
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimitar Djilianov | -- | 2242 | 2024-02-26 09:58:23 | | | |
| 2 | Fanny Huang | Meta information modification | 2242 | 2024-02-27 06:15:37 | | |
Video Upload Options
Resurrection plant species are a group of higher plants whose vegetative tissues are able to withstand long periods of almost full desiccation and recover quickly upon rewatering. Apart from being a model system for studying desiccation tolerance, resurrection plant species appear to be a valuable source of metabolites, with various areas of application. A significant number of papers have been published with respect to the extraction and application of bioactive compounds from higher resurrection plant species in various test systems. Promising results have been obtained with respect to antioxidative and antiaging effects in various test systems, particularly regarding valuable anticancer effects in human cell lines.
1. Introduction
2. Metabolite Profiling and Application of Resurrection Plant Extracts as Bioactive Compounds
References
- Bistgani, Z.E.; Barker, A.V.; Hashemi, M. Physiology of Medicinal and Aromatic Plants under Drought Stress. Crop J. 2024.
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969.
- Gaff, D.F.; Oliver, M. The Evolution of Desiccation Tolerance in Angiosperm Plants: A Rare yet Common Phenomenon. Funct. Plant Biol. 2013, 40, 315–328.
- Dinakar, C.; Bartels, D. Desiccation Tolerance in Resurrection Plants: New Insights from Transcriptome, Proteome and Metabolome Analysis. Front. Plant Sci. 2013, 4, 482.
- Suguiyama, V.; da Silva, E.; Meirelles, S.; Centeno, D.; Braga, M. Leaf Metabolite Profile of the Brazilian Resurrection Plant Barbacenia purpurea Hook. (Velloziaceae) Shows Two Time-Dependent Responses during Desiccation and Recovering. Front. Plant Sci. 2014, 5, 96.
- Legardón, A.; García-Plazaola, J.I. Gesneriads, a Source of Resurrection and Double-Tolerant Species: Proposal of New Desiccation- and Freezing-Tolerant Plants and Their Physiological Adaptations. Biology 2023, 12, 107.
- Rakic, T.; Lazarevic, M.; Jovanovic, Z.; Radovic, S.; Siljak-Yakovlev, S.; Stevanovic, B.; Stevanovic, V. Resurrection Plants of the Genus Ramonda: Prospective Survival Strategies—Unlock Further Capacity of Adaptation, or Embark on the Path of Evolution? Front. Plant Sci. 2014, 4, 550.
- Okemo, P.A.; Njaci, I.; Kim, Y.-M.; McClure, R.S.; Peterson, M.J.; Beliaev, A.S.; Hixson, K.K.; Mundree, S.; Williams, B. Tripogon loliiformis Tolerates Rapid Desiccation after Metabolic and Transcriptional Priming during Initial Drying. Sci. Rep. 2023, 13, 20613.
- Mihailova, G.; Solti, Á.; Sárvári, É.; Hunyadi-Gulyás, É.; Georgieva, K. Protein Changes in Shade and Sun Haberlea rhodopensis Leaves during Dehydration at Optimal and Low Temperatures. Plants 2023, 12, 401.
- Mihaylova, D.; Bahchevanska, S.; Toneva, V. Examination of the Antioxidant Activity of Haberlea rhodopensis Leaf Extracts and Their Phenolic Constituents. J. Food Biochem. 2013, 37, 255–261.
- Gechev, T.; Lyall, R.; Petrov, V.; Bartels, D. Systems Biology of Resurrection Plants. Cell. Mol. Life Sci. 2021, 78, 6365–6394.
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.-R.; Marriott, A.S.; Bergström, E.; et al. Molecular Mechanisms of Desiccation Tolerance in the Resurrection Glacial Relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709.
- Liu, J.; Moyankova, D.; Lin, C.-T.; Mladenov, P.; Sun, R.-Z.; Djilianov, D.; Deng, X. Transcriptome Reprogramming during Severe Dehydration Contributes to Physiological and Metabolic Changes in the Resurrection Plant Haberlea rhodopensis. BMC Plant Biol. 2018, 18, 351.
- Tebele, S.M.; Marks, R.A.; Farrant, J.M. Two Decades of Desiccation Biology: A Systematic Review of the Best Studied Angiosperm Resurrection Plants. Plants 2021, 10, 2784.
