), introduced in 2004, has titanium–niobium–nitride (TiNbN) ceramic surfaces engineered by physical vapor deposition (PVD) to minimize wear and prevent tribocorrosion and metal ion release. Total hip resurfacing represented the fastest-growing section in orthopedic surgery [55][56]. Although some early clinical follow-up studies of this system demonstrated promising results [57], there were also results reporting catastrophic failure of the prosthesis, and an unacceptably high revision rate was demonstrated due to unknown causes that led to cease implanting the ACCIS™ [58][59].
Another step towards biomimetics was the series of ESKA hip resurfacing systems developed by ESKA Implants GmbH & Co. (Lübeck, Germany) beginning in 2006. The first was a metal-on-metal ESKA-BIONIK® hip resurfacing system (also known as Biosurf® hip resurfacing), which has a unique bearing surface hydrodynamic lubrication in the bearings through the concavo–convex pattern designed to reduce abrasive wear. The pattern provided circumferentially distributed escape dimples for wear particles and improved lubrication by providing room for the lubricant [60]. The acetabular component had a spongiosa metal structured surface called Spongiosa Metal® made of a CoCrMo with a titanium niobium (TiNb) coating or an HA coating, available upon request, for cementless anchorage through osseous integration that proved to have excellent long stability in clinical trials [45][46]. The cementless anchorage via the Spongiosa Metal® was also applied in the case of the femoral component. Further, in 2007, based on the composite material ENDOCERAM®, consisting of a polyurethane matrix and a mixed-in glass ceramic powder [49][61], the ceramic-on-ceramic ESKA-CERAM® hip resurfacing system was launched. The ESKA resurfacing implants were the only designs with a cementless acetabular shell in combination with a modular ceramic insert.
Research indicates that the use of cement does not consistently ensure long-term stability of endoprostheses in bones. The most common (ca. 75% of observed) complications of currently used cement resurfacing arthroplasty include the resorption of periarticular bone tissue, loosening at the bone–cement–implant junction zone, migration of endoprosthesis components, and femoral fractures [62][63][64][65]. Additionally, stress-shielding areas near the short stem of the femoral component often lead to loosening and migration [65][66][67][68][69][70]. In cemented HRA, while cement initially anchors the femoral component, it to penetrates deeply into the femoral head’s cancellous bone. Often, the area affected by cement penetration exceeds 30% of the femoral head’s total volume (some studies report over 50% [71]), leading to reduced local blood flow. This, in turn, weakens the internal microstructure of the cancellous bone in the femoral head, resulting in various complications [72][73][74][75][76][77][78].
Currently, available HRA systems exhibit a range of survival rates over five years, from a high of 97.1% to a low of 80.9% [10]. This variability has raised safety concerns regarding certain resurfacing arthroplasty endoprostheses. As a consequence, systems such as the ASR™ by DePuy Orthopaedics Inc., based in Warsaw, IN, USA, have been withdrawn from the market due to their significant rate of postoperative complications [79].
The predominant reason for early HRA failures, accounting for approximately 35% of necessary revision surgeries, is femoral neck fracture [80][81]. Additionally, aseptic loosening of either the femoral or acetabular components is another frequent reason for HRA failures [82][83]. Aseptic bone necrosis (osteonecrosis), often linked with periprosthetic fractures or identified as a contributing factor to such fractures after HRA [65], is also seen as a consequence of using cement for endoprosthesis component fixation [84] or is due to intraoperative damage to the blood vessels supplying the femoral head [85]. The heat generated during cement polymerization can severely damage the surrounding implant tissue, leading to the collapse of the femoral head [86][87]. Moreover, as previously noted, the implantation process of the femoral HRA endoprosthesis component typically forces substantial amounts of cement into the cancellous bone of the femoral head, creating a thick cement layer [88].
