| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Viotti | -- | 1542 | 2024-02-23 09:14:49 |
Video Upload Options
Biochar is a specific carbon obtained by a pyrolysis process from different feedstocks, as an alternative material for heavy metal adsorption from groundwater. Many studies have been conducted regarding the application of innovative materials to water decontamination to develop a more sustainable approach to remediation processes.
1. Introduction
Activated carbon (AC) is one of the most used adsorbents for the removal of contaminants in water due to its properties. AC is primarily prepared from coal, coconut shells, lignite, and wood, and activated by physical and chemical methods. Due to its high specific surface area, chemical stability, durability, high capacity of adsorption, and not selective adsorption capacity, AC has been widely used to remove heavy metals from groundwater [1][2][3]. However, the regeneration costs of AC may limit its extensive use [4][5]; therefore, it is important to develop low-cost adsorbents with a high adsorption capacity for the removal of pollutants from aqueous systems [6].
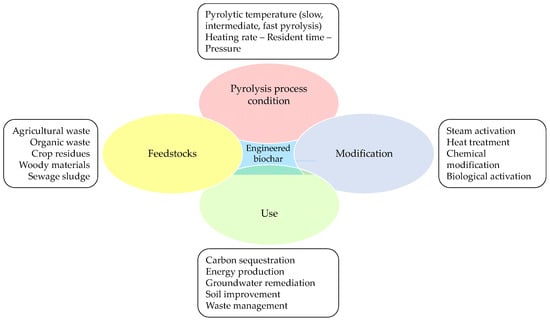
2. Biochar
3. Heavy Metals
4. Applications of Adsorption Process for Heavy Metal Removal
4.1. Adsorption Process
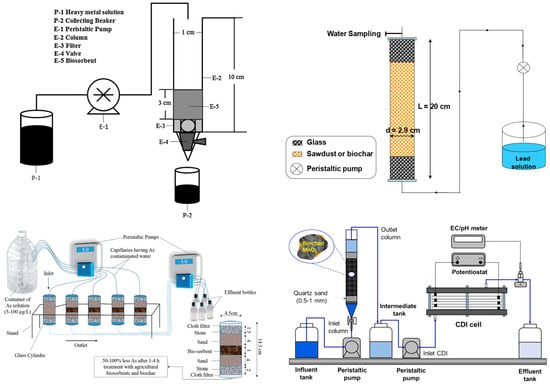
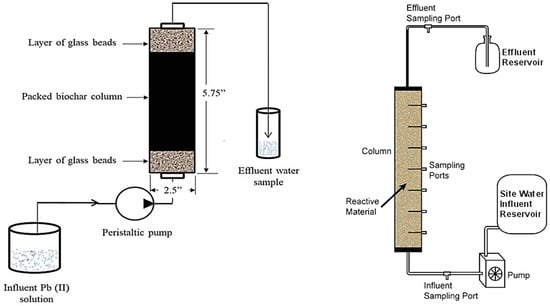
4.2. Column Systems
References
- Liu, Y.; Liu, X.; Zhang, G.; Ma, T.; Du, T.; Yang, Y.; Lu, S.; Wang, W. Adsorptive removal of sulfamethazine and sulfamethoxazole from aqueous solution by hexadecyl trimethyl ammonium bromide modified activated carbon. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 564, 131–141.
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342.
- Baresel, C.; Harding, M.; Fång, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710.
- Nham, N.T.; Tahtamouni, T.M.A.; Nguyen, T.D.; Huong, P.T.; Jitae, K.; Viet, N.M.; Van Noi, N.; Phuong, N.M.; Anh, N.T.H. Synthesis of iron modified rice straw biochar toward arsenic from groundwater. Mater. Res. Express 2019, 6, 115528.
- Liu, X.; Ao, H.; Xiong, X.; Xiao, J.; Liu, J. Arsenic removal from water by iron-modified bamboo charcoal. Water. Air. Soil Pollut. 2012, 223, 1033–1044.
- Tolkou, A.K.; Trikkaliotis, D.G.; Kyzas, G.Z.; Katsoyiannis, I.A.; Deliyanni, E.A. Simultaneous Removal of As(III) and Fluoride Ions from Water Using Manganese Oxide Supported on Graphene Nanostructures (GO-MnO2). Sustainability 2023, 15, 1179.