- Liu, J.; Moyankova, D.; Djilianov, D.; Deng, X. Common and Specific Mechanisms of Desiccation Tolerance in Two Gesneriaceae Resurrection Plants. Multiomics Evidences. Front. Plant Sci. 2019, 10, 1067.
- Mladenov, P.; Zasheva, D.; Planchon, S.; Leclercq, C.C.; Falconet, D.; Moyet, L.; Brugière, S.; Moyankova, D.; Tchorbadjieva, M.; Ferro, M.; et al. Proteomics Evidence of a Systemic Response to Desiccation in the Resurrection Plant Haberlea rhodopensis. Int. J. Mol. Sci. 2022, 23, 8520.
- Mladenov, P.; Wang, X.; Yang, Z.; Djilianov, D.; Deng, X. Dynamics of Chromatin Accessibility and Genome Wide Control of Desiccation Tolerance in the Resurrection Plant Haberlea rhodopensis. BMC Plant Biol. 2023, 23, 654.
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation Tolerance: Avoiding Cellular Damage During Drying and Rehydration. Ann. Rev. Plant Biol. 2020, 71, 435–460.
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895.
- Blomstedt, C.K.; Griffiths, C.A.; Gaff, D.F.; Hamill, J.D.; Neale, A.D. Plant Desiccation Tolerance and Its Regulation in the Foliage of Resurrection “Flowering-Plant” Species. Agronomy 2018, 8, 146.
- Dace, H.J.W.; Adetunji, A.E.; Moore, J.P.; Farrant, J.M.; Hilhorst, H.W.M. A Review of the Role of Metabolites in Vegetative Desiccation Tolerance of Angiosperms. Curr. Opin. Plant Biol. 2023, 75, 102410.
- Moyankova, D.; Djilianov, D. Time- and Space-Saving Procedure to Obtain Extracts with Antioxidative Properties from Haberlea rhodopensis. C. R. L’académie Bulg. Sci. 2016, 69, 879–884.
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N. The Ancient Thracian Endemic Plant Haberlea rhodopensis Friv. and Related Species: A Review. J. Ethnopharmacol. 2020, 249, 112359.
- Rigat, M.; Bonet, M.À.; Garcia, S.; Garnatje, T.; Vallès, J. Studies on Pharmaceutical Ethnobotany in the High River Ter Valley (Pyrenees, Catalonia, Iberian Peninsula). J. Ethnopharmacol. 2007, 113, 267–277.
- Agelet, A.; Vallès, J. Studies on Pharmaceutical Ethnobotany in the Region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or Very Rare Uses of Previously Known Medicinal Plants. J. Ethnopharmacol. 2003, 84, 211–227.
- Nantapo, C.W.T.; Marume, U. Exploring the Potential of Myrothamnus flabellifolius Welw. (Resurrection Tree) as a Phytogenic Feed Additive in Animal Nutrition. Animals 2022, 12, 1973.
- Gahamanyi, N.; Munyaneza, E.; Dukuzimana, E.; Tuyiringire, N.; Pan, C.-H.; Komba, E.V.G. Ethnobotany, Ethnopharmacology, and Phytochemistry of Medicinal Plants Used for Treating Human Diarrheal Cases in Rwanda: A Review. Antibiotics 2021, 10, 1231.
- Erhabor, J.O.; Komakech, R.; Kang, Y.; Tang, M.; Matsabisa, M.G. Ethnopharmacological Importance and Medical Applications of Myrothamnus flabellifolius Welw. (Myrothamnaceae)—A Review. J. Ethnopharmacol. 2020, 252, 112576.
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628.
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection Mechanisms in the Resurrection Plant Xerophyta viscosa (Baker): Both Sucrose and Raffinose Family Oligosaccharides (RFOs) Accumulate in Leaves in Response to Water Deficit. J. Exp. Bot. 2007, 58, 1947–1956.
- Djilianov, D.; Ende, W.V.D.; Alexieva, V.; Moyankova, D. Sugar Ratios, Glutathione Redox Status and Phenols in the Resurrection Species Haberlea rhodopensis and the Closely Related Non-Resurrection Species Chirita eberhardtii. Plant Biol. 2011, 13, 767–776.
