Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benjamin Podmiljsak | -- | 4494 | 2024-02-26 08:57:21 | | | |
| 2 | Sirius Huang | Meta information modification | 4494 | 2024-02-27 01:44:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Podmiljšak, B.; Saje, B.; Jenuš, P.; Tomše, T.; Kobe, S.; Žužek, T.; Šturm, S. Permanent Magnets and How They Dictated History. Encyclopedia. Available online: https://encyclopedia.pub/entry/55436 (accessed on 01 March 2026).
Podmiljšak B, Saje B, Jenuš P, Tomše T, Kobe S, Žužek T, et al. Permanent Magnets and How They Dictated History. Encyclopedia. Available at: https://encyclopedia.pub/entry/55436. Accessed March 01, 2026.
Podmiljšak, Benjamin, Boris Saje, Petra Jenuš, Tomaž Tomše, Spomenka Kobe, Tina Žužek, Sašo Šturm. "Permanent Magnets and How They Dictated History" Encyclopedia, https://encyclopedia.pub/entry/55436 (accessed March 01, 2026).
Podmiljšak, B., Saje, B., Jenuš, P., Tomše, T., Kobe, S., Žužek, T., & Šturm, S. (2024, February 26). Permanent Magnets and How They Dictated History. In Encyclopedia. https://encyclopedia.pub/entry/55436
Podmiljšak, Benjamin, et al. "Permanent Magnets and How They Dictated History." Encyclopedia. Web. 26 February, 2024.
Copy Citation
The most efficient electric motor is a permanent-magnet synchronous motor. Their efficiency makes them popular for drive motors, power steering, stop-start motors, and regenerative braking generators. These motors use permanent magnets based on rare-earth elements (REEs), in particular neodymium-iron-boron (Nd-Fe-B) and samarium-cobalt (Sm-Co), because of their high maximum energy product (BH)max (a measure of the magnet’s performance), which is needed for the high efficiency and the high resistance to demagnetization. But there are still some challenges and gaps in their performance and application.
permanent magnets
rare-earth elements
critical raw materials
electric motor
recycling
additive manufacturing
1. Introduction
The last 30-plus years have been dominated by Nd-Fe-B-type magnets. But for the complete picture, we must look back nearly 50 years and visit the events that led to the discovery of today’s modern metallic magnets. We will divide recent history into decades and add milestones for the triggering events (TE) that could be responsible for the subsequent discoveries (D), as shown in Figure 1.
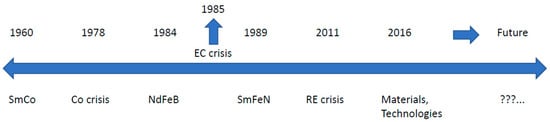
Figure 1. Time scale with triggering events and discoveries in the last 50-plus years.
We will look at the following:
- TE: “Pile of Samarium in the backyard“ at the beginning of the 1960s;
- D: Sm-Co (SmCo5, Sm2Co17) in the mid-1960s and early 1970s;
- TE: Cobalt crisis, which occurred at the end of the 1970s;
- D: Nd-Fe-B development activities at the beginning of the 1980s;
- TE: EC Research Crisis: We have to do something!, which happened in 1985 and resulted in CEAM;
- D: Sm-Fe-N, which appeared at the very end of the 1980s and 1990s;
- TE: Rare Earth crisis, which we all still remember from 2011;
- D: Today’s new materials and technologies with their perspectives.
To be fair to non-metallic, but still hard, magnetic materials, around 80% by weight of today’s permanent magnets are still sintered hard ferrites, as shown in Figure 2 left, and 70% of this ends up in various motors. On the other hand, around 65% of the market value is Nd-Fe-B (Figure 2 right), and again, about 70% of this ends up in motors of all kinds.
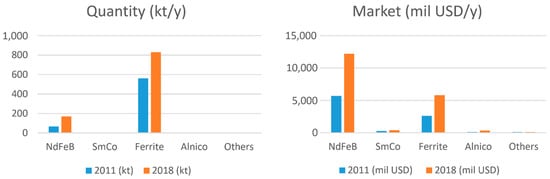
Figure 2. (Left) Permanent-magnet consumption by weight (2011 compared to 2018) in thousands of metric tons. (Right) Permanent-magnet usage in terms of annual turnover (2011 compared to 2018 in millions of US dollars per year) [1].
We will concentrate more on RE-TM magnets, where RE stands for Nd and Sm and TM for Fe and Co, and not so much on ferrites. Before the discovery of Nd-Fe-B magnets in 1984 [2][3], the major players were ferrites, Sm-Co (50 wt.% Co), and Alnico (15 wt.% Ni, 30–35 wt.% Co) magnets. For demanding, high-temperature applications, Sm-Co magnets were dominant (high-performance, high-temperature, defense-related uses). They were developed in Dayton, USA, by the group of K. Strnat [4], who was originally working on the Y-Co system. As recalled by Alden Ray [5], the triggering event was “There was quite some unused Sm pilling up in a backyard, so one day we thought, let us mix it with Co and see what happens”. Eventually, a new compound was discovered—firstly SmCo5; followed by Sm2Co17; both of which proved to be successful [6]. Raw-material prices and availability were of no particular concern then, but at the end of the 1970s, the so-called cobalt crisis occurred and then reappeared in 2008 and 2018 (as shown in Figure 3—collected from references [7][8][9]).
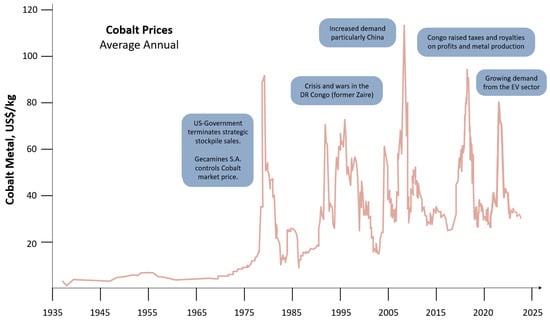
Figure 3. Average annual cobalt prices for the last 80 years.
Supplies of Sm were of no concern, but magnet producers were closely monitoring the Co and Ni markets. Some claim this was the trigger for the development activities on non-cobalt magnets, which resulted in the pioneering work on Nd-Fe-B at the US Naval Labs. At the beginning of the 1980s, N. Koon observed anisotropy in the Tb-La-Fe system that was not as expected [10]. Shortly after, M. Sagawa discovered sintered, anisotropic Nd-Fe-B while working at the Sumitomo Special Metals Company [2], and J.J. Croat and his group [11] discovered isotropic melt-spun ribbons and polymer-bonded magnets at General Motors. These latter materials and technologies still dominate REE-based bonded magnets [3]. Nd-Fe-B magnets have the highest maximum energy product (BH)max at room temperature of over 400 kJ/m3 (Figure 4) [2].
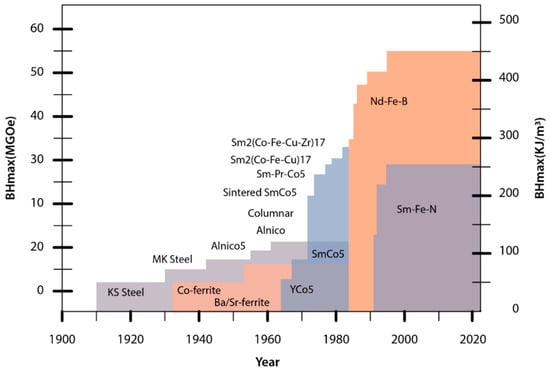
Figure 4. (BH)max values of different permanent magnets developed over the years.
The material itself is possible to describe as follows:
where the Nd2Fe14B phase is the carrier of the magnetic properties, the Nd-rich compound enables sintering, and the NdFe4B4 is difficult to get rid of for thermodynamic reasons. The RE-Fe-B system has a useful property that leads to RE interchangeability and a whole range of magnetic properties. Figure 5a shows the dependence of saturation magnetization (the potential for high remanence in a magnet) for different REs used in RE2Fe14B [12]. We can observe something similar for the magnetocrystalline anisotropy (the basis for high coercivity in the magnet), as shown in Figure 5b. With this interchangeability, we can modify the magnetic properties to fulfill the needs of the application.
