Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valborg Vang Poulsen | -- | 2007 | 2024-02-23 11:22:25 | | | |
| 2 | Fanny Huang | Meta information modification | 2007 | 2024-02-27 06:44:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Poulsen, V.V.; Hadi, A.; Werge, M.P.; Karstensen, J.G.; Novovic, S. Circulating Biomarkers Involved in Chronic Pancreatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/55386 (accessed on 07 February 2026).
Poulsen VV, Hadi A, Werge MP, Karstensen JG, Novovic S. Circulating Biomarkers Involved in Chronic Pancreatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/55386. Accessed February 07, 2026.
Poulsen, Valborg Vang, Amer Hadi, Mikkel Parsberg Werge, John Gásdal Karstensen, Srdan Novovic. "Circulating Biomarkers Involved in Chronic Pancreatitis" Encyclopedia, https://encyclopedia.pub/entry/55386 (accessed February 07, 2026).
Poulsen, V.V., Hadi, A., Werge, M.P., Karstensen, J.G., & Novovic, S. (2024, February 23). Circulating Biomarkers Involved in Chronic Pancreatitis. In Encyclopedia. https://encyclopedia.pub/entry/55386
Poulsen, Valborg Vang, et al. "Circulating Biomarkers Involved in Chronic Pancreatitis." Encyclopedia. Web. 23 February, 2024.
Copy Citation
Chronic pancreatitis (CP) is the end-stage of continuous inflammation and fibrosis in the pancreas evolving from acute- to recurrent acute-, early, and, finally, end-stage CP. Currently, prevention is the only way to reduce disease burden. In this setting, early detection is of great importance. Due to the anatomy and risks associated with direct sampling from pancreatic tissue, most of the information on the human pancreas arises from circulating biomarkers thought to be involved in pancreatic pathophysiology or injury.
chronic pancreatitis
fibrosis
inflammation
oxidative stress
1. Background
Acute pancreatitis (AP), chronic pancreatitis (CP), and pancreatic ductal adenocarcinoma (PDAC) place a significant burden on healthcare systems worldwide. AP is among the three most common benign gastrointestinal diseases, with a mortality rate of 0.9% and an estimated economic burden of USD2.6 billion per year in the US [1]. CP is characterized by gradual irreversible damage to the endocrine and exocrine parenchyma caused by inflammation and subsequent replacement of these tissues with fibrotic tissue and atrophy [2]. Over the last two decades, the incidence of CP has increased by 50%, and there are currently no treatments available to alter this disease’s course, resulting in significantly reduced life expectancy and quality of life. Prevention is the only way to reduce the disease burden, as serious complications including exocrine pancreatic insufficiency, malabsorption, diabetes mellitus, and PDAC may evolve as this disease progresses [3].
Approximately 50% of patients with CP have a history of AP [3]. There is continual replacement of the pancreatic tissue with fibrosis. Individuals who experience first-time AP have a 22% chance of developing recurrent acute pancreatitis (RAP) [4], and patients who experience three episodes of RAP have a 16% chance of developing CP. In addition, patients with four or more episodes of RAP have a much higher risk, around 50%, of developing CP [5].
Thus, the continuum from the first episode of AP to the manifestation of CP provides a framework for epidemiologic studies and the time-dependent evolution of circulating biomarkers involved in the progression of this disease.
2. Inflammation
From 1994 to 2022, 55 articles examined 64 inflammatory biomarkers, of which 23 were examined multiple times. In addition, five of these studies included patients with AP [6][7][8][9][10]. Of the 23 biomarkers, 12 were either not elevated in CP or the findings were inconclusive. Several pro-inflammatory interleukins (IL-6, IL-8, and IL-12) were found to be elevated in patients with CP compared to healthy controls [11][12][13], along with vascular endothelial growth factor (VEGF), intercellular adhesion molecule (ICAM), chemerin, fractalkine, resistin, osteopontin, and neopterin [7][10][14][15][16][17][18]. In contrast, leptin was found to be reduced in patients with CP [16]. IL-1β, IL-6, IL-10, tumor necrosis factor α (TNF-α), adiponectin, and leptin were the most studied inflammatory biomarkers in CP. However, the findings were cohesive only for IL-6, TNF-α, and leptin. IL-10, IL-12, TNF-α, and INF-γ were elevated in patients with AP [6][8]. Figure 1 demonstrates a schematic overview of the inflammatory biomarkers involved in the progression to CP.