- Ghasempour, H.R.; Gaff, D.F.; Williams, R.P.W.; Gianello, R.D. Contents of Sugars in Leaves of Drying Desiccation Tolerant Flowering Plants, Particularly Grasses. Plant Growth Regul. 1998, 24, 185–191.
- Farrant, J.M.; Cooper, K.; Hilgart, A.; Abdalla, K.O.; Bentley, J.; Thomson, J.A.; Dace, H.J.W.; Peton, N.; Mundree, S.G.; Rafudeen, M.S. A Molecular Physiological Review of Vegetative Desiccation Tolerance in the Resurrection Plant Xerophyta viscosa (Baker). Planta 2015, 242, 407–426.
- Zhang, Q.; Bartels, D. Octulose: A Forgotten Metabolite? J. Exp. Bot. 2017, 68, 5689–5694.
- Cheikhyoussef, A.; Summers, R.; Kahaka, G. Qualitative and Quantitative Analysis of Phytochemical Compounds in Namibian Myrothamnus flabellifolius. Int. Sci. Technol. J. Namibia 2015, 5, 71–83.
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471.
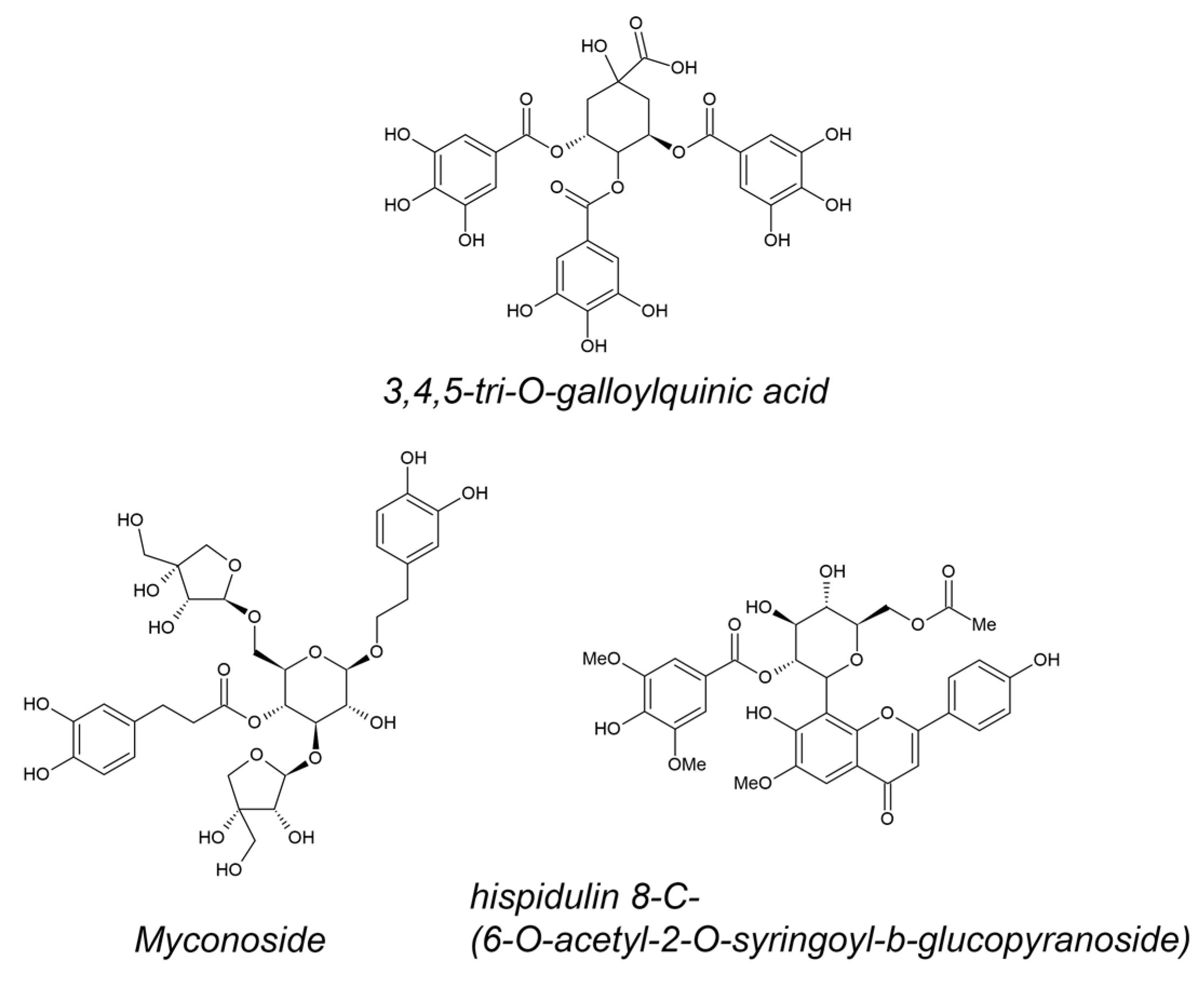
- Moore, J.P.; Westall, K.L.; Ravenscroft, N.; Farrant, J.M.; Lindsey, G.G.; Brandt, W.F. The Predominant Polyphenol in the Leaves of the Resurrection Plant Myrothamnus flabellifolius, 3,4,5 Tri-O-Galloylquinic Acid, Protects Membranes against Desiccation and Free Radical-Induced Oxidation. Biochem. J. 2004, 385, 301–308.