Nd-Fe-B = Nd2Fe14B + Nd-rich + NdFe4B4
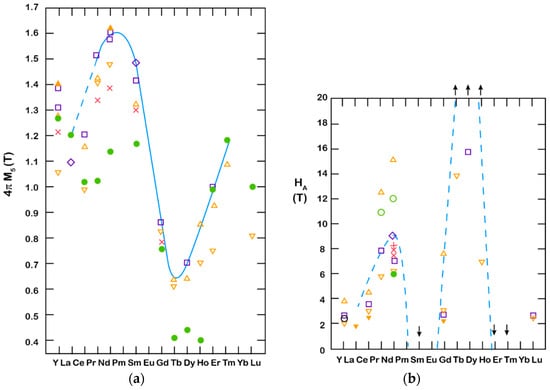
Figure 5. (a) Saturation magnetization vs. RE used in RE2Fe14B; (b) Anisotropy field vs. RE used in RE2Fe14B, reported by various authors. The symbols represent results from different authors. A detailed list can be found in Reference [12].
The discovery of Nd-Fe-B in 1984 led to »shock and awe« when news of this new family of magnetic materials was brought to the European magnetics research community, signaling that researchers in the USA and Japan had effectively pulled ahead [13]. European researchers reacted with a program called the Concerted European Action on Magnets (CEAM), which some claim was the triggering event that led to the discovery of the Sm-Fe-N magnetic phase. In the autumn of 1989, Iriyama and Kobayashi of Asahi Kasei patented a new magnetic compound—Sm-Fe-N [14]; which forms when the Sm2Fe17 binary alloy turns its in-plane anisotropy to uniaxial as nitrogen begins to occupy the interstitial sites in the crystal lattice. Shortly after, H. Sun and M. Coey published a similar result, and this happened within the CEAM program [15]. Sm-Fe-N has magnetic properties very similar to those of Nd-Fe-B at room temperature, with a 150 °C higher Curie temperature. Its major drawback is that it decomposes at around 550 °C, making it suitable mostly for polymer-bonded magnets.
What we today call the rare-earths crisis began at the end of 2010 and reached a peak in March 2011, lasting until August 2011 [16]. It resulted in sharp price rises, especially for the heavy rare earths (HREs) (as shown in Figure 6 for selected REs), and raised many questions about affordability, availability, and the potential monopolization of resources by China.
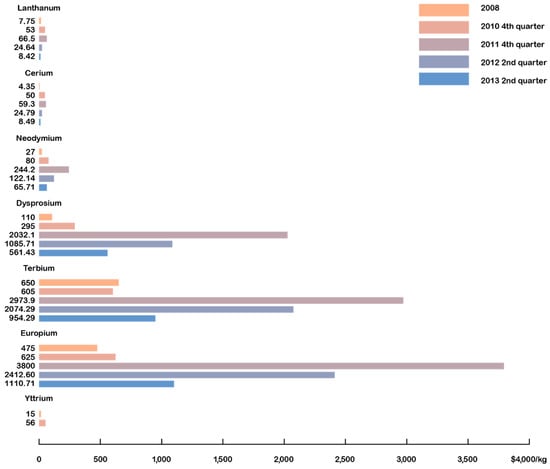
Figure 6. Selected rare-earth oxide prices, 2008–2013.
Today we are at pre-crisis price levels, and post-festum, we can have a closer look at those fears from 5 years ago:
-
There will be a lack of REs: So far, it has not happened because demand has not followed the predictions. The growth that was foreseen in 2009 has not materialized yet;
-
Chinese mining, production, and export quotas: actually never influenced the shortage of material;
-
Shipments failed: the shipment period was longer (particularly between China and Japan), but they never actually failed;
-
China as the only source: If in 2011, 95–97% of Nd for magnets were coming out of China, today (2024), the figure is only 80;
-
Predictions in other fields (phosphor lighting tubes and LEDs—Rare Earth Conference; Shenzhen; 2013)—due to the transition from phosphors to LED; phosphor applications have seen a drop in the consumption of Res; which are then used elsewhere.
A positive outcome of the RE crisis is that it triggered the opening of new and abandoned mines to reduce the dependence on China. Even though the production of rare earth metals will rise to 300.000 metric tons worldwide in 2022, that is up significantly from 190,000 MT in 2018, just four years prior, with 70% still coming from China, but other countries are ramping up their production (Figure 7).
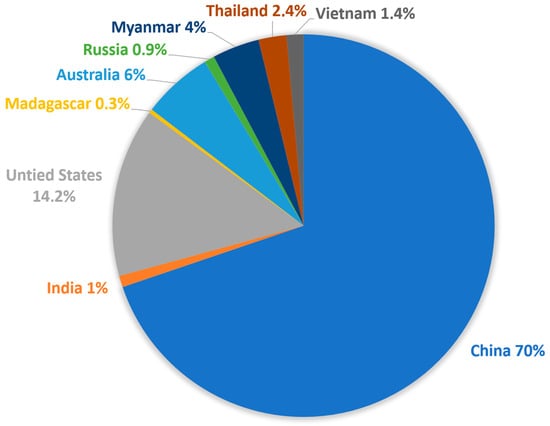
Figure 7. Rare earth metal production by country in 2022 [17].
It also opened up new research fields as to how to reduce dependence on rare earths:
-
Less critical REs and heavy REs, such as Dy and Tb;
-
Recycle REs from end-of-life goods and magnets;
-
New magnetic materials (possibly with no REs);
-
RE-free magnets.
which will be discussed in the following section.
2. Resource-Efficient Nd-Fe-B PMs with Fewer REEs and/or Fewer HREEs
The high operating temperatures of the traction motors for electric vehicles, i.e., approximately 200 °C, mean that Nd–Fe–B ternary magnets cannot be used due to their relatively low Curie temperature (310 °C) and the negative temperature dependence of coercivity. It was calculated that ~2400 kA/m of coercivity at room temperature would be enough to have an appropriate coercivity left that could be used for motor applications [18]. Here heavy rare-earth elements come into play, as in theory, the (Dy/Tb,Nd)2Fe14B phase could reach this value, where we substitute approximately one-third of the Nd atoms with Dy or Tb. They help to reach a higher magneto-crystalline anisotropy in this phase [19]. Applications that contain Dy via traditional alloying are high-temperature motors and generators, hybrid and electric traction drives (up to 11 wt%), commercial and industrial generators up to 6.5 wt%, e-bikes, energy storage systems, magnetically driven transportation, motors, wind-power generators up to 4.2 wt% hard disk drives, and MRI devices, which contain up to 1.4 wt% of Dy [20]. However, if we use conventional powder metallurgy, Dy and Tb antiferromagnetic cations couple with Fe. This reduces the magnetization and thus the energy product because it has the most dominant effect on it. This can be overcome with the grain-boundary diffusion process (GBDP), where Dy and Tb are used only locally [21][22].
The technology of grain surface HRE deployment in magnet microstructure has a different name with different producers, for example:
-
Binary Alloys Method (Grain Boundary Diffusion Process-GBDP-Shin Etsu);
-
High Anisotropy Field Layer (HAL-TDK);
-
DD Magic™ (Deposition and Diffusion-Hitachi).
To achieve those local dispersions of Dy and Tb, their ceramic precursors are used on top of already sintered magnets. These small additions are very much in line with the scarcity of HREEs like Dy and Tb. What is happening is that the heavy rare earths are then diffused into the magnet and substitute Nd in the matrix phase, producing a so-called core-shell structure, which prevents easy demagnetization when exposed to an external reverse magnetic field. By adding less than 1 wt.% Dy, the coercivity can be increased by more than 25%, which is shown in Figure 8 [23]. This was an outcome of the FP7 European project ROMEO (Replacement and Original Magnet Engineering Options), where an innovative approach to minimizing the amount of HRE needed was developed.