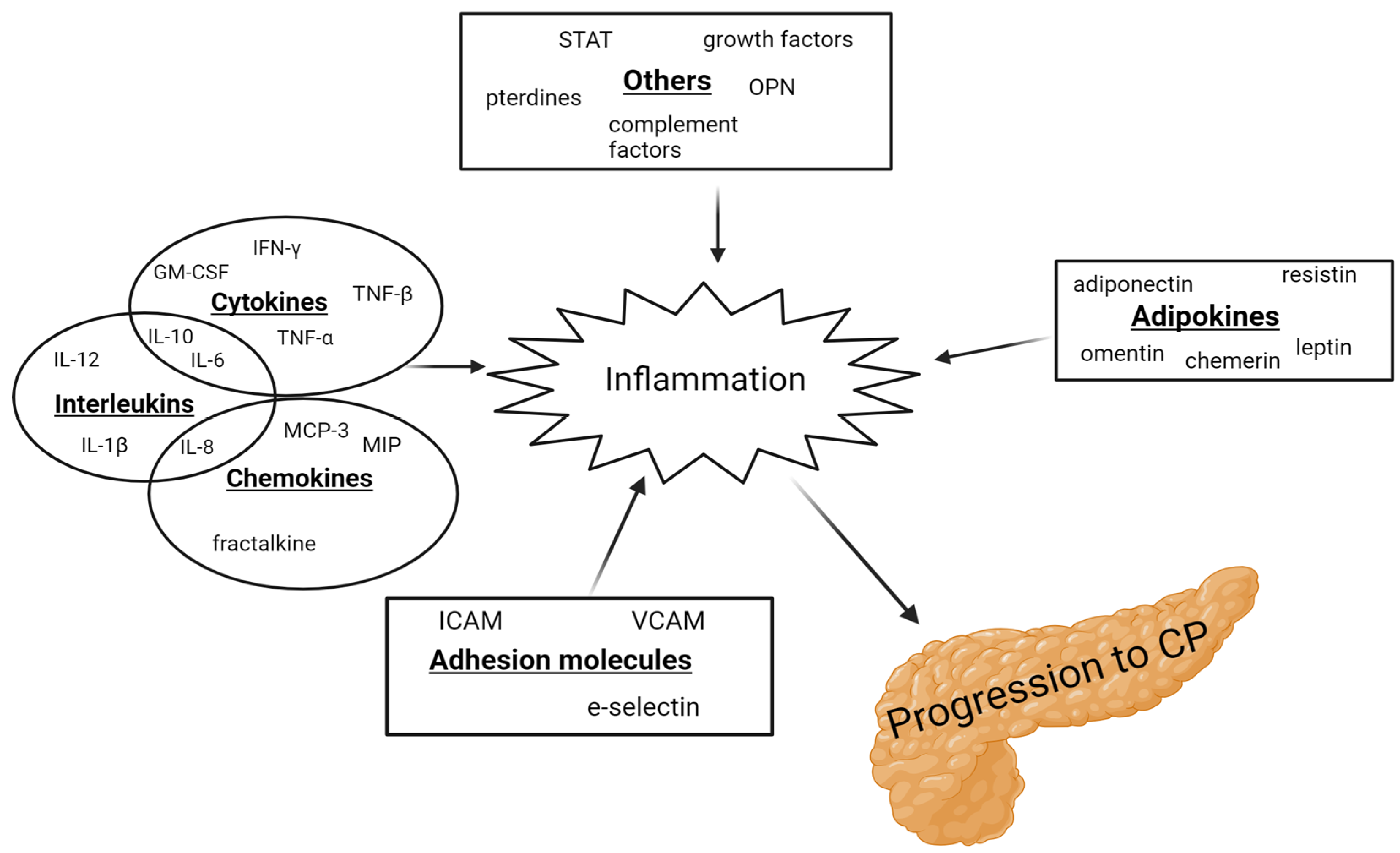
Figure 1. Schematic illustration of the inflammatory biomarkers involved in the progression to CP. GM-CSF: granulocyte-macrophage colony-stimulating factor; ICAM: intracellular adhesion molecule; IFN: interferon; IL: interleukins; MCP: monocyte chemotactic protein; MIP: macrophage inflammatory protein; OPN: osteopontin; STAT: signal transducer and activator of transcription; TNF: tumor necrosis factor; and VCAM: vascular cell adhesion molecule.
2.1. Interleukin 6
IL-6 induces the synthesis of acute-phase proteins and the production of other cytokines, including C-reactive protein (CRP) [19]. Sixteen studies measured IL-6, with twelve observing higher levels in patients with CP compared to healthy controls [6][11][12][20][21][22][23][24][25][26][27][28], although the difference was not significant in three studies [20][25][28]. Four studies found no difference in the IL-6 levels [27][29][30][31]. In one study, a surge in IL-6 serum levels was observed in patients with alcoholic CP after the consumption of alcohol, with a decrease to the pre-stimulatory levels after 4–24 h, suggesting a correlation between alcohol consumption and IL-6 levels [24]. Elevated IL-6 levels were also evident in AP [6]. IL-6 rises 1–2 days before CRP, making it suitable for an early distinction between severe and mild AP [32][33]. Higher concentrations of IL-6 are linked to the increased risk of complications and death in severe AP [34][35][36][37]
2.2. Tumor Necrosis Factor α
TNF-α is a cytokine that facilitates both inflammation and fibrosis formation. It plays a key role in regulating other cytokines towards inflammation and activating pancreatic stellate cells (PSCs). TNF-α triggers the activation of PCSs, which, in turn, start producing extracellular matrix (ECM). This disorganization of the ECM leads to fibrosis formation and chronic inflammation of the pancreas [38][39]. The levels of TNF-α in patients with CP were investigated in 12 studies from 1999 to 2022. Elevated levels were found in six studies [9][11][22][31][39][40][41], one found lower levels [9], while the remaining five found no significant differences [8][20][25][29][42]. Kiyci et al. discovered significantly higher serum levels of TNF-α in AP compared to CP, indicating TNF-α’s potential role in the progression of the disease. However, it is worth noting that this study included only 13 patients with AP, 36 patients with CP, and 14 controls [8].
2.3. Leptin
Leptin, an adipokine with a crucial role in metabolism, obesity, and cardiovascular diseases, has also been found to activate macrophages and T-lymphocytes, stimulating their cytokine secretion [43]. Moreover, it has been demonstrated to induce fibrosis in the liver by inhibiting hepatic stellate cell apoptosis [16][44][45]. Five studies found reduced levels of leptin in patients with CP [16][46][47][48][49], while one study found elevated leptin levels compared to healthy controls [41]. Because leptin is secreted by adipocytes, a higher fat percentage results in a higher amount of circulating leptin. Patients with CP had lower BMI across the involved studies, making it difficult to determine if the reduced levels were due to pancreatitis or to a lower fat mass. Additionally, patients with CP with diabetes mellitus (DM) were found to have higher levels of leptin than patients with CP without DM [41]. Lower levels of leptin may play a protective role in the development of CP by increasing apoptosis of the PSCs.
3. Fibrosis
A total of 46 studies spanning from 1995 to 2022 examined 28 potential biomarkers of fibrosis in patients with CP. Of these, 13 were studied multiple times. Three studies also included patients with AP [7][10][50]. The biomarkers mainly consist of PSCs activators, with the most extensively studied being TGF-β, PDGF, and MIC-1, and components of the ECM, with TIMP-1 and MMP-9 being studied the most. Figure 2 demonstrates a schematic overview of the fibrotic biomarkers associated with the development of CP.