- Veljovic-Jovanovic, S.; Kukavica, B.; Navari-Izzo, F. Characterization of Polyphenol Oxidase Changes Induced by Desiccation of Ramonda serbica Leaves. Physiol. Plant 2008, 132, 407–416.
- Feng, W.-S.; Li, Y.-J.; Zheng, X.-K.; Wang, Y.-Z.; Su, F.-Y. Chemical Constituents of Boea hygrometrica. Chin. J. Nat. Med. 2011, 9, 406–409.
- Moyankova, D.; Mladenov, P.; Berkov, S.; Peshev, D.; Georgieva, D.; Djilianov, D. Metabolic Profiling of the Resurrection Plant Haberlea rhodopensis during Desiccation and Recovery. Physiol. Plant 2014, 152, 675–687.
- Vidović, M.; Battisti, I.; Pantelić, A.; Morina, F.; Arrigoni, G.; Masi, A.; Jovanović, S.V. Desiccation Tolerance in Ramonda serbica Panc.: An Integrative Transcriptomic, Proteomic, Metabolite and Photosynthetic Study. Plants 2022, 11, 1199.
- Gođevac, D.; Ivanović, S.; Simić, K.; Anđelković, B.; Jovanović, Ž.; Rakić, T. Metabolomics Study of the Desiccation and Recovery Process in the Resurrection Plants Ramonda serbica and R. nathaliae. Phytochem. Anal. 2022, 33, 961–970.
- Passon, M.; Weber, F.; Jung, N.U.; Bartels, D. Profiling of Phenolic Compounds in Desiccation-Tolerant and Non-Desiccation-Tolerant Linderniaceae. Phytochem. Anal. 2021, 32, 521–529.
- Cañigueral, S.; Salvía, M.J.; Vila, R.; Iglesias, J.; Virgili, A.; Parella, T. New Polyphenol Glycosides from Ramonda myconi. J. Nat. Prod. 1996, 59, 419–422.
- Jensen, S.R. Caffeoyl Phenylethanoid Glycosides in Sanango racemosum and in the Gesneriaceae. Phytochemistry 1996, 43, 777–783.
- Ebrahimi, S.N.; Gafner, F.; Dell’Acqua, G.; Schweikert, K.; Hamburger, M. Flavone 8-C-Glycosides from Haberlea rhodopensis Friv. (Gesneriaceae). Helv. Chim. Acta 2011, 94, 38–45.
- Gechev, T.S.; Hille, J.; Woerdenbag, H.J.; Benina, M.; Mehterov, N.; Toneva, V.; Fernie, A.R.; Mueller-Roeber, B. Natural Products from Resurrection Plants: Potential for Medical Applications. Biotechnol. Adv. 2014, 32, 1091–1101.
- Nyalo, P.; Omwenga, G.; Ngugi, M. Quantitative Phytochemical Profile and In Vitro Antioxidant Properties of Ethyl Acetate Extracts of Xerophyta spekei (Baker) and Grewia tembensis (Fresen). J. Evid. Based Complement. Altern. Med. 2023, 28, 2515690X231165096.