Aseptic bone necrosis is often seen in the early and middle stages following hip surgery, typically linked to either reduced blood flow to the femoral head or heat damage incurred during the operation [89]. Zustin et al. [89] conducted a histological analysis of 123 bone–implant samples from various resurfacing endoprostheses systems, including ASR™ by DePuy Orthopaedics, BHR™ by Smith & Nephew, Cormet™ by Corin Group, Durom™ by Zimmer Inc., and ReCap™ by Biomet Inc. These samples were collected from patients who had diagnoses other than osteonecrosis prior to surgery. The study found that osteonecrosis occurred in 88% of the cases, often linked with periprosthetic fractures. Of the revisions examined, 85 were due to periprosthetic fractures, with 60% of these fractures exhibiting complete bone necrosis near the fracture line, which are thus classified as post-necrotic fractures. Additionally, 8% of the revisions were due to the loosening of the acetabulum, and the remaining 23% were for various other reasons, including groin pain related to the femoral component. The majority of bone–implant specimens analyzed displayed extensive aseptic necrosis histologically, identified as the reason for 46% of all failures, particularly those linked to post-necrotic periprosthetic fractures and the collapse of the femoral head [90]. Steiger et al. [91] reported that, excluding infections, the leading causes for the primary revision following HRA are hip fractures (43%), loosening/lysis (32%), metal allergic reactions (7%), and pain (6%). Therefore, in cases involving serious postoperative complications, the primary revision focused on the femoral component accounts for 62% of all such revisions in the procedures mentioned [91].
ReCap™ is the only fully porous-coated femoral component currently available. The seven-year follow-up study carried out by Gross [92] suggested that cementless femoral fixation with a short epiphyseal stem may be a viable alternative to the cement fixation of HRA and pointed out that a study on a larger number of patients should be warranted.
In Figure 1, the roentgenogram of a patient (female, 52 y.o.) with a perceived limp requiring a cane for ambulation, showing the loosening and fracture at the femoral neck that occurred after one month.
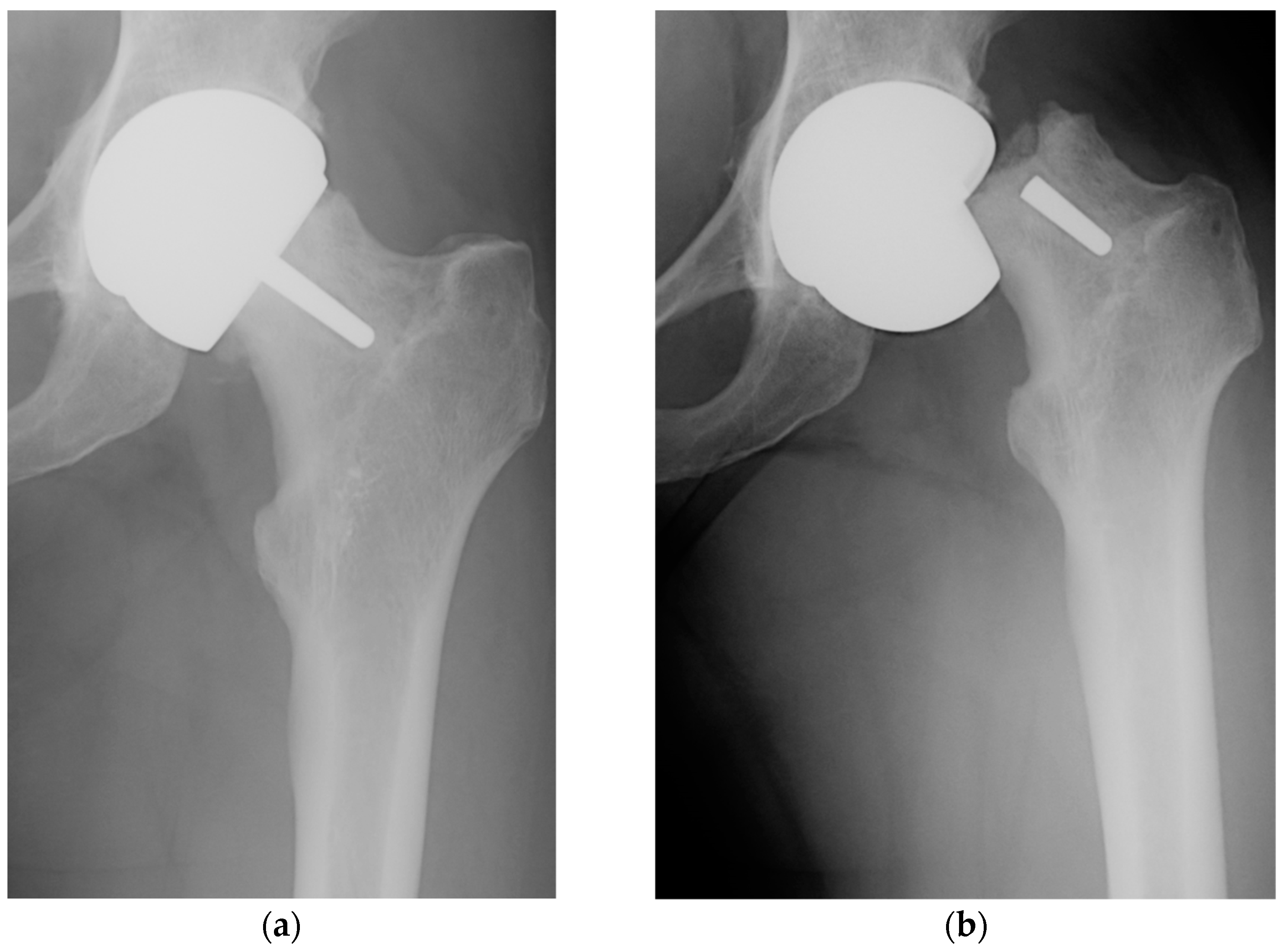
Figure 1. Anteroposterior radiographs demonstrating the Birmingham Hip Resurfacing system in situ (a) with femoral component loosening and (b) femoral neck fracture after one month (from authors' collection).