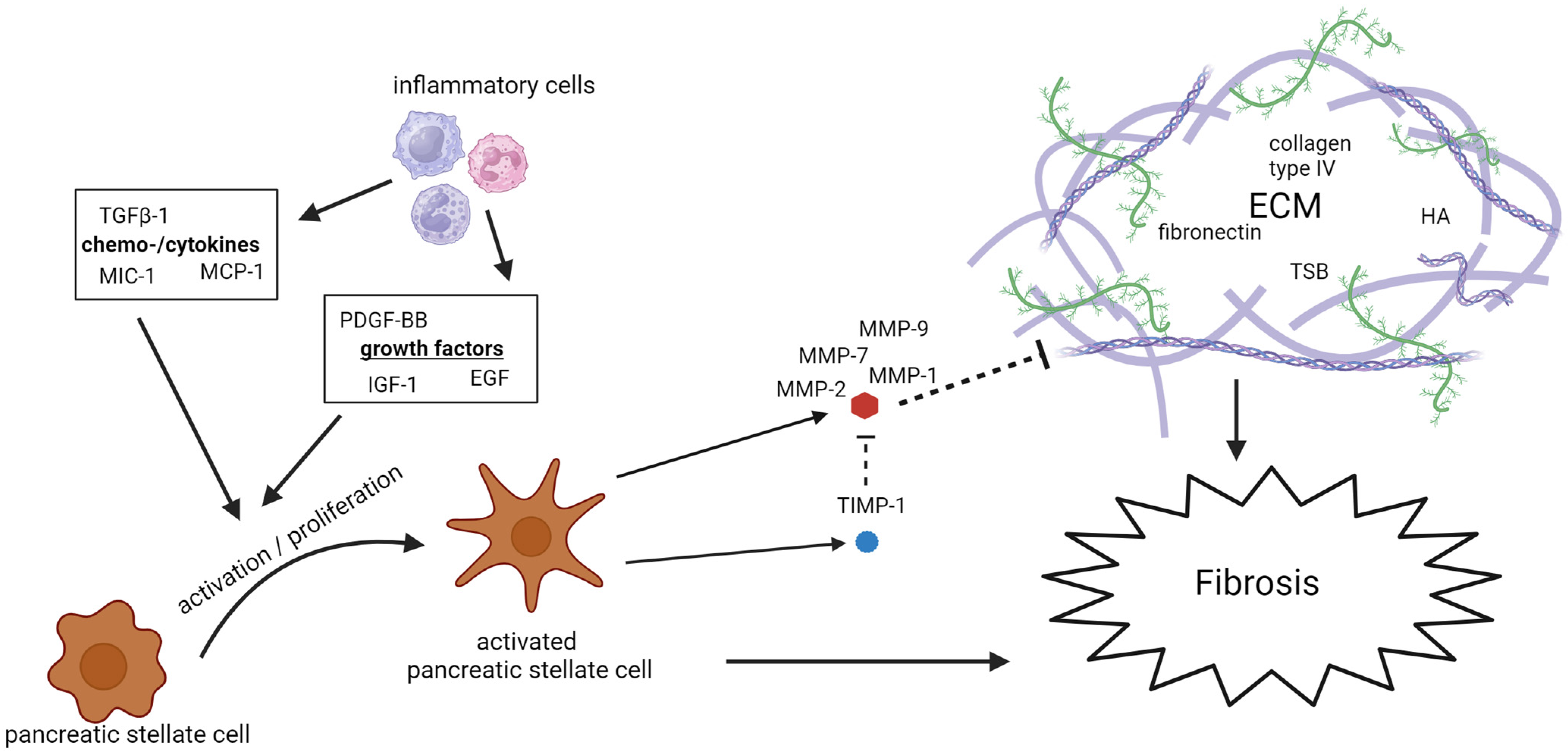
Figure 2. Schematic overview of the fibrotic biomarkers associated with the development of CP. ECM: extracellular matrix; EGF: epidermal growth factor; HA: hyaluronic acid; IGF: insulin-like growth factor; MCP: monocyte chemotactic protein; MIC: macrophage inhibitory cytokine; MMP: matrix metalloproteinase; PDGF: platelet-derived growth factor; TGF: tumor growth factor; TIMP: tissue inhibitors of metalloproteinases; and TSP: tissue polypeptide specific antigen.
3.1. Extracellular Matrix Remodeling
Continuous modulation of the ECM leads to fibrosis. Matrix metalloproteinases (MMPs) degrade the ECM, while tissue inhibitors of matrix metalloproteinases (TIMPs) inhibit MMPs. Numerous studies have measured the concentration of these biomarkers in patients with CP. MMPs are included in seven of the studies researchers reviewed [39][47][48][51][52][53][54]. Elevated levels of MMP-1, MMP-2, MMP-7, and MMP-9 were found in patients with CP compared to the control group, while MMP-3 was not seen to be elevated in patients with CP.
TIMP-1 concentrations in patients with CP were studied in nine of the studies researchers reviewed [15][48][51][53][55][56][57][58][59], all showing elevated concentrations in patients with CP, although three did not reach significance [48][55][56]. Hyaluronic acid (HA), laminin, and fibronectin are also important components of the ECM and are directly associated with the potential role of the ECM in the context of CP, see Figure 2. Elevated levels of all these components of the ECM were found in patients with CP. Four studies found elevated levels of HA [60][61][62][63], a fundamental component of the ECM in the pancreas. The Mac-2-binding protein (M2BP), a ligand which binds to ECM proteins and a novel biomarker of liver fibrosis, has also been found to be elevated in patients with CP.
3.2. Activation of PSCs
The activation and proliferation of PSCs influence the development of pancreatic fibrosis by the synthesis and remodeling of the ECM. The remodeling of the ECM is primarily mediated through the PCSs’ secretion of MMP and TIMP [64].
The cytokine transforming growth factor β1 (TGF-β1), the growth factor platelet-derived growth factor (PDGF), and the chemokine monocyte chemoattractant protein 1 (MCP-1) are among the most important mediators involved in the activation of PSCs. With few exceptions, these biomarkers are all found to be elevated in patients with CP compared to the controls. Elevated levels of TGF-β, PDGF, and MCP-1 were also found in patients with AP [7][10][50]. Macrophage inhibitory cytokine 1 (MIC-1), a cytokine part of the TGF-β family, has also been found to be elevated in patients with CP in five different studies. Its specific role in the pancreas is not extensively studied, but, as a part of the TGF-β family, it can be presumed that it has a role in the activation of PSCs.