- Da Costa, D.J.; Leitão, A.; Faria, R.X.; Anholeti, M.C.; Nunes, M.A.; Oliveira, M.B.P.; Da Costa Santos, W.; De Barros Machado, T. Preliminary Phytochemical Analysis of the Ethanolic Extract of Xerophyta stenophylla Baker. Res. Soc. Dev. 2022, 11, e38211528319.
- Dhillon, J.; Miller, V.; Carter, J.; Badiab, A.; Tang, C.N.; Huynh, A.; Peethambaran, B. Apoptosis-Inducing Potential of Myrothamnus flabellifolius, an Edible Medicinal Plant, on Human Myeloid Leukemia HL-60 Cells. Int. J. Appl. Res. Nat. Prod. 2014, 7, 28–32.
- Brar, J.; Fultang, N.; Askey, K.; Tettamanzi, M.C.; Peethambaran, B. A Novel Anti-Triple Negative Breast Cancer Compound Isolated from Medicinal Herb Myrothamnus flabellifolius. J. Med. Plants Res. 2018, 12, 7–14.
- Fultang, N.; Brar, J.; Mercier, I.; Klase, Z.; Peethambaran, B. Myrothamnus flabellifolius Selectively Targets Triple Negative Breast Cancer in Vitro, Restoring Tamoxifen Sensitivity through Modulation of MiRNAs Associated with Estrogen Receptors. Int. J. Appl. Res. Nat. Prod. 2018, 11, 24–33.
- Kamng’ona, A.; Moore, J.P.; Lindsey, G.; Brandt, W. Inhibition of HIV-1 and M-MLV Reverse Transcriptases by a Major Polyphenol (3,4,5 Tri-O-Galloylquinic Acid) Present in the Leaves of the South African Resurrection Plant, Myrothamnus flabellifolia. J. Enzym. Inhib. Med. Chem. 2011, 26, 843–853.
- Moyankova, D.; Hinkov, A.; Shishkov, S.; Djilianov, D. Inhibitory Effect of Extracts from Haberlea rhodopensis Friv. against Herpes Simplex Virus. C. R. L’académie Bulg. Sci. 2014, 76, 1369–1376.
- Berkov, S.; Nikolova, M.; Hristozova, N.; Momekov, G.; Ionkova, I.; Djilianov, D. GC-MS Profiling of Bioactive Extracts from Haberlea rhodopensis: An Endemic Resurrection Plant. J. Serbian Chem. Soc. 2011, 76, 211–220.
- Radev, R.; Lazarova, G.; Nedialkov, P.; Sokolova, K.; Rukanova, D.; Tsokeva, Z. Study on Antibacterial Activity of Haberlea rhodopensis. Trakia J. Sci. 2008, 7, 34–36.
- Georgieva, S.; Gencheva, D.; Popov, B.; Grozeva, N.; Zhelyazkova, M. Radioprotective Action of Resurrection Plant Haberlea rhodopensis Friv. (Gesneriaceae) and Role of Flavonoids and Phenolic Acids. Bulg. J. Agric. Sci. 2019, 25, 158–168.
- Popov, B.; Georgieva, S.; Oblakova, M.; Bonev, G. Effects of Haberlea rhodopensis Extract on Antioxidation and Lipid Peroxidation in Rabbits after Exposure to 60Co-γ-Rays. Arch. Biol. Sci. 2013, 65, 91–97.
- Popov, B.; Radev, R.; Georgieva, S. In Vitro Incidence of Chromosome Aberrations in Gamma-Irradiated Rabbit Lymphocytes, Treated with Haberlea rhodopensis Extract and Vitamin C. Bulg. J. Vet. Med. 2010, 13, 148–153.
- Dobreva, Z.G.; Popov, B.N.; Georgieva, S.Y.; Stanilova, S.A. Immunostimulatory Activities of Haberlea rhodopensis Leaf Extract on the Specific Antibody Response: Protective Effects against γ-Radiation-Induced Immunosuppression. Food Agric. Immunol. 2015, 26, 381–393.
- Popov, B.; Georgieva, S.; Gadjeva, V. Modulatory Effects of Total Extract of Haberlea rhodopensis against the Cyclophosphamide Induced Genotoxicity in Rabbit Lymphocytes in Vivo. Trakia J. Sci. 2011, 9, 51–57.