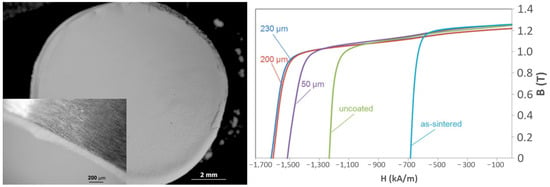
Figure 8. Enhancing the coercivity by deposition of HRE only on the grain surface [23].
Wang et al. report the highest coercivity by separately diffusing Dy70Cu30 and then Pr68Cu32 eutectic alloys through grain boundaries in fine-grained Dy-free sintered magnets. They used a two-step GBD process, which exhibited a coercivity of 2230 kA/m and an excellent temperature coefficient of coercivity of β = −0.447%/°C. During the second Grain Boundary Diffusion (GBD) process, the infiltration of Pr–Cu eutectic markedly enhanced the distribution, thickness, and chemical composition of the essential Dy-rich shell [24].
Because Praseodymium is chemically very similar to Nd, it is used to substitute it in Nd-Fe-B alloys to reduce costs. As it also does not lead to the formation of additional phases, in contrast to other light RE elements such as La [25][26][27], Ce [28][29][30], and Y, it can fully substitute Nd. The problem with a lower Curie temperature is counterbalanced with the addition of Co. A systematic investigation of the Pr-rich Pr-(Fe,Co)-B material system was carried out by Wu et al. [31]. Pr doping is known to increase the coercivity of Nd-Fe-B permanent magnets, but no in-depth study has been conducted on the impact of Pr on phase formation and microstructure. They investigate the phase formation, microstructure, magnetic properties, and coercivity mechanisms in Pr-rich Pr15(Fe1−xCox)78B7 (x = 0 − 1) alloys, where x = 0 and 0.2 produce a Pr-rich phase that hinders reversal magnetization, resulting in a high coercivity of 1400~1600 kA/m with good thermal stability. Micromagnetic modeling reveals that the change in the direction of magnetization at the interfaces of exchange-coupled grains often has a very complex topology, and the transition regions between two interaction domains are “pinned” at the structural inhomogeneities.
Ce and La are also interesting in substituting Nd in the main alloy, as they represent about 70% of the total amount of rare earths in minerals that are excavated and are largely unused and stored. Substitution of Nd by them reduces the performance of the Nd-Fe-B hard magnetic compound, mainly due to the lower intrinsic properties. Delette presented a good overview of substituting with non-critical light rare earth elements [32]. Regarding the Ce and La, it has been demonstrated that an excess in rare earth content and the Co addition limit the formation of some detrimental secondary phases. Coercivity values higher than 1430 kA/m have been achieved for a cerium substitution rate of 20%. These performances correspond to RE contents close to 31% wt. [33]. Co improves the Curie temperature of Ce-substituted magnets and the energy product of melt-spun ribbons from 85 kJ/m3 to 128 kJ/m3 in (Nd1−x Cex)2+yFe14-zCozB with z = 2 [34]. The effectiveness of coercivity in magnets substituted with Ce and La has seen significant improvements through grain boundary engineering techniques. These methods primarily involve creating intergranular secondary phases that are non-ferromagnetic and dispersed around the hard phase. This strategy aids in facilitating magnetic exchange and decoupling among neighboring grains. Techniques such as Infiltration, Grain Boundary Restructuring (GBR), and Grain Boundary Diffusion Process are key examples of this approach. With GBR, an additional powder with a high RE content and/or a low melting temperature is mixed with the RE-Fe-B powder before sintering. The gain in performance with the GBR method is generally lower than the one provided by the Grain Boundary Diffusion Process [35]. These types of magnets are already on the market, as the Inspires project [36] has shown when examining EOL magnets. They are used as lower-quality magnets containing up to 8% cerium in electric scooters. This raises the question of whether this can be as easily recycled as “pure” Nd-Fe-B magnets.
3. Recourse-Efficient Nd-Fe-B PMs via Recycling
The recycling of Nd–Fe–B permanent magnets (mainly sintered) from EOL products is nowadays categorized as a “key enabling technology” for positioning REs within the circular economy. Sintered Nd–Fe–B PMs are really multi-phase materials. The hard magnetic phase represents only 85–87% of the whole magnet; the rest are Nd-rich grain-boundary phases (GBPs) that also contain Nd-oxide phases [37]. Today, there are different techniques available to recycle Nd-Fe-B magnets. They can be divided into reusing, reprocessing, and remelting. The most economically viable route is the reuse of EOL magnets without changing the chemical structure of the magnet. This is only used for generators and motors in wind turbines and EV/HEVs. A mechanical dismantling and separation technique was developed by the Hitachi Group (Japan). They used a rotational drum to separate Nd-Fe-B magnets from hard disk drives (HDDs). Another technique they developed is recycling magnets from air conditioner compressors, which are cut off directly and further demagnetized thermally at 400–500 °C or by resonance damping demagnetization (Figure 9) [38][39].
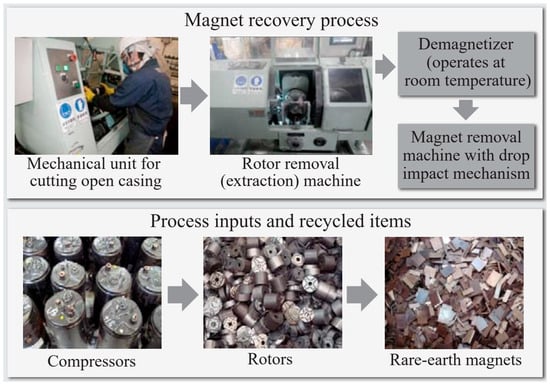
Figure 9. Magnet recovery process [39].
However, this process is very limited as the magnets, after salvaging them, already have a defined shape and properties that are useful only in the same applications. The more common route today is direct re-use via remelting the magnets without generating any waste. This is mostly used by magnet producers to put internal scrap back into the production line. However, this becomes more complicated for EOL magnets in waste electronic equipment (WEE), for example. The recycling rate of permanent magnets is very low because they are too small to be removed from WEE and are just remelted with ferrous or nonferrous [40]. The issue is also that Nd–Fe–B magnet scrap has a higher oxygen content (typically 2000–5000 ppm) compared to virgin magnets (typically 300–400 ppm) [41]. This additional oxygen is mainly trapped in the REE-rich materials; e.g., this is a huge problem as they do not sinter at the low temperatures of regular magnet sintering. This produces low-density parts with low hard magnetic values [42][43]. Additions of either several wt.% NdH2 [44] to the recycled powder or extra Dy [45] are thus needed to induce an increase in the coercivity to the original values. However, if the oxide phase could somehow be removed prior to or during recycling, the addition of extra REEs could be avoided. One way of tackling this problem was suggested by Xu et al. [46]. Using a novel selective electrochemical recycling concept, they could recover pure matrix Nd2Fe14B grains. This presented a very environment-friendly way to remove the unwanted oxide phases directly with a low-energy input. There are other methods of REE recycling. To obtain master alloys, one would use the high-energy-demanding pyrometallurgical route, which is useful only for highly concentrated magnet scrap [47][48][49]. Leaching is also a successful way to recycle these materials using H2SO4 [50] or HCl [51]. Solvent extraction [52][53][54], ion exchange, or ionic-liquid techniques [55][56][57][58] are used to get REE species from it. The final product is achieved by selective precipitation and conversion to REE fluorides or oxides. But this technique is very environmentally problematic because of the large amounts of chemicals used, not to mention the large amount of wastewater that is generated.
Another technique to separate the magnet from its environment was developed by the University of Birmingham. The HPMS (Hydrogen Processing of Magnet Scrap) was developed to retrieve environmentally friendly Nd-Fe-B powder environmentally friendly from end-of-life magnets [59][60]. As Nd-Fe-B magnets break down into a friable, demagnetized, hydrogenated powder that can be separated mechanically from the remaining impurities, like the nickel coating, by, e.g., tumbling electronics in a rotating hydrogen reactor and collecting the powder, the magnet-free component can now be recycled much more easily. This procedure is used by the company Hypromag Ltd., Birmingham, UK to develop a full recycling supply chain for rare-earth magnets. Figure 10 illustrates how the HPMS process pulverizes the Nd-Fe-B magnet of a hard disk drive.