It should be noted that the cementless femoral fixation applied in the ReCap™ using a porous coating with sintered beads allows only shallow ingrowth of bone tissue into the pore space of the coating, and this requires the use of a short epiphyseal stem to ensure the proper fixation of the cap. Further studies [93][94] justify the validity of striving for more biomimetic bone ingrowth fixation methods in the case of the femoral component of hip resurfacing versus non-biomimetic approaches in terms of the cemented fixation methods.
References
- Callaghan, J.J.; Rosenberg, A.G.; Rubash, H.E.; Clohisy, J.C.; Beaulé, P.E.; DellaValle, C.J. The Adult Hip (Two Volume Set): Hip Arthroplasty Surgery, 3rd ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2015.
- Amstutz, H.C.; Grigoris, P.; Dorey, F.J. Evolution and future of surface replacement of the hip. J. Orthop. Sci. 1998, 3, 169–186.
- Amstutz, H.C.; Sparling, E.A.; Grigoris, P.; Campbell, P.A.; Dorey, F.J. Surface Replacement: The Hip Replacement of the Future? HIP Int. 1998, 8, 187–207.
- Quesada, M.J.; Marker, D.R.; Mont, M.A. Metal-on-metal hip resurfacing: Advantages and disadvantages. J. Arthroplast. 2008, 23, 69–73.
- Daniel, J.; Pradhan, C.; Ziaee, H.; Pynsent, P.B.; McMinn, D.J. Results of Birmingham hip resurfacing at 12 to 15 years: A single-surgeon series. Bone Jt. J. 2014, 96-B, 1298–1306.
- Ford, M.C.; Hellman, M.D.; Kazarian, G.S.; Clohisy, J.C.; Nunley, R.M.; Barrack, R.L. Five to Ten-Year Results of the Birmingham Hip Resurfacing Implant in the US: A Single Institution’s Experience. J. Bone Jt. Surg. 2018, 100, 1879–1887.
- Hellman, M.D.; Ford, M.C.; Barrack, R.L. Is there evidence to support an indication for surface replacement arthroplasty?: A systematic review. Bone Jt. J. 2019, 101-B (Suppl. A), 32–40.
- Amstutz, H.C.; Ball, S.T.; Le Duff, M.J.; Dorey, F.J. Resurfacing THA for patients younger than 50 year: Results of 2- to 9-year followup. Clin. Orthop. Relat. Res. 2007, 460, 159–164.
- Lawrie, C.M.; Barrack, R.L. Hip resurfacing arthroplasty—What has history taught us? Ann. Jt. 2020, 5, 20.
- Cadossi, M.; Tedesco, G.; Sambri, A.; Mazzotti, A.; Giannini, S. Hip Resurfacing Implants. Orthopedics 2015, 38, 504–509.
- Amstutz, H.C.; Le Duff, M.J. Hip resurfacing: History, current status, and future. HIP Int. 2015, 25, 330–338.
- Clough, E.J.; Clough, T.M. Metal on metal hip resurfacing arthroplasty: Where are we now? J. Orthop. 2020, 23, 123–127.
- Al-Jabri, T.; Ridha, M.; McCulloch, R.A.; Kayani, B.; Arif, A.; Habad, M.; Kosuge, D.; Jayadev, C.; Donaldson, J.; Skinner, J.A. Hip Resurfacing Arthroplasty: Past, Present and Future. Orthop. Rev. 2023, 15, 77745.