4. Oxidative Stress
Twenty-three studies from 1981 to 2022 examined 34 biomarkers of oxidative stress, of which 23 were studied multiple times. Four articles also included patients with AP [65][66][67][68]. A potential relationship between oxidative stress and pancreatic inflammation has been extensively studied. Research indicates an early occurrence of pancreatic oxidative stress in AP. Free oxygen radicals play a crucial role in regulating the extent of necrosis in acinar cells, the development of pancreatic edema, the sequestration of inflammatory cells within the pancreas, and the release of inflammatory mediators [69]. Additionally, there is growing evidence connecting oxidative stress and CP. The use of antioxidant therapy has been shown to reduce the severity of CP, resulting in less fibrosis in murine models [70], as well as improve the well-being, decrease pain, and improve the overall functioning of patients with CP [71][72]. The biomarkers of oxidative stress are challenging to evaluate, primarily due to their complex metabolism and high turnover, making them difficult to measure in systemic circulation. The low blood antioxidant levels could be attributed to poor nutritional status due to malabsorption, maldigestion, and reduced food intake, often observed in patients with CP. Figure 3 gives a schematic overview of oxidative stress biomarkers associated with CP development.
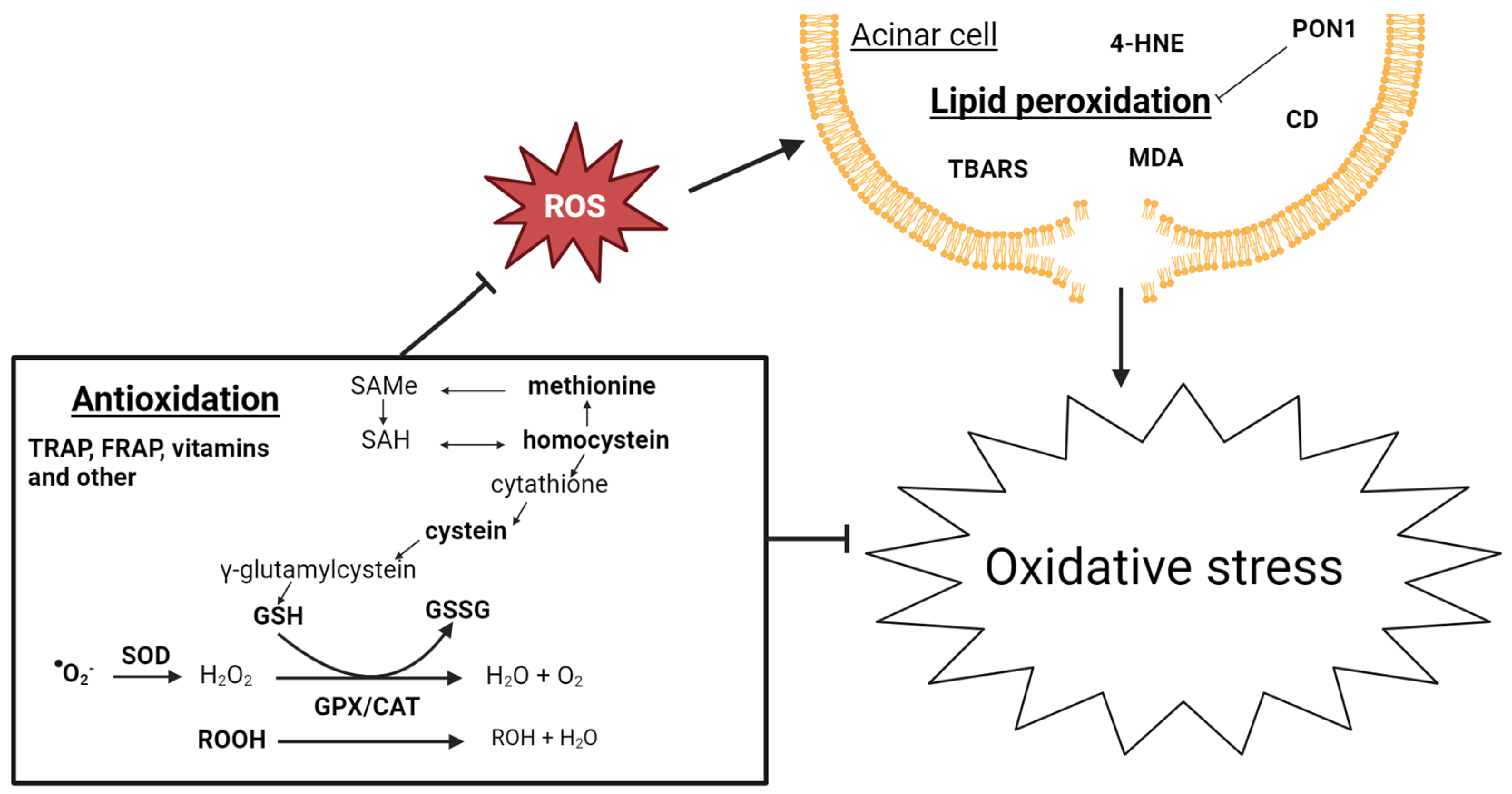
Figure 3. Schematic overview of biomarkers of oxidative stress associated with CP development. CAT: catalase; GPX: glutathione peroxidase; GSH: glutathione; GSSG: glutathione disulfide; ROOH: hydroperoxides; SAH: S-adenosyl homocysteine; SAMe: S-adenyl methionine; and SOD: superoxide dismutase.
4.1. Lipid Peroxidation
Prolonged exposure to oxygen radicals results in lipid peroxidation and the oxidation of fatty acids in cell membranes. Lipid peroxidation has been the focus of several studies investigating oxidative stress in CP [73]. Fourteen studies have included one of these biomarkers, and, apart from a few non-significant results, all these biomarkers are found to be elevated in patients with CP. Few studies include oxygen radicals in patients with CP. Superoxides, main reactive oxygen species (ROS) in cells, and ROS production in cells after phorbol myristate acetate (PMA) stimulation have all been investigated in patients with CP. These biomarkers are difficult to measure in the blood as they have a very short half-life. Elevated levels were found in all four studies; however, in two of them, the elevation was not significant. On the other hand, PON1 is a free radical-scavenging molecule contributing to the detoxification of free radicals involved in lipid peroxidation [74], and consistently reduced levels of PON1 were found in patients with CP in the studies researchers analyzed, indicating elevated lipid peroxidation in patients with CP. MDA, superoxide anion, and CAT were also found to be elevated in AP [65][66][67][68].