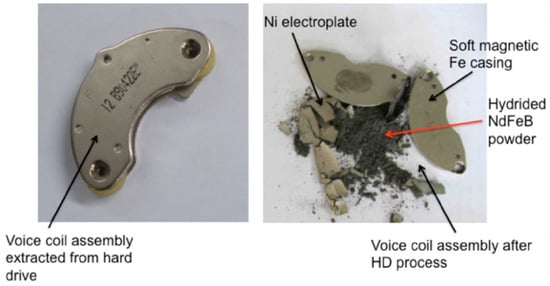
Figure 10. Illustration of the HPMS process applied to the voice-coil assembly of a hard disk drive [61].
Because of the difference in composition [62], it is not so easy to use the recycled powders directly in the normal production line by just mixing them with a fresh powder. Sometimes additional REEs have to be added to compensate for the loss during their lifetime. This can also alter the end properties of the magnet. To avoid these problems, a more standardized production method should be implemented, as suggested by Ueberschaar et al. [63]. These problems are also tackled in the ongoing ERA-Min 2 project MaXycle, which will classify EOL magnets for recyclability in a standardized grading system and develop a labeling system for newly produced magnets for easy recyclability at their end of life [64]. By investigating the EOL magnets from different fields, it will also give recommendations for a more recycle-friendly design for dismantling and suggest new coatings for easy removal. To make these recycling routes viable, large-scale production has to lower the prices for these kinds of recycled magnets. Currently, four pilot plants are being constructed in Europe in the scope of the Susmagpro project to increase the output of recycled magnets via the HPMS route [65]. They are developing automated sensing and robotic sorting lines to identify, sort, and extract Nd-Fe-B magnets more efficiently. They will use the HPMS method to harvest the magnetic powder, which will be used in four different reprocessing routes and will be installed by partners in electric motors, water pumps, loudspeakers, and headphones. Although the core technology for creating powder from recycled magnets is mature and ready for deployment, certain segments of the value chain still require refinement to fully realize a genuinely cost-effective circular economy approach [66].
4. Non-REE-Based PMs
High-energy magnets without REEs have not been produced. To use PMs without REEs, we can choose among ferrites, alnico, and Fe-Co-Cr, but they do not match the properties that REE-based magnets can achieve. Because of the REE crisis, research in the EU was focused on finding alternatives to low-performance REE PMs in the intermediate energy-product range between 50 and 200 kJ/m3 [67][68][69][70]. Novel, low-cost hybrid magnets based on ferrites/alnicos, or any of their combinations represent an interesting and viable solution to close the above-mentioned gap in magnetic performance between ferrites and RE magnets. A promising development for low-temperature applications was doping ferrites with B2O3, affecting the temperature coefficient of coercivity, which, unexpectedly, switched sign, enhancing the effect of the temperature on the magnetic properties (Figure 11) [71]. Guzmán-Mínguez et al. improved the magnetic performance of strontium ferrite sintered magnets using silica by tuning sintering parameters [72].
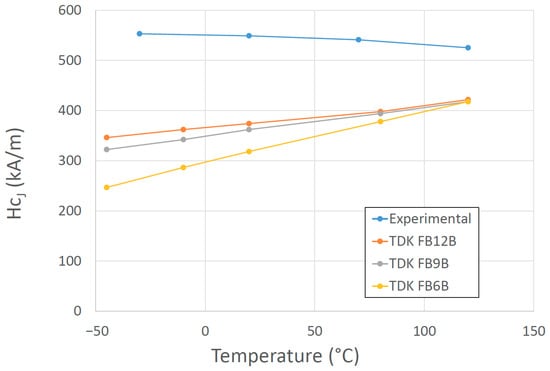
Figure 11. Temperature dependence of the intrinsic coercive force for a ferrite sintered magnet (Ca0.5La0.5Fe9.1Co0.4O19-δ) with 0.22 wt% B2O3 and several commercial magnets from the company TDK [71].
Exchange coupling between the hard and soft magnetic phases is one of the magnetic interactions that could lead to an increase in (BH)max [73][74]. The latter tends to increase with an increase in the coercivity, HcJ (the contribution of the hard phase), and/or the saturation magnetization, MS (the contribution of the soft phase) [75][76]. Therefore, by choosing wisely the constituent phases of the hybrid magnet, the energy product can be increased beyond the energy product of the hard phase alone [74]. Although this seems like an elegant solution, several requirements and restrictions have to be considered for an effective exchange. First, the constituent phases have to be in close contact, e.g., in the form of a core-shell structure [77][78][79], a layered structure [80][81][82], or as particulate composites [83][84][85][86]. Second, the particle size of the soft phase is limited by the selected hard phase. So, to effectively exchange the hard and soft magnetic phases, the particle size of the soft phase should not exceed twice that of the domain-wall width of the hard-phase material [74][79]. Some recent studies even indicated that for an efficient exchange coupling, the phases also need to have some degree of structural matching [87][88]. Furthermore, to increase the (BH)max of the composite as much as possible, the particle size of the hard phase should be at the limit of the single-domain particle size (to obtain the maximum coercivity). This means that for Sr-ferrite (or Ba-ferrite), if used in hard-soft magnetic composites, the preferred particle size would be around 1 µm, and the particle size of the soft-phase material should not exceed 28 nm [89]. The majority of the published research in the field of exchange-coupled composites is on powders only [86][90][91][92], and papers reporting increased (BH)max values based on exchange-coupled hard-soft bulk magnetic materials are scarce. Nevertheless, a (BH)max of 14.3 kJ/m3 for a hard-soft magnetic ceramic composite was reported by Debangsu Roy and P. S. Anil Kumar [93], in which they presented a hard-soft magnetic composite consisting of BaCa2Fe16O27 as the hard phase and Fe3O4 as the soft phase. Torkian and Ghasemi [94] presented SrFe10Al2O19/(x)Co0.8Ni0.2Fe2O4 composites with a (BH)max of 29.5 kJ/m3. During two European projects (the FP7 project Nanopyme [95] (GA 310516) and the H2020 project AMPHIBIAN [96] (GA 720853)), some encouraging results on sintered hybrid composites were obtained. Sr-ferrite/Co-ferrite bulk composites with a (BH)max of 26.1 kJ/m3 (a schematic and the actual prepared sample are presented in Figure 12) and hard-soft Sr-ferrite/(Mn, Zn)-ferrite hybrid magnets with a (BH)max of 23.7 kJ/m3 were prepared. A lot of efforts have been devoted to the research of modified or new magnetic materials that would fill the gap between REEs-based PMs and ferrites, but the vast combination of possible candidate materials, the preparation and consolidation conditions, and the fact that some options are viable only in theory make this task a highly demanding one, and so the quest to find a new magnet with improved properties continues.
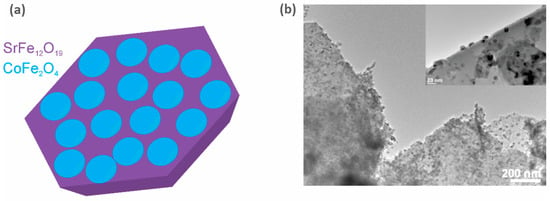
Figure 12. (a) schematic representation of CoFe2O4 nanoparticles distributed on the surface of a SrFe12O19 platelet and (b) TEM micrograph of the composite schematically presented in (a) prepared at the Department for Nanostructural Materials, Jozef Stefan Institute [97].