- Shan, R.; Shi, Y.; Gu, J.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383.
- Safaei Khorram, M.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279.
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544.
- Ho, S.-H.; Chen, Y.; Yang, Z.; Nagarajan, D.; Chang, J.-S.; Ren, N. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149.
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481.
- Hussin, F.; Aroua, M.K.; Szlachta, M. Biochar derived from fruit by-products using pyrolysis process for the elimination of Pb(II) ion: An updated review. Chemosphere 2022, 287, 132250.
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969.
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85.
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Earthscan Publications Ltd.: London, UK; Routledge: Oxfordshire, UK, 2015; ISBN 978-0-415-70415-1.
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230.
- Manasa, M.R.K.; Katukuri, N.R.; Darveekaran Nair, S.S.; Haojie, Y.; Yang, Z.; Guo, R.B. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737.
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87.
- Basinas, P.; Rusín, J.; Chamrádová, K.; Kaldis, S.P. Pyrolysis of the anaerobic digestion solid by-product: Characterization of digestate decomposition and screening of the biochar use as soil amendment and as additive in anaerobic digestion. Energy Convers. Manag. 2023, 277, 116658.
- Marzeddu, S.; Décima, M.A.; Camilli, L.; Bracciale, M.P.; Genova, V.; Paglia, L.; Marra, F.; Damizia, M.; Stoller, M.; Chiavola, A.; et al. Physical-Chemical Characterization of Different Carbon-Based Sorbents for Environmental Applications. Materials 2022, 15, 7162.
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Gikas, P.; Chong, K.-K.; Chew, K.W. Challenges and opportunities for biochar to promote circular economy and carbon neutrality. J. Environ. Manag. 2023, 332, 117429.
- Marzeddu, S.; Cappelli, A.; Paoli, R.; Ambrosio, A.; Decima, M.A.; Boni, M.R.; Romagnoli, F. LCA Sensitivity Analysis of an Energy-Biochar Chain from an Italian Gasification Plant: Environmental Trade-offs Assessment. CONECT. Int. Sci. Conf. Environ. Clim. Technol. 2023, 63.
- Shrestha, P.; Chun, D.D.; Kang, K.; Simson, A.E.; Klinghoffer, N.B. Role of Metals in Biochar Production and Utilization in Catalytic Applications: A Review. Waste Biomass Valorization 2022, 13, 797–822.
- International Biochar Initiative List of Frequently Asked Questions. Available online: https://biochar-international.org/about-biochar/faqs/ (accessed on 20 April 2023).
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440.
- Terrón-Sánchez, J.; Martín-Franco, C.; Vicente, L.A.; Fernández-Rodríguez, D.; Albarrán, Á.; Rato Nunes, J.M.; Peña, D.; López-Piñeiro, A. Combined use of biochar and alternative management systems for imazamox induced pollution control in rice growing environments. J. Environ. Manag. 2023, 334, 117430.
- Viotti, P.; Tatti, F.; Rossi, A.; Luciano, A.; Marzeddu, S.; Mancini, G.; Boni, M.R. An Eco-Balanced and Integrated Approach for a More-Sustainable MSW Management. Waste and Biomass Valorization 2020, 11, 5139–5150.
- Matsagar, B.M.; Wu, K.C.-W. Agricultural waste-derived biochar for environmental management. In Biochar in Agriculture for Achieving Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–13.
- Wijitkosum, S. Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341.
- Gong, X.; Zou, L.; Wang, L.; Zhang, B.; Jiang, J. Biochar improves compost humification, maturity and mitigates nitrogen loss during the vermicomposting of cattle manure-maize straw. J. Environ. Manag. 2023, 325, 116432.
- Kumari, K.; Kumar, R.; Bordoloi, N.; Minkina, T.; Keswani, C.; Bauddh, K. Unravelling the Recent Developments in the Production Technology and Efficient Applications of Biochar for Agro-Ecosystems. Agriculture 2023, 13, 512.
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36.
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental Benefits of Biochar. J. Environ. Qual. 2012, 41, 967–972.
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Hosseini Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755.
- Tan, X.-f.; Liu, S.-b.; Liu, Y.-g.; Gu, Y.-l.; Zeng, G.-m.; Hu, X.-j.; Wang, X.; Liu, S.-h.; Jiang, L.-h. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372.
