2. The Cell Can Repair a Wounded Cell Membrane
The presence of a cell membrane wound repair mechanism must be noticed during the initial phases of single-cell microsurgery and microinjection experiments
[30][31][53][54][55][56]. For example, when a
Dictyostelium cell is divided into two fragments using a microneedle, the nucleate fragment exhibits normal migration, whereas the anucleate fragment is incapable of doing so
[57]. This experiment underscores the nucleus’s indispensable role in cell migration and simultaneously emphasizes the prompt repair of the cell membrane, which was wounded during microsurgery—a pioneering observation in
Dictyostelium cells.
The majority of wound experiments have focused on a limited life stage of cells. The life cycle of
Dictyostelium discoideum is broadly categorized into four stages: vegetative, aggregation, multicellular, and culmination. After the starvation of vegetative cells, individual cells aggregate to form streams towards the aggregation center. Aggregation is mediated by the chemotaxis of cells toward cAMP excreted from the aggregation centers. This process results in the formation of a multicellular organism and eventually leads to the development of fruiting bodies consisting of spores and stalks. Wound repair is observed at all stages in
Dictyostelium cells (
Figure 1A), including spore cells, which are dormant cells with a rigid cell wall. Furthermore, wound repair is noted at different stages of the cell cycle, such as interphase and the mitotic stage, in
Dictyostelium cells
[2].
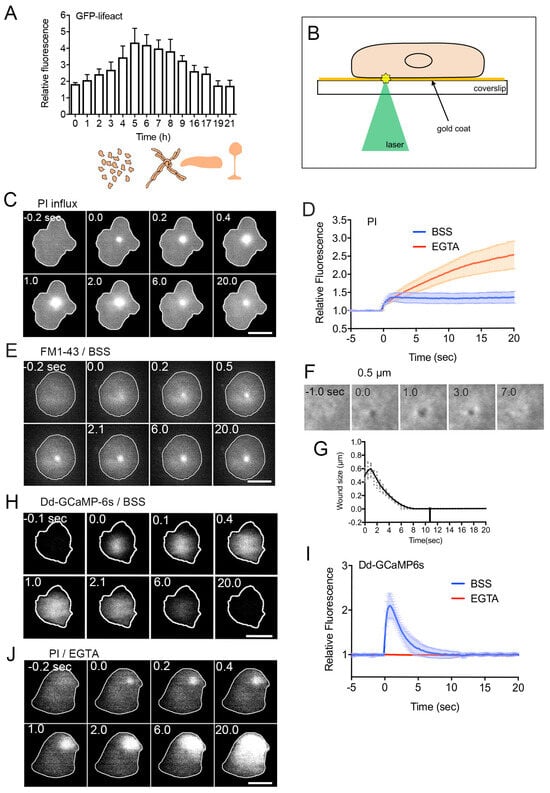
Figure 1. Wound repair of the cell membrane. (
A) Relative amplitudes of actin accumulation at the wound site over time following starvation (0 h). As illustrated in the lower drawings, upon initiation of vegetative cell starvation, individual cells aggregate, forming streams direct toward the aggregation center. This process leads to the creation of a multicellular structure, culminating in the development of a fruiting body. Importantly, wound repair is observed at every stage of the lifecycle in
Dictyostelium discoideum. (
B) Schematic representation of the enhanced laserporation with gold coating. The wound diameter is usually set at 0.5 μm for
Dictyostelium cells. (
C) A representative sequence of fluorescence images capturing PI influx after laserporation. (
D) Temporal profiles of PI influx in the presence (BSS, control) and absence (EGTA) of external Ca
2+. The wound laser beam was applied at 0 sec. (
E) A typical sequence of fluorescence images illustrating FM dye influx after laserporation. (
F) Laserporation of a cell expressing GFP-cAR1 resulted in the appearance of a black spot on the cell membrane. The black spot transiently expanded, then contracted, and finally closed. (
G) The time course of the black spot diameter. (
H) A sequence of fluorescence images featuring a cell expressing GCAMP6s after laserporation. (
I) Temporal profiles of GCAMP6s fluorescence intensities in the presence (BSS) and absence (EGTA) of external Ca
2+. (
J) A typical sequence of fluorescence images illustrating PI influx after laserporation in the absence (EGTA) of external Ca
2+. Scale bars, 10 µm. Figures are posted from
[48][51][58][59] with CC BY licence.