4.2. Antioxidation
The reactive superoxide is catalyzed to hydrogen peroxide by superoxide dismutase (SOD). The main ROS scavenger molecule is glutathione (GSH), which is used by glutathione peroxidase (GPX) and catalase (CAT) to reduce/neutralize ROS [75]; see Figure 3. GSH, GPX, CAT, and SOD have been measured in patients with CP in, respectively six, eight, four, and six studies. GSH, GPX, and SOD levels were reduced in patients with CP, while the results on CAT were contradictive, as two studies found no difference, one found lower levels, and one study found elevated levels. The antioxidant capacity in the blood is measured by the ferrin-reducing ability of the plasma (FRAP) and the total peroxyl radical-trapping antioxidant parameter (TRAP). While FRAP is reduced in patients with CP, the TRAP concentrations are no different from the controls. Reduced levels of GSH and elevated levels of CAT and TRAP were found in patients with AP [65][66][67].
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11.
- Schneider, A.; Löhr, J.M.; Singer, M.V. The M-ANNHEIM Classification of Chronic Pancreatitis: Introduction of a Unifying Classification System Based on a Review of Previous Classifications of the Disease. J. Gastroenterol. 2007, 42, 101–119.
- Cai, Q.Y.; Tan, K.; Zhang, X.L.; Han, X.; Pan, J.P.; Huang, Z.Y.; Tang, C.W.; Li, J. Incidence, Prevalence, and Comorbidities of Chronic Pancreatitis: A 7-Year Population-Based Study. World J. Gastroenterol. 2023, 29, 4671–4684.
- Sankaran, S.J.; Xiao, A.Y.; Wu, L.M.; Windsor, J.A.; Forsmark, C.E.; Petrov, M.S. Frequency of Progression from Acute to Chronic Pancreatitis and Risk Factors: A Meta-Analysis. Gastroenterology 2015, 149, 1490–1500.e1.
- Hegyi, P.J.; Soós, A.; Tóth, E.; Ébert, A.; Venglovecz, V.; Márta, K.; Mátrai, P.; Mikó, A.; Bajor, J.; Sarlós, P.; et al. Evidence for Diagnosis of Early Chronic Pancreatitis after Three Episodes of Acute Pancreatitis: A Cross-Sectional Multicentre International Study with Experimental Animal Model. Sci. Rep. 2021, 11, 1367.
- Bhatnagar, A.; Wig, J.D.; Majumdar, S. Immunological Findings in Acute and Chronic Pancreatitis. ANZ J. Surg. 2003, 73, 59–64.
- Ito, T. Can Measurement of Chemokines Become Useful Biological and Functional Markers of Early-Stage Chronic Pancreatitis? J. Gastroenterol. 2007, 42, 72–77.
- Kıyıcı, A.; İbiş, M.; Akbulut, Ş.; Köklü, S.; Uçar, E.; Ünlü, A. Serum TNF-Alpha Levels in Acute and Chronic Pancreatitis. Eur. J. Gen. Med. 2009, 6, 103–107.
- Sandström, A.; Andersson, R.; Segersvärd, R.; Löhr, M.; Borrebaeck, C.A.K.; Wingren, C. Serum Proteome Profiling of Pancreatitis Using Recombinant Antibody Microarrays Reveals Disease-Associated Biomarker Signatures. Proteom. Clin. Appl. 2012, 6, 486–496.
- Stojek, M.; Adrych, K.; Rojek, L.; Smoczynski, M.; Sledzinski, T.; Szrok, S.; Swierczynski, J. Decreased Serum Platelet Derived Growth Factor BB Levels in Acute and Increased in Chronic Pancreatitis. World J. Gastroenterol. 2014, 20, 13127–13132.
- Miron, N.; Miron, M.-M.; Milea, V.G.I.; Cristea, V. Proinflammatory Cytokines: An Insight into Pancreatic Oncogenesis. Rom. Arch. Microbiol. Immunol. 2010, 69, 183–189.
- Shaw, V.E.; Lane, B.; Jenkinson, C.; Cox, T.; Greenhalf, W.; Halloran, C.M.; Tang, J.; Sutton, R.; Neoptolemos, J.P.; Costello, E. Serum Cytokine Biomarker Panels for Discriminating Pancreatic Cancer from Benign Pancreatic Disease. Mol. Cancer 2014, 13, 114.
- Schneider, A.; Haas, S.L.; Hildenbrand, R.; Siegmund, S.; Reinhard, I.; Nakovics, H.; Singer, M.V.; Feick, P. Enhanced Expression of Interleukin-18 in Serum and pancreas of Patients with Chronic Pancreatitis. World J. Gastroenterol. 2006, 12, 6507.
- Berindan-Neagoe, I.; Burz, C.; Balacescu, O.; Balacescu, L.; Seicean, A.; Cristea, V.; Irimie, A. Molecular Angiogenesis Profile as a Tool to Discriminate Chronic Pancreatitis (CP) from Pancreatic Cancer (PC). CA Cancer J. Clin. 2011, 40, 482–483.
- Jenkinson, C.; Elliott, V.; Menon, U.; Apostolidou, S.; Fourkala, O.E.; Gentry-Maharaj, A.; Pereira, S.P.; Jacobs, I.; Cox, T.F.; Greenhalf, W.; et al. Evaluation in Pre-Diagnosis Samples Discounts ICAM-1 and TIMP-1 as Biomarkers for Earlier Diagnosis of Pancreatic Cancer. J. Proteom. 2015, 113, 400–402.
- Adrych, K.; Smoczynski, M.; Sledzinski, T.; Dettlaff-Pokora, A.; Goyke, E.; Swierczynski, J. Increased Serum Resistin Concentration in Patients With Chronic Pancreatitis Possible Cause of Pancreatic Fibrosis. J. Clin. Gastroenterol. 2008, 43, 63–68.
- Song, J.; Sokoll, L.J.; Pasay, J.J.; Rubin, A.L.; Li, H.; Bach, D.M.; Chan, D.W.; Zhang, Z. Identification of Serum Biomarker Panels for the Early Detection of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 174–182.
- Talar-Wojnarowska, R.; Gasiorowska, A.; Olakowski, M.; Lekstan, A.; Lampe, P.; Malecka-Panas, E. Clinical Value of Serum Neopterin, Tissue Polypeptide-Specific Antigen and CA19-9 Levels in Differential Diagnosis between Pancreatic Cancer and Chronic Pancreatitis. Pancreatology 2011, 10, 689–694.