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544.
- Uchimiya, M.; Klasson, K.T.; Wartelle, L.H.; Lima, I.M. Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere 2011, 82, 1431–1437.
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480.
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287.
- Chiavola, A.; Marzeddu, S.; Boni, M.R. Remediation of Water Contaminated by Pb(II) Using Virgin Coniferous Wood Biochar as Adsorbent. In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Naddeo, V., Balakrishnan, M., Choo, K.-H., Eds.; Springer: Salerno, Italy, 2020; pp. 363–366. ISBN 978-3-030-13067-1.
- Cao, Q.; Wang, C.; Tang, D.; Zhang, X.; Wu, P.; Zhang, Y.; Liu, H.; Zheng, Z. Enhanced elemental mercury removal in coal-fired flue gas by modified algal waste-derived biochar: Performance and mechanism. J. Environ. Manag. 2023, 325, 116427.
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis, applications, and mechanisms. Chemosphere 2020, 246, 125609.
- Liu, X.; Dong, X.; Chang, S.; Xu, X.; Li, J.; Pu, H. Remediation of lead-contaminated groundwater by oyster shell powder–peanut shell biochar mixture. Environ. Geochem. Health 2023, 45, 9599–9619.
- Liu, F.; Liu, H.; Zhu, H.; Xie, Y.; Zhang, D.; Cheng, Y.; Zhang, J.; Feng, R.; Yang, S. Remediation of petroleum hydrocarbon-contaminated groundwater by biochar-based immobilized bacteria. Biochem. Eng. J. 2023, 197, 108987.
- Zhang, J.B.; Dai, C.; Wang, Z.; You, X.; Duan, Y.; Lai, X.; Fu, R.; Zhang, Y.; Maimaitijiang, M.; Leong, K.H.; et al. Resource utilization of rice straw to prepare biochar as peroxymonosulfate activator for naphthalene removal: Performances, mechanisms, environmental impact and applicability in groundwater. Water Res. 2023, 244, 120555.
- Nguyen, T.M.; Chen, H.H.; Chang, Y.C.; Ning, T.C.; Chen, K.F. Remediation of groundwater contaminated with trichloroethylene (TCE) using a long-lasting persulfate/biochar barrier. Chemosphere 2023, 333, 138954.
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, ISBN 978-3-7643-8339-8.
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216.
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253.
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807.
- Abdallah, M.M.; Ahmad, M.N.; Walker, G.; Leahy, J.J.; Kwapinski, W. Batch and Continuous Systems for Zn, Cu, and Pb Metal Ions Adsorption on Spent Mushroom Compost Biochar. Ind. Eng. Chem. Res. 2019, 58, 7296–7307.
- Bradl, H.B. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier, Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2005; ISBN 0-12-088381-3.
- Oyeku, O.T.; Eludoyin, A.O. Heavy metal contamination of groundwater resources in a Nigerian urban settlement. Afr. J. Environ. Sci. Technol. 2010, 4, 201–214.
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528.
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388.
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419.
- Roane, T.M.; Pepper, I.L.; Gentry, T.J. Microorganisms and Metal Pollutants. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 415–439. ISBN 9780123946263.
- Gadd, G. Metals and microorganisms: A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203.
- Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules 2023, 28, 719.
- Tiller, K.G. Heavy Metals in Soils and Their Environmental Significance. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1989; Volume 9, pp. 113–142. ISBN 978-1-4612-3532-3.
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; IntechOpen: London, UK, 2018; pp. 115–134. ISBN 978-1-78923-361-2.
- Tolkou, A.K.; Katsoyiannis, I.A.; Zouboulis, A.I. Removal of Arsenic, Chromium and Uranium from Water Sources by Novel Nanostructured Materials Including Graphene-Based Modified Adsorbents: A Mini Review of Recent Developments. Appl. Sci. 2020, 10, 3241.
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate pollution of groundwater; all right…, but nothing else? Sci. Total Environ. 2016, 539, 241–251.
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553.
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A review of threats to groundwater quality in the anthropocene. Sci. Total Environ. 2019, 684, 136–154.
- Grimshaw, P.; Calo, J.M.; Hradil, G. Cyclic electrowinning/precipitation (CEP) system for the removal of heavy metal mixtures from aqueous solutions. Chem. Eng. J. 2011, 175, 103–109.