3. Monitoring of Wound Repair
Various methods have been employed to investigate the wound repair mechanism. In early experiments, the cell membrane was wounded mainly by microneedle poking in large cells such as protozoan amebae
[41][56][60], amphibian eggs
[30][53][55], and echinoderm eggs
[50]. For small cells like yeast, animal cultured cells, and
Dictyostelium cells, laser ablation has been predominantly used due to the technical challenges and time constraints associated with microneedle poking in such small cells. Recent research also utilizes laser ablation for large cells, offering precise-sized wounds and accurate timing. However, both laser ablation and previous methods not only damage the cell membrane but also impact intracellular structures, including cortical actin networks, microtubules, and organelles.
To address this, researchers have developed an improved laser ablation method that selectively injures only the cell membrane. As depicted in
Figure 1B, after placing cells on a carbon or gold-coated coverslip, a laser beam is focused on the coat underneath the cells. The laser energy absorbed by the coat generates heat and/or plasmon
[48][61], selectively injuring the cell membrane attached to the coat
[48]. This method has been originally invented for the introduction of extracellular substances into cells
[62]. Instead of wounding individual cells, for biochemical analysis, a large number of cells can be wounded by treating with pore-forming agents or detergents
[63][64][65].
For monitoring the wounding process, propidium iodide (PI) or FM1-43 has been widely used. PI, a cell-impermeant dye emitting fluorescence upon binding to RNA or DNA, and FM1-43, a cell-impermeable fluorescent lipid analog emitting fluorescence upon insertion into the membrane, are placed in the external medium. Their entry into the cytosol is monitored by the increase in fluorescence upon wounding. As shown in Figure 1C, PI fluorescence begins to increase at the wound site upon injury, spreading over the cytosol, suggesting PI entry through the wound pores. Figure 1D (BSS) illustrates the time course of PI fluorescence intensity in the cytosol of wounded cells, indicating that PI influx ceases within 2–3 s after injury, terminating urgent wound repair within this timeframe. Figure 1E demonstrates the influx of FM1-43 dye upon wounding, also showing that the dye enters from the wound pore and spreads across the cytoplasm.
To visualize the wound pore in Dictyostelium cells, cells expressing GFP-cAR1 (cAMP receptor) as a membrane protein marker are wounded. Immediately after wounding, a black spot appears at the laser application site (Figure 1F). This black spot is not generated by photobleaching, as it transiently expands slightly, then shrinks, and eventually closes (Figure 1G). This closure is not uniform but occurs from the wound edge to the center.
4. Ca2+ Influx as the First Signal
The initial signal common to all examined cells across various species is the influx of Ca
2+ from the wound pore
[30][32][41][50][66]. Monitoring this influx is feasible using a Ca
2+ indicating fluorescent dye or a GFP-based Ca
2+ indicator.
Figure 1H presents a time series of fluorescence images of
Dictyostelium cells expressing GCAMP6s, a GFP-based fluorescent Ca
2+ indicator. In
Figure 1I (BSS), the time course of fluorescence intensities in the cytosol is depicted. Intracellular Ca
2+ concentration (Ca
i2+) promptly rises upon wounding, returning to resting levels within approximately 7 s. In the absence of external Ca
2+, Ca
i2+ remains unchanged upon wounding (
Figure 1I, EGTA), indicating that the influx of Ca
2+ triggers the increase in Ca
i2+. Without external Ca
2+, PI influx persists, leading to the eventual death of wounded cells (
Figure 1D, EGTA, and
Figure 1J). Additionally, the black spot observed in experiments using GFP-cAR1 does not close without Ca
2+ influx. A concentration higher than 0.1 mM of Ca
2+ in the external medium is necessary for wound repair in
Dictyostelium cells
[51].
Ca
2+ influx induces the release of Ca
2+ from intracellular stores through the calcium-induced calcium release (CICR) mechanism
[67][68]. Deleting CICR reduces the amplitude of Ca
i2+ but does not impact wound repair, indicating that a local increase in Ca
i2+ is crucial, not a global one
[58]. On the other hand, MCOLN1, an endosomal and lysosomal Ca
2+-channel, is crucial for cell membrane repair in muscle cells, emphasizing the significance of Ca
2+ release from intracellular stores in wound repair
[69].
Various intracellular targets of Ca2+ for wound repair include dysfelin, mitsugmin 53 (MG53), neuroblast differentiation-associated protein (AHNAK), calpain, calmodulin, annexins, the endosomal sorting complex required for transport (ESCRT), protein kinase C, and actin-related proteins. These will be discussed in detail later.