- Wagner, M.; Wagner, H. Preliminary results of uncemented metal on metal stemmed and resurfacing hip replacement arthroplasty. Clin. Orthop. Relat. Res. 1996, 329, S78–S88.
- McMinn, D.J.W.; Treacy, R.; Lin, K.; Pynsent, P. Metal on metal surface replacement of the hip. Experience of the McMinn prosthesis. Clin. Orthop. Relat. Res. 1996, 329, S89–S98.
- Beaulé, P.; Le Duff, M.; Campbell, P.; Dorey, F.; Park, S.; Amstutz, H.C. Metal-on-metal surface arthroplasty with a cemented femoral component: A 7–10 year follow-up study. J. Arthroplast. 2004, 19 (Suppl. 3), 17–22.
- Southgate, R.D.; Vail, T.P. Resurfacing Hip Arthroplasty: Evolution, Design, Indications, and Results. In Surgery of the Hip, 2nd ed.; Berry, D.J., Lieberman, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 779–788.
- Amstutz, H.C.; Le Duff, M.J. Hip resurfacing: A 40-year perspective. HSS J. 2012, 8, 275–282.
- Bohm, R.; Schraml, A.; Schuh, A. Long-term results with the Wagner metal-on-metal hip resurfacing prosthesis. HIP Int. 2006, 16 (Suppl. 4), 58–64.
- McMinn, D.J.W. Modern Hip Resurfacing; Springer: London, UK, 2009.
- Amstutz, H.C.; Le Duff, M.J. Background of metal-on-metal resurfacing. Proc. Inst. Mech. Eng. Part H 2006, 220, 85–94.
- Treacy, R.B.C.; McBryde, C.W.; Shears, E.; Pynsent, P.B. Birmingham hip resurfacing: A minimum follow-up of ten years. J. Bone Jt. Surg. 2011, 93-B, 27–33.
- Coulter, G.; Young, D.A.; Dalziel, R.E.; Shimmin, A.J. Birmingham hip resurfacing at a mean of ten years: Results from an independent centre. J. Bone Jt. Surg. 2012, 94-B, 315–321.
- Murray, D.W.; Grammatopoulos, G.; Pandit, H.; Gundle, R.; Gill, H.S.; McLardy-Smith, P. The ten-year survival of the Birmingham hip resurfacing: An independent series. J. Bone Jt. Surg. 2012, 94-B, 1180–1186.
- Amstutz, H.C.; Le Duff, M.J.; Campbell, P.A.; Gruen, T.A.; Wisk, L.E. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J. Bone Jt. Surg. 2010, 92, 2663–2671.
- De Smet, K.; Campbell, P.; Van Der Straeten, C. (Eds.) The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; Woodhead Publishing Ltd.: Sawston, UK, 2013.
- Amstutz, H.C.; Le Duff, M.J. Eleven years of experience with metal-on-metal hybrid hip resurfacing: A review of 1000 conserve plus. J. Arthroplasty 2008, 23 (Suppl. 1), 36–43.
- McMinn, D.J. Development of metal/metal hip resurfacing. HIP Int. 2003, 13, 41–53.
- Amstutz, H.C. The Conserve® Plus hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 31–38.
- Campbell, P.; De Smet, K. The Birmingham Hip Resurfacing (BHR) prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 25–30.
- Dhawan, R.; Young, D.A.; Van Eemeren, A.; Shimmin, A. Birmingham Hip Resurfacing at 20 years. Bone Jt. J. 2023, 105-B, 946–952.
- Costa, C.R.; Johnson, A.J.; Naziri, Q.; Mont, M.A. The outcomes of Cormet hip resurfacing compared to standard primary total hip arthroplasty. Bull. NYU Hosp. Jt. Dis. 2011, 69 (Suppl. 1), S12–S15.
- Norton, M. The Cormet™ hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 39–43.
- Gravius, S.; Wirtz, D.; Maus, U.; Andereya, S.; Müller-Rath, R.; Mumme, T. Durom-Hip-Oberflächenersatz am Hüftgelenk: Erste klinische Ergebnisse mit dem lateralen Zugang . Z. Orthop. Unfall. 2007, 145, 461–467.
- Campbell, P.; Girard, J. The Durom hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 44–47.
- Siebel, T.; Maubach, S.; Morlock, M.M. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc. Inst. Mech. Eng. Part H 2006, 220, 345–353.