- Levchuk, I.; Rueda Márquez, J.J.; Sillanpää, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104.
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304.
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104.
- Ye, J.; Chen, X.; Chen, C.; Bate, B. Emerging sustainable technologies for remediation of soils and groundwater in a municipal solid waste landfill site—A review. Chemosphere 2019, 227, 681–702.
- Dialynas, E.; Diamadopoulos, E. Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination 2009, 238, 302–311.
- Gavrilescu, M. Removal of Heavy Metals from the Environment by Biosorption. Eng. Life Sci. 2004, 4, 219–232.
- Otero, M.; Rozada, F.; Morán, A.; Calvo, L.F.; García, A.I. Removal of heavy metals from aqueous solution by sewage sludge based sorbents: Competitive effects. Desalination 2009, 239, 46–57.
- González-González, A.; Cuadros, F.; Ruiz-Celma, A.; López-Rodríguez, F. Influence of heavy metals in the biomethanation of slaughterhouse waste. J. Clean. Prod. 2014, 65, 473–478.
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. CLEAN-Soil, Air, Water 2018, 46, 1800120.
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027.
- Jusoh, A.; Su Shiung, L.; Ali, N.; Noor, M.J.M.M. A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 2007, 206, 9–16.
- Taghavi, M.; Zazouli, M.A.; Yousefi, Z.; Akbari-adergani, B. Kinetic and isotherm modeling of Cd (II) adsorption by l-cysteine functionalized multi-walled carbon nanotubes as adsorbent. Environ. Monit. Assess. 2015, 187, 682.
- Zazouli, M.A.; Yousefi, Z.; Taghavi, M.; Akbari-Adergani, B.; Cherati, J.Y. Cadmium Removal from Aqueous Solutions using L-cysteine Functionalized Single-Walled Carbon Nanotubes. J. Maz. Univ. Med. Sci. 2013, 22, 37–47.
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245.
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202.
- Petrella, A.; Notarnicola, M. Recycled Materials in Civil and Environmental Engineering. Materials 2022, 15, 3955.
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II), and Ni(II) From Single- and Multimetal Solutions by Recycled Waste Porous Glass. Chem. Eng. Commun. 2016, 203, 940–947.
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C 2018, 4, 62.
- Tran, H.N.; Thanh Trung, N.P.; Lima, E.C.; Bollinger, J.; Dat, N.D.; Chao, H.; Juang, R. Revisiting the calculation of thermodynamic parameters of adsorption processes from the modified equilibrium constant of the Redlich–Peterson model. J. Chem. Technol. Biotechnol. 2023, 98, 462–472.
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196.
- He, E.; Liu, N.; Zhou, Y.; Wang, Z.; Lu, X.; Yu, L. Adsorption properties and mechanism of zinc acrylic carbon nanosphere aggregates for perfluorooctanoic acid from aqueous solution. Environ. Pollut. 2023, 316, 120540.
- Feng, C.; Huang, M.; Huang, C. Specific chemical adsorption of selected divalent heavy metal ions onto hydrous γ-Fe2O3-biochar from dilute aqueous solutions with pH as a master variable. Chem. Eng. J. 2023, 451, 138921.
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712.
- Fu, S.; Tan, B.; Cheng, G.; Wang, H.; Fang, X.; Li, Z.; Guo, M.; Zan, X. Molecular model construction of Chifeng lignite and analysis of adsorption mechanism of O2 at low temperature. J. Mol. Struct. 2023, 1276, 134613.
- Nguyen, T.-B.; Nguyen, T.-K.-T.; Chen, W.-H.; Chen, C.-W.; Bui, X.-T.; Patel, A.K.; Dong, C.-D. Hydrothermal and pyrolytic conversion of sunflower seed husk into novel porous biochar for efficient adsorption of tetracycline. Bioresour. Technol. 2023, 373, 128711.
- Gouvêa, D.; Ushakov, S.V.; Navrotsky, A. Energetics of CO2 and H2O adsorption on zinc oxide. Langmuir 2014, 30, 9091–9097.
- Patra, J.M.; Panda, S.S.; Dhal, N.K. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. Biosci. 2017, 6, 105081.
- McNaught, A.D.; Ilkinson, A. IUPAC. Compendium of Chemical Terminology. In Gold Book; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8.