5. Closing Wound Pores
5.1. Spontaneous Self-Sealing
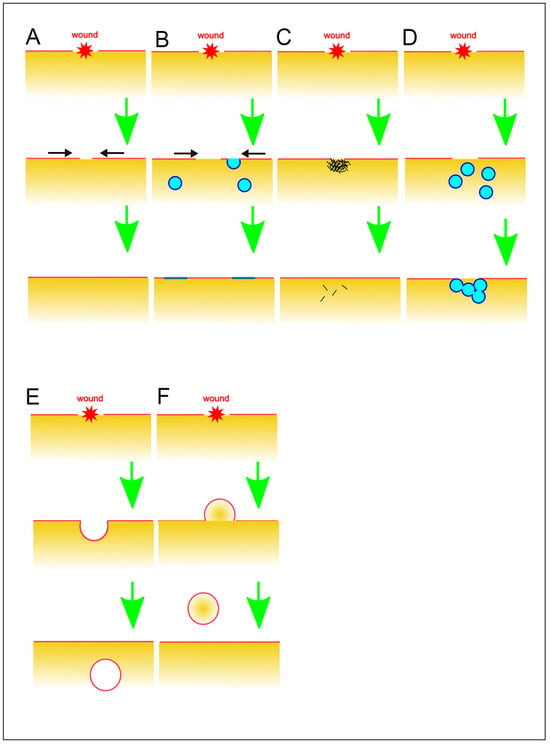
Ruptured artificial lipid bilayer membranes exhibit spontaneous resealing
[70]. Similarly, extremely small wound pores, such as those generated by electroporation in live cells at the nanometer scale, are thought to undergo spontaneous resealing due to thermodynamically unfavorable lipid disorder (
Figure 2A). However, even with such small pores, there may be a necessity for an active wound repair mechanism
[71]. Additionally, membrane pores created by pore-forming toxins, despite being nanometer-sized, require an active wound repair mechanism
[7][23][72].
Figure 2. Various models for wound repair mechanisms. (A) Spontaneous self-sealing. (B) Self-sealing by regulation of surface tension. Black arrows indicate the direction of the membrane flow. (C) Sealing by protein aggregation. (D) Sealing by membrane patch. (E) Endocytosis of damaged membrane. (F) Vesicle budding and shedding to the outside. These illustrations are simplified for a better understanding of basic concepts.
5.2. Self-Sealing by Regulation of Surface Tension
Given the tension on the cell surface, larger pores cannot spontaneously reseal against the cell surface tension. The wound-induced influx of Ca
2+ triggers the fusion of exocytic vesicles with the cell membrane, extending beyond the wound site and enlarging the plasma membrane (
Figure 2B). This process results in a reduction of cell surface tension, facilitating spontaneous resealing and closure of the wounded pore
[73]. In large cells, although the actomyosin ring exerts force to close the wound pore against the opening force of the cell surface tension, self-sealing alone is insufficient, and a membrane patch is also required for sealing, as described later.
5.3. Sealing by Protein Aggregation
The wounded pores have been suggested to be clogged by the aggregation of proteins, including annexins and actin (
Figure 2C). Annexin A5 self-assembles into two-dimensional arrays on the membrane upon Ca
2+ activation, a crucial aspect of its role in plasma membrane repair in mammalian cells
[74]. Similar clogging phenomena have been reported for other annexins, which collaborate with wound repair-related proteins such as actin, dysferin, and MG53
[75][76][77][78]. Actin accumulates at the wound site, potentially serving a clogging function through actin gelation. However, this accumulation does not happen immediately upon wounding but occurs after the cessation of PI influx.
5.4. Sealing by Membrane Patch
Mammalian red blood cells, lacking endomembranes, including nuclei, take a longer time to reseal wound membranes or fail to repair in physiological conditions, suggesting that endomembranes are necessary for wound repair
[79]. The membrane patch hypothesis was initially proposed in large cells like echinoderm and frog oocytes (
Figure 2D). A local increase in Ca
2+ induces the fusion of small cytoplasmic vesicles with each other, creating a continuous membrane plug at the wound site along with the plasma membrane
[34][35]. More recently, cortical granules in Xenopus oocytes have been identified as such intracellular compartments, and their fusion was visualized in live cells
[80][81]. This wound repair process is succeeded by the constriction of an actomyosin ring, akin to the contractile ring involved in cytokinesis.
Various sources for the membrane patch, including lysosomes
[82][83], endosomes
[84][85], MG53-rich vesicles
[66], dysferlin-containing vesicles
[86][87], or AHNAK-positive “enlargeosomes”
[88][89], have been proposed. However, these vesicles and organelles might not meet the spatiotemporal requirements for rapid and efficient wound repair, considering the possibility of multiple wounds with very short intervals
[58].