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Sanders, N.L.; Mishra, A. Pathogenic Mechanisms of Pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 10–25.
- Manes, G.; Spada, O.A.; Rabitti, P.G.; Feola, B.; Misso, S.; Minerva, A.; Uomo, G. Neopterin Serum Levels in Pancreatic Adenocarcinoma. Int. J. Pancreatol. 1999, 25, 31–37.
- Talar-Wojnarowska, R.; Gasiorowska, A.; Smolarz, B.; Romanowicz-Makowska, H.; Kulig, A.; Malecka-Panas, E. Clinical Significance of Interleukin-6 (Il-6) Gene Polymorphism and Il-6 Serum Level in Pancreatic Adenocarcinoma and Chronic Pancreatitis. Dig. Dis. Sci. 2009, 54, 683–689.
- Gasiorowska, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E. Subclinical Inflammation and Endothelial Dysfunction in Patients with Chronic Pancreatitis and Newly Diagnosed Pancreatic Cancer. Dig. Dis. Sci. 2016, 61, 1121–1129.
- Tanţău, A.; Leucuţa, D.C.; Tanţău, M.; Boţan, E.; Zaharie, R.; Mândruţiu, A.; Tomuleasa, I.C. Inflammation, Tumoral Markers and Interleukin-17, -10, and -6 Profiles in Pancreatic Adenocarcinoma and Chronic Pancreatitis. Dig. Dis. Sci. 2021, 66, 3427–3438.
- Pedersen, N.; Larsen, S.; Seidelin, J.B.; Nielsen, O.H. Alcohol Modulates Circulating Levels of Interleukin-6 and Monocyte Chemoattractant Protein-1 in Chronic Pancreatitis. Scand. J. Gastroenterol. 2004, 39, 277–282.
- Zhang, J.; Fan, H.; Gross, M.; Liu, N.; Carlson, H.; Wood, A.; Hoffman, K.; Petrosino, J.; Pankratz, N.; Thyagarajan, B.; et al. Progressive Reduction in Circulating Levels of Carotenoids and Other Micronutrients in Patients with Chronic Pancreatitis. Pancreatology 2022, 22, 1126–1133.
- Hansen, M.; Rinnov Nielsen, A.; Vilsbøll, T.; Lund, A.; Krarup, T.; Knop, F.K.; Vestergaard, H. Increased Levels of YKL-40 and Interleukin 6 in Patients With Chronic Pancreatitis and Secondary Diabetes. Pancreas 2012, 41, 1316–1318.
- Mroczko, B.; Groblewska, M.; Gryko, M.; Kȩdra, B.; Szmitkowski, M. Diagnostic Usefulness of Serum Interleukin 6 (IL-6) and C-Reactive Protein (CRP) in the Differentiation between Pancreatic Cancer and Chronic Pancreatitis. J. Clin. Lab. Anal. 2010, 24, 256–261.
- Singh, N.; Gupta, S.; Rashid, S.; Saraya, A. Association of Inflammatory Markers with the Disease & Mutation Status in Pancreatic Cancer. Indian J. Med. Res. 2022, 155, 49–55.
- Chung, H.W.; Jang, S.; Lim, J.B. Clinical Implications and Diagnostic Usefulness of Correlation between Soluble Major Histocompatibility Complex Class i Chain-Related Molecule a and Protumorigenic Cytokines in Pancreatic Ductal Adenocarcinoma. Cancer 2013, 119, 233–244.
- Bamba, T.; Yoshioka, U.; Hosoda, S. Serum Levels of Interleukin-Lp and Interleukin-6 in Patients with Chronic Pancreatitis. J. Gastroenterol. 1994, 29, 314–319.
- Dima, S.O.; Tanase, C.; Albulescu, R.; Herlea, V.; Chivu-Economescu, M.; Purnichescu-Purtan, R.; Dumitrascu, T.; Duda, D.G.; Popescu, I. An Exploratory Study of Inflammatory Cytokines as Prognostic Biomarkers in Patients With Ductal Pancreatic Adenocarcinoma. Pancreas 2012, 41, 1001–1007.
- Cho, I.R.; Do, M.Y.; Han, S.Y.; Jang, S.I.; Cho, J.H. Comparison of Interleukin-6, C-Reactive Protein, Procalcitonin, and the Computed Tomography Severity Index for Early Prediction of Severity of Acute Pancreatitis. Gut Liver 2023, 17, 629–637.
- Heresbach, D.; Letourneur, J.P.; Bahon, I.; Pagenault, M.; Guillou, Y.M.; Dyard, F.; Fauchet, R.; Mallédant, Y.; Bretagne, J.F.; Gosselin, M. Value of Early Blood Th-1 Cytokine Determination in Predicting Severity of Acute Pancreatitis. Scand. J. Gastroenterol. 1998, 33, 554–560.
- Berney, T.; Gasche, Y.; Robert, J.; Jenny, A.; Mensi, N.; Grau, G.; Vermeulen, B.; Morel, P. Serum Profiles of Interleukin-6, Interleukin-8, and Interleukin-10 in Patients with Severe and Mild Acute Pancreatitis. Pancreas 1999, 18, 37–38.
- Inagaki, T.; Hoshino, M.; Hayakawa, T.; Ohara, H.; Yamada, H.; Iida, M.; Nakazawa, T.; Ogasawara, T.; Uchida, A.; Hasegawa, C.; et al. Interleukin-6 Is a Useful Marker for Early Prediction of the Severity of Acute Pancreatitis. Pancreas 1997, 14, 1–8.
- Brivet, F.G.; Emilie, D.; Galanaud, P. Pro- and Anti-Inflammatory Cytokines during Acute Severe Pancreatitis: An Early and Sustained Response, Although Unpredictable of Death. Crit. Care Med. 1999, 27, 749–755.
- Leser, H.G.; Gross, V.; Scheibenbogen, C.; Heinisch, A.; Salm, R.; Lausen, M.; Rückauer, K.; Andreesen, R.; Farthmann, E.H.; Schölmerich, J. Elevation of Serum Interleukin-6 Concentration Precedes Acute-Phase Response and Reflects Severity in Acute Pancreatitis. Gastroenterology 1991, 101, 782–785.