- Georgieva, S.; Popov, B.; Bonev, G. Radioprotective Effect of Haberlea rhodopensis (Friv.) Leaf Extract on Gamma-Radiation-Induced DNA Damage, Lipid Peroxidation and Antioxidant Levels in Rabbit Blood. Indian. J. Exp. Biol. 2013, 51, 29–36.
- Staneva, D.; Dimitrova, N.; Popov, B.; Alexandrova, A.; Georgieva, M.; Miloshev, G. Haberlea rhodopensis Extract Tunes the Cellular Response to Stress by Modulating DNA Damage, Redox Components, and Gene Expression. Int. J. Mol. Sci. 2023, 24, 15964.
- Hayrabedyan, S.; Todorova, K.; Zasheva, D.; Moyankova, D.; Georgieva, D.; Todorova, J.; Djilianov, D. Haberlea rhodopensis Has Potential as a New Drug Source Based on Its Broad Biological Modalities. Biotechnol. Biotechnol. Equip. 2013, 27, 3553–3560.
- Spyridopoulou, K.; Kyriakou, S.; Nomikou, A.; Roupas, A.; Ermogenous, A.; Karamanoli, K.; Moyankova, D.; Djilianov, D.; Galanis, A.; Panayiotidis, M.I.; et al. Chemical Profiling, Antiproliferative and Antimigratory Capacity of Haberlea rhodopensis Extracts in an In Vitro Platform of Various Human Cancer Cell Lines. Antioxidants 2022, 11, 2305.
- Moyankova, D.; Lyubenova, A.; Slavov, S.; Djilianov, D. Extracts of the Endemic Resurrection Plant Haberlea rhodopensis Stimulate In Vitro Growth of Various Phytophthora Spp. Pathogens. Eur. J. Plant Pathol. 2014, 138, 149–155.
- Moyankova, D.; Georgieva, D.; Batchvarova, R.; Slavov, S.; Djilianov, D. Effect of Extracts from the Resurrection Plant Haberlea rhodopensis on in Vitro Growth of Plant Pathogens. C. R. L’académie Bulg. Sci. 2013, 66, 1269–1272.
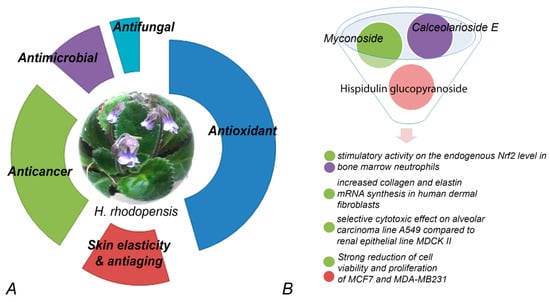
- Dell’Acqua, G.; Schweikert, K. Skin Benefits of a Myconoside-Rich Extract from Resurrection Plant Haberlea rhodopensis. Int. J. Cosmet. Sci. 2012, 34, 132–139.
- Kostadinova, A.; Doumanov, J.; Moyankova, D.; Ivanov, S.; Mladenova, K.; Djilianov, D.; Topuzova-Hristova, T. Haberlea rhodopensis Extracts Affect Cell Periphery of Keratinocytes. C. R. L’académie Bulg. Sci. 2016, 69, 439–448.
- Georgieva, M.; Moyankova, D.; Djilianov, D.; Uzunova, K.; Miloshev, G. Methanol Extracts from the Resurrection Plant Haberlea rhodopensis Ameliorate Cellular Vitality in Chronologically Ageing Saccharomyces Cerevisiae Cells. Biogerontology 2015, 16, 461–472.
- Kondeva-Burdina, M.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Mitcheva, M. Cytoprotective and Antioxidant Effects of Phenolic Compounds from Haberlea rhodopensis Friv. (Gesneriaceae). Pharmacogn. Mag. 2013, 9, 294–301.
- Amirova, K.M.; Dimitrova, P.A.; Marchev, A.S.; Krustanova, S.V.; Simova, S.D.; Alipieva, K.I.; Georgiev, M.I. Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. Int. J. Mol. Sci. 2021, 22, 1759.