- De Smet, K. The DePuy Articular Surface Replacement (ASR™) hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 20–24.
- Trč, T.; Šťastný, E.; Kopečný, Z.; Kos, P.; Přidal, J.; Havlas, V. Naše zkušenosti s povrchovou náhradou kyčelního kloubu ICON . Acta Chir. Orthop. Traumatol. Cechoslov. 2022, 89, 323–331.
- Van Der Straeten, C. The ICON hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 53–55.
- Delport, H.P. The BIOMET ReCap hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 69–77.
- Hamelynck, K.J.; Woering, R. The advanced ceramic coated implant systems (ACCIS) hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 3–10.
- Mancino, F.; Finsterwald, M.A.; Jones, C.W.; Prosser, G.H.; Yates, P.J. Metal-on-Metal Hips: Ten-Year Clinical and Radiographic Outcomes of the ADEPT Metal-on-Metal Hip Resurfacing and Modular Total Hip Arthroplasty. J. Clin. Med. 2023, 12, 889.
- Tuke, M. The ADEPT® hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 11–19.
- Young, D.; Burke, J. The MITCH hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 65–68.
- Gollwitzer, H.; Gerdesmeyer, L.; Horn, C.; Diehl, P.; Töpfer, A.; Gradinger, R. 8-year follow-up after cementless hip arthroplasty with a second generation spongy metal total hip replacement. HIP Int. 2009, 19, 359–366.
- Götze, C.; Tschugunow, A.; Wiegelmann, F.; Osada, N.; Götze, H.G.; Böttner, F. Langfristiger Einfluss der anatomisch angepassten spongiösen Endoprothese auf den periprothetischen Knochen . Z. Orthop. Ihre. Grenzgeb. 2006, 144, 192–198. (In German)
- Van Der Straeten, C. The ESKA hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 48–52.
- Quack, G.; Willmann, G.; Krahl, H.; Grundei, H. Konzeptionelle Uberlegungen zur Verbesserung der Pfanne der ESKA-Hüftendoprothese durch die Gleitpaarung Keramik/Keramik . Biomed. Technol. 1996, 41, 253–259.
- Scholz, J.; Grundei, H.; Klingbeil, K. ESKA-Ceram®—Ein neuer Werkstoff in der Endoprothetik des Hüftgelenkes . Biomed. Technol. 2000, 45, 377–379.
- Gross, T.P.; Liu, F.; Webb, L.A. Clinical outcome of the metal-on-metal hybrid Corin Cormet 2000 hip resurfacing system: An up to 11-year follow-up study. J. Arthroplast. 2012, 27, 533–538.e1.
- Giannini, S.; Laude, F.; Menge, M.; Sadri, H.; Siccardi, F. The ROMAX® hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 78–84.
- Cadossi, M.; Tedesco, G.; Savarino, L.; Baldini, N.; Mazzotti, A.; Greco, M.; Giannini, S. Effect of acetabular cup design on metal ion release in two designs of metal-on-metal hip resurfacing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1595–1601.
- Girard, J. The Tornier DynaMoM hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 85–87.
- Singh Chana, G.; Palmer, S. The MIHR International® hip resurfacing prosthesis. In The Hip Resurfacing Handbook. A Practical Guide to the Use and Management of Modern Hip Resurfacings; De Smet, K., Campbell, P., Van Der Straeten, C., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2013; pp. 59–64.
- Heisel, C.; Kleinhans, J.A.; Menge, M.; Kretzer, J.P. Ten different hip resurfacing systems: Biomechanical analysis of design and material properties. Int. Orthop. 2009, 33, 939–943.
- Australian Orthopaedic Association National Joint Replacement Registry. Annu. Rep. 2006, 57–87.
- Hamelynck, K.J.; Woering, R.G. Ceramic Surface Engineered Metal-on-Metal Hips system for Total Hip Arthroplasty and Resurfacing Hip Arthroplasty. The design rationale, pre-clinical testing and interim report on 2–7 years of clinical results. In White Paper; Implantcast: Buxtehude, Germany, 2009; pp. 1–8. Available online: https://silo.tips/download/ceramic-surface-engineered-metal-on-metal-hips-system-for-total-hip-arthroplasty (accessed on 19 December 2023).
- Jemmett, P.; Parfitt, D.; Rice, R. Early clinical failure of the ACCIS® metal on metal hip arthroplasty system—A metal on metal hip with a difference. Acta Orthop. Belg. 2016, 82, 491–496.