- Norman, J. The Role of Cytokines in the Pathogenesis of Acute Pancreatitis. Am. J. Surg. 1998, 175, 76–83.
- Manjari, K.S.; Jyothy, A.; Vidyasagar, A.; Prabhakar, B.; Nallari, P.; Venkateshwari, A. Matrix Metalloproteinase-9, Transforming Growth Factor-Β1, and Tumor Necrosis Factor-α Plasma Levels in Chronic Pancreatitis. Indian J. Gastroenterol. 2013, 32, 103–107.
- Sri Manjari, K.; Jyothy, A.; Shravan Kumar, P.; Prabhakar, B.; Uma Devi, M.; Ramanna, M.; Nallari, P.; Venkateshwari, A. A Single-Nucleotide Polymorphism in Tumor Necrosis Factor-α (-308 G/A) as a Biomarker in Chronic Pancreatitis. Gene 2014, 539, 186–189.
- Hontsariuk, D.O.; Ferfetska, K.V.; Khrystych, T.M.; Fediv, O.I.; Temerivska, T.G.; Jiguleva, E.O.; Honcharuk, L.M.; Olinik, O.Y. Incides of C-Reactive Protein, Tumor Necrosis Factor-α, Adiponectin, Leptin and Resistin in the Blood of Patients Suffering from Chronic Pancreatitis and Type 2 Diabetes Mellitus. J. Med. Life 2020, 13, 568–571.
- Greer, J.B.; Greer, P.; Sandhu, B.S.; Alkaade, S.; Wilcox, C.M.; Anderson, M.A.; Sherman, S.; Gardner, T.B.; Lewis, M.D.; Guda, N.M.; et al. Nutrition and Inflammatory Biomarkers in Chronic Pancreatitis Patients. Nutr. Clin. Pract. 2019, 34, 387–399.
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149.
- Saxena, N.K.; Titus, M.A.; Ding, X.; Floyd, J.; Srinivasan, S.; Sitaraman, S.V.; Anania, F.A. Leptin as a Novel Profibrogenic Cytokine in Hepatic Stellate Cells: Mitogenesis and Inhibition of Apoptosis Mediated by Extracellular Regulated Kinase (Erk) and Akt Phosphorylation. FASEB J. 2004, 18, 1612–1614.
- Petrescu, A.D.; Grant, S.; Williams, E.; An, S.Y.; Seth, N.; Shell, M.; Amundsen, T.; Tan, C.; Nadeem, Y.; Tjahja, M.; et al. Leptin Enhances Hepatic Fibrosis and Inflammation in a Mouse Model of Cholestasis. Am. J. Pathol. 2022, 192, 484–502.
- Park, W.G.; Li, L.; Appana, S.; Wei, W.; Stello, K.; Andersen, D.K.; Hughes, S.J.; Whitcomb, D.C.; Brand, R.E.; Yadav, D.; et al. Unique Circulating Immune Signatures for Recurrent Acute Pancreatitis, Chronic Pancreatitis and Pancreatic Cancer: A Pilot Study of These Conditions with and without Diabetes: Immune Profiling of Pancreatic Disorders. Pancreatology 2020, 20, 51–59.
- Dranka-Bojarowska, D.; Lekstan, A.; Olakowski, M.; Jablonska, B.; Lewinski, A.; Musialski, P.; Sobczyk, W.; Kapalka, A.; Lampe, P. The Assessment of Serum Concentration of Adiponectin, Leptin and Serum Carbonhydrate Antigen-19.9 in Patients with Pancreatic Cancer and Chronic Pancreatitis. J. Physiol. Pharmacol. 2015, 66, 653–663.
- Hrabák, P.; Šoupal, J.; Kalousová, M.; Krechler, T.; Vočka, M.; Hanuš, T.; Petruželka, L.; Svačina, Š.; Žák, A.; Zima, T. Novel Biochemical Markers for Non-Invasive Detection of Pancreatic Cancer. Neoplasma 2022, 69, 474–483.
- Adrych, K.; Smoczynski, M.; Stojek, M.; Sledzinski, T.; Slominska, E.; Goyke, E.; Smolenski, R.T.; Swierczynski, J. Decreased Serum Essential and Aromatic Amino Acids in Patients with Chronic Pancreatitis. World J. Gastroenterol. 2010, 16, 4422–4427.
- Cavestro, G.M.; Zuppardo, R.A.; Bertolini, S.; Sereni, G.; Frulloni, L.; Okolicsanyi, S.; Calzolari, C.; Singh, S.K.; Sianesi, M.; Del Rio, P.; et al. Connections between Genetics and Clinical Data: Role of Mcp-1, Cftr, and Spink-1 in the Setting of Acute, Acute Recurrent, and Chronic Pancreatitis. Am. J. Gastroenterol. 2010, 105, 199–206.
- Resovi, A.; Bani, M.R.; Porcu, L.; Anastasia, A.; Minoli, L.; Allavena, P.; Cappello, P.; Novelli, F.; Scarpa, A.; Morandi, E.; et al. Soluble Stroma-related Biomarkers of Pancreatic Cancer. EMBO Mol. Med. 2018, 10, e8741.
- Venkateshwari, A.; Sri Manjari, K.; Krishnaveni, D.; Nallari, P.; Vidyasagar, A.; Jyothy, A. Role of Plasma MMP 9 Levels in the Pathogenesis of Chronic Pancreatitis. Indian J. Clin. Biochem. 2011, 26, 136–139.
- Mroczko, B.; Lukaszewicz-Zajac, M.; Wereszczynska-Siemiatkowska, U.; Groblewska, M.; Gryko, M.; Kedra, B.; Jurkowska, G.; Szmitkowski, M. Clinical Significance of the Measurements of Serum Matrix Metalloproteinase-9 and Its Inhibitor (Tissue Inhibitor of Metalloproteinase-1) in Patients With Pancreatic Cancer Metalloproteinase-9 as an Independent Prognostic Factor. Pancreas 2009, 38, 613–618.
- Dranka-Bojarowska, D.; Lewinski, A.; Lekstan, A.; Gajda, M.; Ciosek, J.; Mrowiec, S. The Assessment of Serum and Diagnostic Peritoneal Lavage Concentration of Matrix Metalloproteinase-2, Matrix Metalloproteinase-9, Carbohydrate Antigen 19-9, and Carcinoembryonic Antigen in Patients with Pancreatic Cancer and Chronic Pancreatitis. J. Physiol. Pharmacol. 2020, 71, 689–704.