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570.
- Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Thermodynamic and kinetic investigation of heavy metals sorption in packed bed columns by recycled lignocellulosic materials from olive oil production. Chem. Eng. Commun. 2019, 206, 1715–1730.
- Kumkum, P.; Kumar, S. Evaluation of Lead (Pb(II)) Removal Potential of Biochar in a Fixed-bed Continuous Flow Adsorption System. J. Health Pollut. 2020, 10, 201210.
- Shabalala, A.N.; Ekolu, S.O.; Diop, S. Permeable reactive barriers for acid mine drainage treatment: A review. Constr. Mater. Struct. 2014, 1416–1426.
- Powell, R.M.; Puls, R.W.; Blowes, D.W.; Vogan, J.L.; Gillham, R.W.; Powell, P.D.; Schultz, D.; Landis, R.; Sivavec, T. Permeable Reactive Barrier Technologies for Contaminant Remediation; EPA/600/R-98/125 (NTIS 99-105702); U.S. Environmental Protection Agency: Washington, DC, USA, 1998.
- Mutharasu, L.C.; Kalaga, D.V.; Sathe, M.; Turney, D.E.; Griffin, D.; Li, X.; Kawaji, M.; Nandakumar, K.; Joshi, J.B. Experimental study and CFD simulation of the multiphase flow conditions encountered in a Novel Down-flow bubble column. Chem. Eng. J. 2018, 350, 507–522.
- Bouissonnié, A.; Daval, D.; Marinoni, M.; Ackerer, P. From mixed flow reactor to column experiments and modeling: Upscaling of calcite dissolution rate. Chem. Geol. 2018, 487, 63–75.
- Sirini, P. Ingegneria Sanitaria-Ambientale: Principi, Teoria e Metodi di Rappresentazione, 1st ed.; McGraw-Hill Education: Milano, Italy, 2011; ISBN 88-386-0897-0.
- YEO, K.F.H.; Dong, Y.; Xue, T.; Yang, Y.; Chen, Z.; Han, L.; Zhang, N.; Mawignon, F.J.; Kolani, K.; Wang, W. Fixed-bed column method for removing arsenate from groundwater using aluminium-modified kapok fibres. J. Porous Mater. 2023, 1, 1221–1232.
- Cao, R.; Liu, S.; Yang, X.; Wang, C.; Wang, Y.; Wang, W.; Pi, Y. Enhanced remediation of Cr(VI)-contaminated groundwater by coupling electrokinetics with ZVI/Fe3O4/AC-based permeable reactive barrier. J. Environ. Sci. 2022, 112, 280–290.
- Wawrzkiewicz, M.; Kebir, M.; Tahraoui, H.; Chabani, M.; Trari, M.; Noureddine, N.; Assadi, A.A.; Amrane, A.; Hamadi, N.B.; Khezami, L.; et al. Water Cleaning by a Continuous Fixed-Bed Column for Cr(VI) Eco-Adsorption with Green Adsorbent-Based Biomass: An Experimental Modeling Study. Processes 2023, 11, 363.
- Fila, D.; Kołodyńska, D. Fixed-Bed Column Adsorption Studies: Comparison of Alginate-Based Adsorbents for La(III) Ions Recovery. Materials 2023, 16, 1058.
- Tabassum, R.A.; Shahid, M.; Niazi, N.K.; Dumat, C.; Zhang, Y.; Imran, M.; Bakhat, H.F.; Hussain, I.; Khalid, S. Arsenic removal from aqueous solutions and groundwater using agricultural biowastes-derived biosorbents and biochar: A column-scale investigation. Int. J. Phytoremediat. 2019, 21, 509–518.
- Chao, H.P.; Chang, C.C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414.
- Jellali, S.; Diamantopoulos, E.; Haddad, K.; Anane, M.; Durner, W.; Mlayah, A. Lead removal from aqueous solutions by raw sawdust and magnesium pretreated biochar: Experimental investigations and numerical modelling. J. Environ. Manag. 2016, 180, 439–449.
- Cuong, D.V.; Wu, P.C.; Liou, S.Y.H.; Hou, C.H. An integrated active biochar filter and capacitive deionization system for high-performance removal of arsenic from groundwater. J. Hazard. Mater. 2022, 423, 127084.