5. New Permanent Magnetic Materials
In the past 10 years, a lot of scientific effort has been applied to develop a new magnetic material that would overcome the problems associated with REEs and have sufficient magnetic properties. Developing a new material to challenge anisotropic Nd–Fe–B magnets is very demanding. To replace Nd2Fe14B with a new hard magnetic compound, we need a material with a higher Ms (μ0Ms > 1.6 T) and a sufficient magnetic hardness of Ha > 1.35 Ms. Some of the promising results are summarized in Figure 13, where alternatives to REE-containing compounds are presented. To have superior magnetic properties, we want to be in the upper right-hand corner of the graph. If we take Nd2Fe14B as a reference point, we can see that REEFe12 compounds with the ThMn12 structure are very interesting and have been extensively studied as a potential high-performance permanent-magnet material. Since the molar fraction of Fe in the REE-Fe12 compounds is the highest among various REE(m−n)Fe(5m+2n) compounds, a high spontaneous magnetization μ0MS is expected for REE-Fe12. However, most of the REE-Fe12 binary compounds are not thermodynamically stable. To obtain the REE-Fe12 phase in bulk form, the Fe must be substituted with a stabilizing element M, such as Al, Cr, V, Ti, Mo, W, Si, and Nb, which reduces the μ0MS [98]. If the REEFe12(N) phase could be synthesized without M, it is clear that NdFe12N is a very promising magnetic material—it has a higher anisotropy field and a higher magnetization (see Figure 14a) than Nd2Fe14B. Unfortunately, it decomposes at elevated temperatures (see Figure 14b, just as Sm-Fe-N does), which makes it suitable only for bonded applications [99]. Unlike NdFe12, SmFe12 shows a large uniaxial anisotropy without nitrogenation. Hirayama et al. [99] found that Sm(Fe0.8Co0.2)12 has excellent intrinsic hard-magnetic properties as a thin film by doping it with 3.7 at.% of B, which achieved a spontaneous magnetization of 1.78 T and a Curie temperature of 859 K. H. Sepehri-Amin et al. [100] achieved a high remanent magnetization of 1.50 T simultaneously with a high coercivity of 950 kA/m. Mn-based compounds have also been intensively studied, but compounds such as MnBi [101] and MnAl(C) [102] have only a small μ0MS of ∼0.7 T. Recent studies by Jia et al. [103] showed that a twin-free microstructure that negatively affects the magnetic properties of conventionally fabricated L10-type and RETM12 can be suppressed by particle sizes below a critical size of Dt~300 nm. The problem with all these mentioned materials—Fe16N2 particles [104]; tetragonal-FeCo thin films epitaxially grown on substrates [105][106]; and L10-FeNi thin films [107]—is the production in bulk form. Production methods using these types of nano-sized materials, either by bonding or sintering techniques, have to be developed [103].
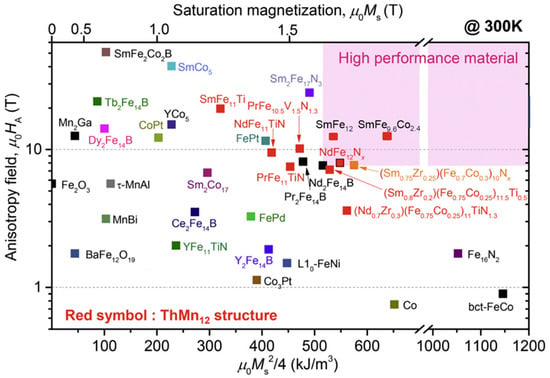
Figure 13. Anisotropy field (Ha) vs. theoretical (BH)max for selected new magnetic materials [98].
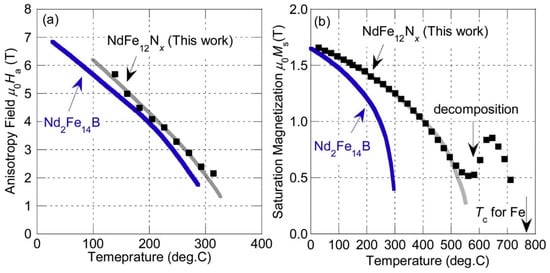
Figure 14. Anisotropy field (a) and magnetization (b) vs. temperature for NdFe12N [99].
The current status of developments can be summarized as follows:
-
With new materials, we are achieving a (BH)max of around 100 kJ/m3);
-
This is better than ferrite (40 kJ/m3) and isotropically bonded Nd-Fe-B (40–80 kJ/m3);
-
It is on par with anisotropically bonded Nd-Fe-B and Sm-Fe-N.
Unfortunately, it is still far from sintered Nd-Fe-B or Sm-Co.
References
- Kolektor d.o.o. Interna Document; Kolektor d.o.o.: Idrija, Slovenia, 2018.
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsuura, Y. New Material for Permanent Magnets on a Base of Nd and Fe (Invited). J. Appl. Phys. 1984, 55, 2083–2087.
- Croat, J.J.; Herbst, J.F.; Lee, R.W.; Pinkerton, F.E. Pr-Fe and Nd-Fe-based Materials: A New Class of High-performance Permanent Magnets (Invited). J. Appl. Phys. 1984, 55, 2078–2082.
- Ray, A.E.; Strnat, K.J. Research and Development of Rare Earth-Transition Metal Alloys as Permanent-Magnet Materials; Defense Technical Information Center: Fort Belvoir, VA, USA, 1973.
- Ray, A.E. University of Dayton: Dayton, TX, USA, Personal communication. 1994.
- Gschneidner, K.A., Jr.; Capellen, J. Two Hundred Years of Rare Earths, 1787–1987; Technical Report; Iowa State University of Science and Technology: Ames, IA, USA, 1987.
- Global Cobalt Corp.; Supply & Demand of the Strategic Metal—COBALT. 2011. Available online: https://www.slideshare.net/globalcobaltcorp/supply-demand-of-the-strategic-metal-cobalt-7958509 (accessed on 14 January 2024).
- LME Cobalt Price Chart. Available online: https://www.mining-bulletin.com/index.php/read_151125.html (accessed on 14 January 2024).
- Adams, W. Strong Growth for Cobalt, but Some Headwinds Too. Available online: https://www.fastmarkets.com/insights/strong-growth-for-cobalt-but-some-headwinds-too-lme-week-2021/#:~:text=Cobalt%20got%20off%20to%20a%20quick%20start%20in,lb%20by%20mid-March%2C%20a%20rise%20of%20some%2065%25 (accessed on 14 January 2024).
- Koon, N.C.; Das, B.N. Magnetic Properties of Amorphous and Crystallized (Fe0.82B0.18)0.9Tb0.05La0.05. Appl. Phys. Lett. 1981, 39, 840–842.
- Croat, J.J.; Herbst, J.F.; Lee, R.W.; Pinkerton, F.E. High-energy Product Nd-Fe-B Permanent Magnets. Appl. Phys. Lett. 1984, 44, 148–149.
- Livingston, J.D. Low-Cost Alloy Permanent Magnet Materials; AD-A-171264/5/XAB; -86SRD020; General Electric Corporate Research and Development: Schenectady, NY, USA, 1986.
- Hatch, G. The Concerted European Action On Magnets: A Model For Facing The Rare Earths Challenge?—Technology Metals Research. Available online: https://www.techmetalsresearch.net/the-concerted-european-action-on-magnets-a-model-for-facing-the-rare-earths-challenge/ (accessed on 4 April 2022).
- Iriyama, T.; Kobayashi, K.; Imai, H. Magnetic Materials Containing Rare Earth Element Iron Nitrogen and Hydrogen. U.S. Patent 5186766, 16 February 1993.
- Coey, J.M.D.; Sun, H. Improved Magnetic Properties by Treatment of Iron-Based Rare Earth Intermetallic Compounds in Anmonia. J. Magn. Magn. Mater. 1990, 87, L251–L254.
- Humphries, M. Rare Earth Elements: The Global Supply Chain; Rare Earth Elements 31; Diane Publishing: Darby, PA, USA, 2010.
- Pistilli, M. Top 10 Countries for Rare Earth Metal Production. Available online: https://investingnews.com/daily/resource-investing/critical-metals-investing/rare-earth-investing/rare-earth-metal-production/ (accessed on 14 January 2024).
- Hono, K.; Sepehri-Amin, H. Strategy for High-Coercivity Nd–Fe–B Magnets. Scr. Mater. 2012, 67, 530–535.