- Pan, S.; Chen, R.; Crispin, D.A.; May, D.; Stevens, T.; McIntosh, M.W.; Bronner, M.P.; Ziogas, A.; Anton-Culver, H.; Brentnall, T.A. Protein Alterations Associated with Pancreatic Cancer and Chronic Pancreatitis Found in Human Plasma Using Global Quantitative Proteomics Profiling. J. Proteome Res. 2011, 10, 2359–2376.
- Poruk, K.E.; Firpo, M.A.; Scaife, C.L.; Adler, D.G.; Emerson, L.L.; Boucher, K.M.; Mulvihill, S.J. Serum Osteopontin and Tissue Inhibitor of Metalloproteinase 1 as Diagnostic and Prognostic Biomarkers for Pancreatic Adenocarcinoma. Pancreas 2013, 42, 193–197.
- Koopmann, J.; Rosenzweig, C.N.W.; Zhang, Z.; Canto, M.I.; Brown, D.A.; Hunter, M.; Yeo, C.; Chan, D.W.; Breit, S.N.; Goggins, M. Serum Markers in Patients with Resectable Pancreatic Adenocarcinoma: Macrophage Inhibitory Cytokine 1 versus CA19-9. Clin. Cancer Res. 2006, 12, 442–446.
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011–1024.e7.
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated Systemic Levels of the Matrix Metalloproteinase Inhibitor TIMP-1 Correlate with Clinical Markers of Cachexia in Patients with Chronic Pancreatitis and Pancreatic Cancer. BMC Cancer 2018, 18, 128.
- Kozak, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E.; Gąsiorowska, A. Utility of Different Serum Fibrosis Markers in Diagnosing Patients with Chronic Pancreatitis and Pancreatic Adenocarcinoma. World J. Gastrointest. Oncol. 2016, 8, 635–641.
- Kamath, M.G.; Pai, C.G.; Kamath, A.; Kurien, A. Monocyte Chemoattractant Protein-1, Transforming Growth Factor-Β1, Nerve Growth Factor, Resistin and Hyaluronic Acid as Serum Markers: Comparison between Recurrent Acute and Chronic Pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 209–215.
- Adrych, K.; Smoczynski, M.; Stojek, M.; Sledzinski, T.; Korczynska, J.; Goyke, E.; Swierczynski, J. Coordinated Increase in Serum Platelet-Derived Growth Factor-BB and Transforming Growth Factor-Β1 in Patients with Chronic Pancreatitis. Pancreatology 2011, 11, 434–440.
- Chen, I.M.; Willumsen, N.; Dehlendorff, C.; Johansen, A.Z.; Jensen, B.V.; Hansen, C.P.; Hasselby, J.P.; Bojesen, S.E.; Pfeiffer, P.; Nielsen, S.E.; et al. Clinical Value of Serum Hyaluronan and Propeptide of Type III Collagen in Patients with Pancreatic Cancer. Int. J. Cancer 2020, 146, 2913–2922.
- Suda, K. Distribution, Pathogenesis and Progression of Human Pancreatic Fibrosis. Gastroenterology 2007, 33, 67–79.
- Sajewicz, W.; Milnerowicz, S.; Nabzdyk, S. Blood Plasma Antioxidant Defense in Patients With Pancreatitis. Pancreas 2006, 32, 139–144.
- Tsuji, N.; Watanabe, N.; Okamoto, T.; Niitsu, Y. Specific Interaction of Pancreatic Elastase and Leucocytes to Produce Oxygen Radicals and Its Implication in Pancreatitis. Gut 1994, 35, 1659–1664.
- Schoenberg, M.H.; Buchler, M.; Pietrzyk, C.; Uhl, W.; Birk, D.; Eisele, S.; Marzinzig, M.; Beger, H.G. Lipid Peroxidation and Glutathione Metabolism in Chronic Pancreatitis. Pancreas 1995, 10, 36–43.
- Fukui, M.; Kanoh, M.; Takamatsu, Y.; Arakawa, Y. Analysis of Serum Catalase Activities in Pancreatic Diseases. J. Gastroenterol. 2004, 39, 469–474.
- López, M.A.; Alcaraz, C.A. Oxidative Stress and Acute Pancreatitis. World J. Gastroenterol. 2011, 103, 559–562.
- Tasci, I.; Deveci, S.; Isik, A.T.; Comert, B.; Akay, C.; Mas, N.; Inal, V.; Yamanel, L.; Mas, M.R. Allopurinol in Rat Chronic Pancreatitis: Effects on Pancreatic Stellate Cell Activation. Pancreas 2007, 35, 366–371.
- Kirk, G.R.; White, J.S.; McKie, L.; Stevenson, M.; Young, I.; Clements, W.D.B.; Rowlands, B.J. Combined Antioxidant Therapy Reduces Pain and Improves Quality of Life in Chronic Pancreatitis. J. Gastrointest. Surg. 2006, 10, 499–503.
- Salim, A.S. Role of Oxygen-Derived Free Radical Scavengers in the Treatment of Recurrent Pain Produced by Chronic Pancreatitis. A New Approach. Arch. Surg. 1991, 126, 1109–1114.
- Santini, S.A.; Spada, C.; Bononi, F.; Foschia, F.; Mutignani, M.; Perri, V.; Giardina, B.; Gentiloni Silveri, N.; Costamagna, G. Enhanced Lipoperoxidation Products in Pure Pancreatic Juice: Evidence for Organ-Specific Oxidative Stress in Chronic Pancreatitis. Dig. Liver Dis. 2003, 35, 888–892.
- Zhang, L.; Lin, B. Decreased Serum Paraoxonase Activity in Patients With Chronic Pancreatitis. Am. J. Med. Sci. 2013, 346, 363–365.
- Kodydkova, J.; Vavrova, L.; Stankova, B.; Macasek, J.; Krechler, T.; Zak, A. Antioxidant Status and Oxidative Stress Markers in Pancreatic Cancer and Chronic Pancreatitis. Pancreas 2013, 42, 614–621.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
521
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