- Kim, W.Y.; Ko, M.S.; Lee, S.W.; Kim, K.S. Short-term Outcomes of Ceramic Coated Metal-on-Metal Large Head in Total Hip Replacement Arthroplasty. HIP Pelvis 2018, 30, 12–17.
- Gerdesmeyer, L.; Gollwitzer, H.; Al Muderis, M.; Fletcher, S.; Böhling, U. Alternative Bearing Designs for Hip Resurfacing Arthroplasty. Tech. Orthop. 2010, 25, 67–72.
- Bader, R.; Klüss, D.; Gerdesmeyer, L.; Steinhauser, E. Biomechanische Aspekte zur Implantatverankerung und Kinematik von Oberflächenersatzhüftendoprothesen . Orthopade 2008, 37, 634–643.
- Shimmin, A.J.; Back, D. Femoral neck fractures following Birmingham hip resurfacing: A national review of 50 cases. J. Bone Jt. Surg. 2005, 87, 463–464.
- Gupta, S.; New, A.M.; Taylor, M. Bone remodelling inside a cemented resurfaced femoral head. Clin. Biomech. 2006, 21, 594–602.
- Long, J.P.; Santner, T.J.; Bartel, D.L. Hip resurfacing increases bone strains associated with short-term femoral neck fracture. J. Orthop. Res. 2009, 27, 1319–1325.
- Steffen, R.T.; Foguet, P.R.; Krikler, S.J.; Gundle, R.; Beard, D.J.; Murray, D.W. Femoral neck fractures after hip resurfacing. J. Arthroplast. 2009, 24, 614–619.
- Fowble, V.A.; Schuh, A.; Hoke, R.; Bitsch, R.G.; Beaulé, P.E. Clinical correlation of femoral component migration in hip resurfacing arthroplasty analyzed by Einzel-Bild-Röntgen-analyze-femoral component analysis. Orthop. Clin. N. Am. 2005, 36, 243–250.
- Amstutz, H.C.; Le Duff, M.J.; Campbell, P.A.; Dorey, F.J. The effects of technique changes on aseptic loosening of the femoral component in hip resurfacing. Results of 600 Conserve Plus with a 3 to 9 year follow-up. J. Arthroplast. 2007, 22, 481–489.
- Falez, F.; Favetti, F.; Casella, F.; Panegrossi, G. Hip resurfacing: Why does it fail? Early results and critical analysis of our first 60 cases. Int. Orthop. 2008, 32, 209–216.
- Zustin, J.; Hahn, M.; Morlock, M.M.; Rüther, W.; Amling, M.; Sauter, G. Femoral component loosening after hip resurfacing arthroplasty. Skelet. Radiol. 2010, 39, 747–756.
- Baad-Hansen, T.; Storgaard Jakobsen, S.; Soballe, K. Two-year migration results of the ReCap hip resurfacing system-a radiostereometric follow-up study of 23 hips. Int. Orthop. 2011, 35, 497–502.
- Beaulé, P.; White, C.; Lopez-Castellaro, J.; Kim, P. Acetabular component migration analysis of hip resurfacing using ein bild roentegen analyse (EBRA). Orthop. Procs. 2012, 94-B, 214.
- de Haan, R.; Buls, N.; Scheerlinck, T. Impact of implant size on cement filling in hip resurfacing arthroplasty. Proc. Inst. Mech. Eng. Part H 2014, 228, 3–10.
- Howald, R.; Kesteris, U.; Klabunde, R.; Krevolin, J. Factors affecting the cement penetration of a hip resurfacing implant: An in vitro study. HIP Int. 2006, 16 (Suppl. 4), 82–89.
- Beaulé, P.E.; Lu, Z.; Luck, J.V.; Campbell, P. Bone thermal necrosis and cement penetration in femoral head resurfacing. J. Bone Jt. Surg. 2005, 90-B, 97.
- Beaulé, P.E.; Campbell, P.; Lu, Z.; Leunig-Ganz, K.; Beck, M.; Leunig, M.; Ganz, R. Vascularity of the arthritic femoral head and hip resurfacing. J. Bone Jt. Surg. 2006, 88 (Suppl. 4), 85–96.
- Beaulé, P.E.; Campbell, P.; Shim, P. Femoral head blood flow during hip resurfacing. Clin. Orthop. Relat. Res. 2007, 456, 148–152.