- Sagawa, M.; Fujimura, S.; Yamamoto, H.; Matsuura, Y.; Hiraga, K. Permanent Magnet Materials Based on the Rare Earth-Iron-Boron Tetragonal Compounds. IEEE Trans. Magn. 1984, 20, 1584–1589.
- Constantinides, S. The Important Role of Dysprosium in Modern Permanent Magnets; Arnold Magnetic Technologies Corp.: Rochester, NY, USA, 2012.
- Nakamura, H.; Hirota, K.; Shimao, M.; Minowa, T.; Honshima, M. Magnetic Properties of Extremely Small Nd-Fe-B Sintered Magnets. IEEE Trans. Magn. 2005, 41, 3844–3846.
- Li, D.; Suzuki, S.; Kawasaki, T.; Machida, K. Grain Interface Modification and Magnetic Properties of Nd–Fe–B Sintered Magnets. Jpn. J. Appl. Phys. 2008, 47, 7876–7878.
- Soderžnik, M.; Rožman, K.Ž.; Kobe, S.; McGuiness, P. The Grain-Boundary Diffusion Process in Nd–Fe–B Sintered Magnets Based on the Electrophoretic Deposition of DyF3. Intermetallics 2012, 23, 158–162.
- Wang, Z.; Sasaki, T.T.; Une, Y.; Ohkubo, T.; Hono, K. Substantial Coercivity Enhancement in Dy-Free Nd-Fe-B Sintered Magnet by Dy Grain Boundary Diffusion. Acta Mater. 2023, 248, 118774.
- Tang, X.; Sepehri-Amin, H.; Bolyachkin, A.; Ohkubo, T.; Hono, K. (Nd,La,Ce)-Fe-B Hot-Deformed Magnets for Application of Variable-Magnetic-Force Motors. Acta Mater. 2022, 228, 117747.
- Liao, X.; Zhao, L.; Zhang, J.; Xu, K.; Zhou, B.; Yu, H.; Zhang, X.; Greneche, J.-M.; Aubert, A.; Skokov, K.; et al. Textured (Ce,La,Y)–Fe–B Permanent Magnets by Hot Deformation. J. Mater. Res. Technol. 2022, 17, 1459–1468.
- Kim, G.-Y.; Kim, T.-H.; Cha, H.-R.; Lee, S.; Kim, D.-H.; Kim, Y.-D.; Lee, J.-G. Texture Development and Grain Boundary Phase Formation in Ce- and Ce-La-Substituted Nd-Fe-B Magnets during Hot-Deformation Process. J. Mater. Sci. Technol. 2022, 126, 71–79.
- Wu, Y.; Skokov, K.P.; Schäfer, L.; Maccari, F.; Aubert, A.; Xu, H.; Wu, H.; Jiang, C.; Gutfleisch, O. Microstructure, Coercivity and Thermal Stability of Nanostructured (Nd,Ce)-(Fe,Co)-B Hot-Compacted Permanent Magnets. Acta Mater. 2022, 235, 118062.
- Zhao, L.; He, J.; Li, W.; Liu, X.; Zhang, J.; Wen, L.; Zhang, Z.; Hu, J.; Zhang, J.; Liao, X.; et al. Understanding the Role of Element Grain Boundary Diffusion Mechanism in Nd–Fe–B Magnets. Adv. Funct. Mater. 2022, 32, 2109529.
- Sun, Q.; Zhu, M.; Wang, Q.; Zhu, C.; Yang, J.; Li, W. Design of Novel Quasi-Trivalent Dual-Main-Phase Ce Magnets with High Performance by Manipulating the Chemical State of Ce. Acta Mater. 2023, 246, 118703.
- Wu, Y.; Skokov, K.P.; Schäfer, L.; Maccari, F.; Xu, H.; Wang, X.; Jiang, C.; Gutfleisch, O. A Systematic Investigation of Pr-Rich Pr-(Fe,Co)-B Material System: Phase Formation, Microstructure and Magnetic Property. Acta Mater. 2024, 263, 119517.
- Delette, G. Nd2Fe14B Permanent Magnets Substituted with Non-Critical Light Rare Earth Elements (Ce, La): A Review. J. Magn. Magn. Mater. 2023, 577, 170768.
- Yang, M.; Wang, H.; Hu, Y.; Yang, L.; Maclennan, A.; Yang, B. Increased Coercivity for Nd-Fe-B Melt Spun Ribbons with 20 at.% Ce Addition: The Role of Compositional Fluctuation and Ce Valence State. J. Alloys Compd. 2017, 710, 519–527.
- Pathak, A.K.; Khan, M.; Gschneidner, K.A.; McCallum, R.W.; Zhou, L.; Sun, K.; Kramer, M.J.; Pecharsky, V.K. Magnetic Properties of Bulk, and Rapidly Solidified Nanostructured (Nd1−xCex)2Fe14-yCoyB Ribbons. Acta Mater. 2016, 103, 211–216.
- Liu, Y.; Jin, J.; Wang, X.; Yan, M. Comparison of (Pr, Nd)Hx Grain Boundary Restructuring and Diffusion on the Magnetic Properties of Nd–La–Ce–Fe–B Sintered Magnet. J. Alloys Compd. 2021, 868, 159154.
- EIT RawMaterials Project INSPIRES. INtelligent and Sustainable Processing of Innovative Rare-Earth magnetS. Available online: https://inspires-magnet.eu/ (accessed on 24 January 2024).
- Holc, J.; Beseničar, S.; Kolar, D. A Study of Nd2Fe14B and a Neodymium-Rich Phase in Sintered NdFeB Magnets. J. Mater. Sci. 1990, 25, 215–219.
- Baba, K.; Nemoto, T.; Maruyama, H.; Taketani, N.; Itayagoshi, K.; Hirose, Y. Hitachi’s Involvement in Material Resource Recycling. Hitachi Rev. 2010, 59, 180–187.
- Baba, K.; Hiroshige, Y.; Nemoto, T. Rare-Earth Magnet Recycling. Hitachi Rev. 2013, 62, 452–455.
- Bandara, H.M.D.; Mantell, M.A.; Darcy, J.W.; Emmert, M.H. Closing the Lifecycle of Rare Earth Magnets: Discovery of Neodymium in Slag from Steel Mills. Energy Technol. 2015, 3, 118–120.
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149.
- Awais, M.; Coelho, F.; Degri, M.; Herraiz, E.; Walton, A.; Rowson, N. Hydrocyclone Separation of Hydrogen Decrepitated NdFeB. Recycling 2017, 2, 22.
- Lalana, E.H.; Degri, M.; Bradshaw, A.; Walton, A. Recycling of Rare Earth Magnets by Hydrogen Processing and Re-Sintering. In European Congress and Exhibition on Powder Metallurgy, European PM Conference Proceedings; The European Powder Metallurgy Association: Shrewsbury, UK, 2016.
- Diehl, O.; Schönfeldt, M.; Brouwer, E.; Dirks, A.; Rachut, K.; Gassmann, J.; Güth, K.; Buckow, A.; Gauss, R.; Stauber, R.; et al. Towards an Alloy Recycling of Nd–Fe–B Permanent Magnets in a Circular Economy. J. Sustain. Metall. 2018, 4, 163–175.
- Zakotnik, M.; Tudor, C. Commercial-Scale Recycling of NdFeB-Type Magnets with Grain Boundary Modification Yields Products with “designer Properties” That Exceed Those of Starting Materials. Waste Manag. 2015, 44, 48–54.
- Xu, X.; Sturm, S.; Samardzija, Z.; Vidmar, J.; Scancar, J.; Rozman, K.Z. Direct Recycling of Nd–Fe–B Magnets Based on the Recovery of Nd2Fe14B Grains by Acid-Free Electrochemical Etching. ChemSusChem 2019, 12, 4754–4758.
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22.
- Takeda, O.; Okabe, T.H. Current Status on Resource and Recycling Technology for Rare Earths. Metall. Mater. Trans. E 2014, 1, 160–173.
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of Rare Earths from Scrap. Handb. Phys. Chem. Rare Earths 2013, 43, 159–211.
- Lyman, J.W.; Palmer, G.R. Recycling of Rare Earths and Iron from NdFeB Magnet Scrap. High Temp. Mater. Process. 1993, 11, 175–188.
- Bandara, H.M.D.; Field, K.D.; Emmert, M.H. Rare Earth Recovery from End-of-Life Motors Employing Green Chemistry Design Principles. Green Chem. 2016, 18, 753–759.
- Kraikaew, J.; Srinuttrakul, W.; Chayavadhanakur, C. Solvent Extraction Study of Rare Earth from Nitrate Medium by the Mixtures of TBP and D2EHPA in Kerosene. J. Met. Mater. Miner. 2005, 15, 89–95.
- Nash, K.L.; Choppin, G.R. Introduction: A Brief History of f Element Separations. In Separations of f Elements; Nash, K.L., Choppin, G.R., Eds.; Springer: Boston, MA, USA, 1995; pp. 1–8. ISBN 978-1-4899-1406-4.
- Yoon, H.-S.; Kim, C.-J.; Chung, K.-W.; Kim, S.-D.; Lee, J.-Y.; Kumar, J.R. Solvent Extraction, Separation and Recovery of Dysprosium (Dy) and Neodymium (Nd) from Aqueous Solutions: Waste Recycling Strategies for Permanent Magnet Processing. Hydrometallurgy 2016, 165, 27–43.
- Nakashima, K.; Kubota, F.; Maruyama, T.; Goto, M. Ionic Liquids as a Novel Solvent for Lanthanide Extraction. Anal. Sci. 2003, 19, 1097–1098.
- Kubota, F.; Baba, Y.; Goto, M. Application of Ionic Liquids for the Separation of Rare Earth Metals. Solvent Extr. Res. Dev. Jpn. 2012, 19, 17–28.
- Makanyire, T.; Sanchez-Segado, S.; Jha, A. Separation and Recovery of Critical Metal Ions Using Ionic Liquids. Adv. Manuf. 2016, 4, 33–46.
- Riaño, S.; Binnemans, K. Extraction and Separation of Neodymium and Dysprosium from Used NdFeB Magnets: An Application of Ionic Liquids in Solvent Extraction towards the Recycling of Magnets. Green Chem. 2015, 17, 2931–2942.
- Walton, A.; Williams, A. Rare Earth Recovery. Mater World 2011, 19, 24–26.
- Walton, A.; Yi, H.; Rowson, N.A.; Speight, J.D.; Mann, V.S.J.; Sheridan, R.S.; Bradshaw, A.; Harris, I.R.; Williams, A.J. The Use of Hydrogen to Separate and Recycle Neodymium–Iron–Boron-Type Magnets from Electronic Waste. J. Clean. Prod. 2015, 104, 236–241.
- Rare Earth Magnet Recycling. Available online: https://hypromag.com/rare-earth-magnet-recycling/ (accessed on 14 January 2024).
- Walton, A.; Campbell, A.; Sheridan, R.S.; Mann, V.S.J.; Speight, J.D.; Harris, I.R.; Guerrero, B.; Bagan, C.; Conesa, A.; Schaller, V. Recycling of rare earth magnets. In Proceeding of the 23rd International Workshop on Rare Earth Magnets and Their Applications, Annapolis, MD, USA, 17–21 August 2014.
- Ueberschaar, M.; Rotter, V.S. Enabling the Recycling of Rare Earth Elements through Product Design and Trend Analyses of Hard Disk Drives. J. Mater. Cycles Waste Manag. 2015, 17, 266–281.
- European project Maxycle—A Novel Circular Economy for Sustainable RE-Based Magnets. Available online: http://www.maxycle.eu/ (accessed on 14 January 2024).
- European project Susmagpro—Sustainable Recovery, Reprocessing and Reuse of Rare-Earth Magnets in the Circular Economy. Available online: https://www.susmagpro.eu/ (accessed on 14 January 2024).
- Burkhardt, C.; van Nielen, S.; Awais, M.; Bartolozzi, F.; Blomgren, J.; Ortiz, P.; Xicotencatl, M.B.; Degri, M.; Nayebossadri, S.; Walton, A. An Overview of Hydrogen Assisted (Direct) Recycling of Rare Earth Permanent Magnets. J. Magn. Magn. Mater. 2023, 588, 171475.
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842.
- Lewis, L.H.; Jiménez-Villacorta, F. Perspectives on Permanent Magnetic Materials for Energy Conversion and Power Generation. Metall. Mater. Trans. A 2013, 44, 2–20.
- Coey, J.M.D. Permanent Magnets: Plugging the Gap. Scr. Mater. 2012, 67, 524–529.
- Bollero, A.; Palmero, E.M. Chapter 03—Recent Advances in Hard ferrite Magnets. In Modern Permanent Magnets; Croat, J., Ormerod, J., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 65–112. ISBN 978-0-323-88658-1.
- Nagaoka, J.; Miwa, M. High-Performance Ferrite Magnets Overcoming Irreversible Low Temperature Demagnetization: Temperature Dependence of Magnetic Properties. In Proceedings of the Rare Earth Permanent Magnets and Advanced Magnetic Materials and Their Applications REPM2018, Beijing, China, 26–30 August 2018.
- Guzmán-Mínguez, J.C.; Vicente-Arche, L.M.; Granados-Miralles, C.; Fernández, J.F.; Quesada, A. Improvement of the Magnetic Properties of SrFe12O19 Ceramics by Tailored Sintering with SiO2 Addition. J. Alloys Compd. 2021, 860, 157890.
- Kneller, E.F.; Hawig, R. The Exchange-Spring Magnet: A New Material Principle for Permanent Magnets. IEEE Trans. Magn. 1991, 27, 3560–3588.
- Jenuš, P.; Učakar, A.; Repše, S.; Sangregorio, C.; Petrecca, M.; Albino, M.; Cabassi, R.; de Julián Fernández, C.; Belec, B. Magnetic Performance of SrFe12O19–Zn0.2Fe2.8O4 Hybrid Magnets Prepared by Spark Plasma Sintering. J. Phys. D Appl. Phys. 2021, 54, 204002.
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2008; ISBN 978-0-471-47741-9.
- Fullerton, E.E.; Jiang, J.S.; Bader, S.D. Hard/Soft Magnetic Heterostructures: Model Exchange-Spring Magnets. J. Magn. Magn. Mater. 1999, 200, 392–404.
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of Exchange Coupled Bi-Magnetic Hard/Soft and Soft/Hard Magnetic Core/Shell Nanoparticles. Phys. Rep. 2015, 553, 1–32.
- Soares, J.M.; Galdino, V.B.; Conceição, O.L.A.; Morales, M.A.; de Araújo, J.H.; Machado, F.L.A. Critical Dimension for Magnetic Exchange-Spring Coupled Core/Shell CoFe2O4/CoFe2 Nanoparticles. J. Magn. Magn. Mater. 2013, 326, 81–84.
- Cabreira-Gomes, R.; Silva, F.G.; Aquino, R.; Bonville, P.; Tourinho, F.A.; Perzynski, R.; Depeyrot, J. Exchange Bias of MnFe2O4@γFe2O3 and CoFe2O4@γFe2O3 Core/Shell Nanoparticles. J. Magn. Magn. Mater. 2014, 368, 409–414.
- Ichitsubo, T.; Takashima, S.; Matsubara, E.; Tamada, Y.; Ono, T. Exchange-Coupling of c-Axis Oriented L10–FePd and Fe in FePd/Fe Thin Films. Appl. Phys. Lett. 2010, 97, 182508.
- Liu, X.; Yuan, H.; Liu, P.; Shi, J.; Wang, H.; Nie, S.; Jin, F.; Zheng, Z.; Yu, X.; Zhao, J.; et al. Ultrafast Control of Interfacial Exchange Coupling in Ferromagnetic Bilayer. Adv. Electron. Mater. 2023, 9, 2201025.