- Beaulé, P.E.; Matar, W.Y.; Poitras, P.; Smit, K.; May, O. 2008 Otto Aufranc Award: Component design and technique affect cement penetration in hip resurfacing. Clin. Orthop. Relat. Res. 2009, 467, 84–93.
- Krause, M.; Breer, S.; Hahn, M.; Rüther, W.; Morlock, M.M.; Amling, M.; Zustin, J. Cementation and interface analysis of early failure cases after hip-resurfacing arthroplasty. Int. Orthop. 2012, 36, 1333–1340.
- Hug, K.T.; Watters, T.S.; Vail, T.P.; Bolognesi, M.P. The withdrawn ASR™ THA and hip resurfacing systems: How have our patients fared over 1 to 6 years? Clin. Orthop. Relat. Res. 2013, 471, 430–438.
- Davis, E.T.; Olsen, M.; Zdero, R.; Smith, G.M.; Waddell, J.P.; Schemitsch, E.H. Predictors of femoral neck fracture following hip resurfacing: A cadaveric study. J. Arthroplast. 2013, 28, 110–116.
- Matharu, G.S.; McBryde, C.W.; Revell, M.P.; Pynsent, P.B. Femoral neck fracture after Birmingham Hip Resurfacing Arthroplasty: Prevalence, time to fracture, and outcome after revision. J. Arthroplast. 2013, 28, 147–153.
- Haughom, B.D.; Erickson, B.J.; Hellman, M.D.; Jacobs, J.J. Do complication rates differ by gender after metal-on-metal hip resurfacing arthroplasty? A systematic review. Clin. Orthop. Relat. Res. 2015, 473, 2521–2529.
- Amstutz, H.C.; Le Duff, M.J. Aseptic loosening of cobalt chromium monoblock sockets after hip resurfacing. HIP Int. 2015, 25, 466–470.
- Gill, H.S.; Campbell, P.A.; Murray, D.W.; De Smet, K.A. Reduction of the potential for thermal damage during hip resurfacing. J. Bone Jt. Surg. 2007, 89, 16–20.
- Steffen, R.T.; Fern, D.; Norton, M.; Murray, D.W.; Gill, H.S. Femoral oxygenation during hip resurfacing through the trochanteric flip approach. Clin. Orthop. Relat. Res. 2009, 467, 934–939.
- Scheerlinck, T.; Delport, H.; Kiewitt, T. Influence of the cementing technique on the cement mantle in hip resurfacing: An in vitro computed tomography scan-based analysis. J. Bone Jt. Surg. 2010, 92, 375–387.
- Girard, J. Is it time for cementless hip resurfacing? HSS J. 2012, 8, 245–250.
- Baker, R.; Whitehouse, M.; Kilshaw, M.; Pabbruwe, M.; Spencer, R.; Blom, A.; Bannister, G. Maximum temperatures of 89 °C recorded during the mechanical preparation of 35 femoral heads for resurfacing. Acta Orthop. 2011, 82, 669–673.
- Zustin, J.; Sauter, G.; Morlock, M.M.; Rüther, W.; Amling, M. Association of osteonecrosis and failure of hip resurfacing arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 756–761.
- Ullmark, G.; Sundgren, K.; Milbrink, J.; Nilsson, O.; Sörensen, J. Osteonecrosis following resurfacing arthroplasty. Acta Orthop. 2009, 80, 670–674.
- de Steiger, R.N.; Miller, L.N.; Prosser, G.H.; Graves, S.E.; Davidson, D.C.; Stanford, T.E. Poor outcome of revised resurfacing hip arthroplasty. Acta Orthop. 2010, 81, 72–76.
- Gross, T.P.; Liu, F. Metal-on-metal hip resurfacing with an uncemented femoral component. A seven-year follow-up study. J. Bone Jt. Surg. 2008, 90 (Suppl. 3), 32–37.
- O’Leary, R.J.; Gaillard, M.D.; Gross, T.P. Comparison of Cemented and Bone Ingrowth Fixation Methods in Hip Resurfacing for Osteonecrosis. J. Arthroplast. 2017, 32, 437–446.
- Uklejewski, R.; Rogala, P.; Winiecki, M. Prototype of a Biomimetic Multi-Spiked Connecting Scaffold for a New Generation of Resurfacing Endoprostheses, 1st ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2024.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
709
Revisions:
3 times
(View History)
Update Date:
29 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