- Nayyef, H.; Świerkosz, E.; Janus, W.; Klimeczek, A.; Szpytma, M.; Zając, M.; Dróżdż, P.; Kozioł-Rachwał, A.; Ślęzak, T.; Ślęzak, M. Tunable Interplay between Exchange Coupling and Uniaxial Magnetic Anisotropy in Epitaxial CoO/Au/Fe Trilayers. Sci. Rep. 2023, 13, 10902.
- Jenuš, P.; Topole, M.; McGuiness, P.; Granados-Miralles, C.; Stingaciu, M.; Christensen, M.; Kobe, S.; Žužek Rožman, K. Ferrite-Based Exchange-Coupled Hard–Soft Magnets Fabricated by Spark Plasma Sintering. J. Am. Ceram. Soc. 2016, 99, 1927–1934.
- Nikmanesh, H.; Moradi, M.; Kameli, P.; Bordbar, G.H. Effects of Annealing Temperature on Exchange Spring Behavior of Barium Hexaferrite/Nickel Zinc Ferrite Nanocomposites. J. Electron. Mater. 2017, 46, 5933–5941.
- Mohseni, F.; Pullar, R.C.; Vieira, J.M.; Amaral, J.S. Enhancement of Maximum Energy Product in Exchange-Coupled BaFe12O19/Fe3O4 Core-Shell-like Nanocomposites. J. Alloys Compd. 2019, 806, 120–126.
- Xia, A.; Ren, S.; Lin, J.; Ma, Y.; Xu, C.; Li, J.; Jin, C.; Liu, X. Magnetic Properties of Sintered SrFe12O19–CoFe2O4 Nanocomposites with Exchange Coupling. J. Alloys Compd. 2015, 653, 108–116.
- Belec, B.; Dražić, G.; Gyergyek, S.; Podmiljšak, B.; Goršak, T.; Komelj, M.; Nogués, J.; Makovec, D. Novel Ba-Hexaferrite Structural Variations Stabilized on the Nanoscale as Building Blocks for Epitaxial Bi-Magnetic Hard/Soft Sandwiched Maghemite/Hexaferrite/Maghemite Nanoplatelets with out-of-Plane Easy Axis and Enhanced Magnetization. Nanoscale 2017, 9, 17551–17560.
- D Soria, G.; Granados-Miralles, C.; Mandziak, A.; Jenuš, P.; Saura-Múzquiz, M.; Christensen, M.; Foerster, M.; Aballe, L.; Fernández, J.F.; de la Figuera, J.; et al. Uncorrelated Magnetic Domains in Decoupled SrFe12O19/Co Hard/Soft Bilayers. J. Phys. D Appl. Phys. 2020, 54, 054003.
- Smit, J.; Wijn, H.P.J. Ferrites; Philips’ Technical Library: Natlab, The Netherlands, 1959.
- Pan, L.; Cao, D.; Jing, P.; Wang, J.; Liu, Q. A Novel Method to Fabricate CoFe2O4/SrFe12O19 Composite Ferrite Nanofibers with Enhanced Exchange Coupling Effect. Nanoscale Res. Lett. 2015, 10, 131.
- Granados-Miralles, C.; Quesada, A.; Saura-Múzquiz, M.; Andersen, H.L.; Fernández, J.F.; Christensen, M. Expanding the Tunability and Applicability of Exchange-Coupled/Decoupled Magnetic Nanocomposites. Mater. Chem. Front. 2020, 4, 1222–1230.
- Esir, S.; Junejo, Y.; Baykal, A.; Toprak, M.; Sözeri, H. SrFe12O19/Zn0.65Ni0.25Cu0.1Fe2O4 Core–Shell Nanocomposite: Synthesis, Chracterization and Catalytic Activity in Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2014, 24, 722–728.
- Roy, D.; Kumar, P.S.A. Enhancement of (BH)Max in a Hard-Soft-Ferrite Nanocomposite Using Exchange Spring Mechanism. J. Appl. Phys. 2009, 106, 073902.
- Torkian, S.; Ghasemi, A. Energy Product Enhancement in Sufficiently Exchange-Coupled Nanocomposite Ferrites. J. Magn. Magn. Mater. 2019, 469, 119–127.
- FP7 Project Nanocrystalline Permanent Magnets Based on Hybrid Metal-Ferrites. Available online: https://cordis.europa.eu/article/id/165081-solutions-to-permanent-magnet-problem (accessed on 23 January 2024).
- H2020 Project Anisometric Permanent Hybrid Magnets Based on Inexpensive and Non-Critical Materials. Available online: https://cordis.europa.eu/project/id/720853 (accessed on 23 January 2024).
- Jenuš, P. Internal Document; Jožef Stefan Institute: Ljubljana, Slovenia, 2021.
- Hirayama, Y.; Takahashi, Y.K.; Hirosawa, S.; Hono, K. Intrinsic Hard Magnetic Properties of Sm(Fe1−xCox)12 Compound with the ThMn12 Structure. Scr. Mater. 2017, 138, 62–65.
- Hirayama, Y.; Takahashi, Y.K.; Hirosawa, S.; Hono, K. NdFe12Nx Hard-Magnetic Compound with High Magnetization and Anisotropy Field. Scr. Mater. 2015, 95, 70–72.
- Sepehri-Amin, H.; Tamazawa, Y.; Kambayashi, M.; Saito, G.; Takahashi, Y.K.; Ogawa, D.; Ohkubo, T.; Hirosawa, S.; Doi, M.; Shima, T.; et al. Achievement of High Coercivity in Sm(Fe0.8Co0.2)12 Anisotropic Magnetic Thin Film by Boron Doping. Acta Mater. 2020, 194, 337–342.
- Poudyal, N.; Liu, X.; Wang, W.; Nguyen, V.V.; Ma, Y.; Gandha, K.; Elkins, K.; Liu, J.P.; Sun, K.; Kramer, M.J.; et al. Processing of MnBi Bulk Magnets with Enhanced Energy Product. AIP Adv. 2016, 6, 056004.
- Sakamoto, Y.; Ibata, A.; Kojima, S.; Ohtani, T. New MnAlC Permanent Magnets Exhibiting Macroscopically-Plane Magnetic-Anisotropy. IEEE Trans. Magn. 1980, 16, 1056–1058.
- Jia, Y.; Wu, Y.; Xu, Y.; Zheng, R.; Zhao, S.; Skokov, K.P.; Maccari, F.; Aubert, A.; Gutfleisch, O.; Wang, J.; et al. Roadmap towards Optimal Magnetic Properties in L10-MnAl Permanent Magnets. Acta Mater. 2023, 245, 118654.
- Ogawa, T.; Ogata, Y.; Gallage, R.; Kobayashi, N.; Hayashi, N.; Kusano, Y.; Yamamoto, S.; Kohara, K.; Doi, M.; Takano, M.; et al. Challenge to the Synthesis of α’’-Fe16N2Compound Nanoparticle with High Saturation Magnetization for Rare Earth Free New Permanent Magnetic Material. Appl. Phys. Express 2013, 6, 073007.
- Winkelmann, A.; Przybylski, M.; Luo, F.; Shi, Y.; Barthel, J. Perpendicular Magnetic Anisotropy Induced by Tetragonal Distortion of FeCo Alloy Films Grown on Pd(001). Phys. Rev. Lett. 2006, 96, 257205.
- Reichel, L.; Schultz, L.; Fähler, S. Lattice Relaxation Studies in Strained Epitaxial Fe-Co-C Films. J. Appl. Phys. 2015, 117, 17C712.
- Kojima, T.; Ogiwara, M.; Mizuguchi, M.; Kotsugi, M.; Koganezawa, T.; Ohtsuki, T.; Tashiro, T.-Y.; Takanashi, K. Fe–Ni Composition Dependence of Magnetic Anisotropy in Artificially Fabricated L10-Ordered FeNi Films. J. Phys. Condens. Matter 2014, 26, 064207.
More
Information
Subjects:
Physics, Condensed Matter
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