Recently, researchers proposed that the vesicles for the membrane plug are newly generated at the wound site in
Dictyostelium cells
[51]. In influx experiments using FM dye, most of the FM fluorescence diffuses in the cytosol, but a portion of FM dye remains at the wounded site and increases in size (
Figure 1E), indicating membrane accumulation at the wound site. In the PI influx experiment (
Figure 1C), a portion of PI fluorescence also persists at the wound site, suggesting that cytoplasm, including PI dye, is entrapped in the newly enclosed vesicles. It is improbable that the membrane plug originates from the broken cell membrane due to the limited amount of the broken cell membrane. Additionally, vesicles are unlikely to be transported from other locations since pharmacological disruption of microtubules and actin did not impede membrane accumulation. Therefore, researchers propose that the vesicles for the membrane plug are generated de novo at the wound site, although the mechanism for this generation remains unclear.
As described earlier, experiments using GFP-cAR1 show that the wound pore is not repaired from the wound edge to the center. Therefore, the wound edge grows toward the center through vesicles repeatedly fusing with the edge of the cell membrane, rather than forming a fused large patch to plug the wound pore.
5.5. Endocytosis of Damaged Membrane
Ca
2+-triggered endocytosis is suggested to eliminate damaged membrane (
Figure 2E). The membrane, including the damaged portion, invaginates inward, and the resulting bud is removed by releasing it into the cell, dependent on Ca
2+ [65][90]. Upon wounding, acid sphingomyelinase is secreted into the extracellular space through Ca
2+-dependent lysosomal exocytosis. This enzyme hydrolyzes sphingomyelin in the cell membrane into ceramide, facilitating membrane invagination and vesiculation
[91]. Ceramide formation by sphingomyelinase also induces caveolae-mediated endocytosis, internalizing the wounded membrane
[90][92][93][94][95][96]. It has been also reported that clathrin- and dynamin-mediated endocytosis facilitates removing the wounds by pore-forming proteins or toxins
[97][98].
In
Dictyostelium cells, neither endocytosis nor exocytosis appears to contribute to membrane accumulation for wound repair
[51], despite the rapid turnover of the cell membrane through endocytosis–exocytosis coupling
[99][100][101][102]. Notably, caveolin proteins are not present in
Dictyostelium [103] and inhibitors of sphingomyelinase do not affect the wound repair in
Dictyostelium cells (our preliminary observations). Additionally, clathrin- and dynamin-mediated endocytosis does not contribute to wound repair in
Dictyostelium cells
[48].
5.6. Vesicle Budding and Shedding to the Outside
Rather than endocytosis involving the inward budding and scission of the damaged membrane, vesicle budding or blebbing toward the outside of the cell, followed by scission, facilitates the removal and shedding of damaged membrane or pore-forming reagents (
Figure 2F). Membrane-binding proteins for wound repair, such as the endosomal sorting complex required for transport (ESCRT) and annexins, facilitate this type of shedding in a Ca
2+-dependent manner in mammal cells
[10][44][75][90][104][105].
In
Dictyostelium cells, FM dye that accumulates at the wound site remains there for the duration of observation. The wounded sites do not move relative to the substrate and eventually shed onto the substrate as cells migrate
[1].
6. Membrane-Binding Proteins in Wound Repair
For wound repair, various membrane-binding proteins, including annexins, the ESCRT complex, synaptotagmin, and dysferin, have been proposed. These proteins also act as sensors, detecting damage to the cell membrane due to their calcium-dependency
[106].
6.1. Annexins
Annexins, a highly conserved and ubiquitous family of Ca
2+- and phospholipid-binding proteins, play a crucial role in wound repair
[107][108][109][110][111][112]. In vertebrates, 12 annexin subfamilies (A1–A11 and A13) have been identified. Annexins such as A1, A2, A5, and A6 accumulate at the wound site by binding to the inner cell membrane, particularly acidic phospholipids like phosphatidylserine, in response to a Ca
2+ influx. Through membrane binding and interactions with other proteins, such as S100 family proteins, annexin prevents further expansion of the wound pore, reduces membrane tension, and prepares the membrane for resealing
[113][114][115].
Annexins induce curvature in the free-edge membranes and generate constriction force to close the wound pore through annexin crosslinking
[116][117][118]. Annexins can also be cross-linked by transglutamilases in a Ca
2+-dependent manner
[119], which have also been implicated in plasma membrane repair
[120].
Moreover, annexins have been proposed to assemble into multimeric lattice structures, recruiting M53-laden vesicles and mini-dysferlin72, effectively clogging the wound pore
[13][74][111][121]. Some annexins, such as annexin 1 and 2, can bind to actin and stabilize actin filaments at the wound site
[76][110][122][123][124].
Dictyostelium possesses two annexin genes, annexin C1 (annexin VII or synexin) and annexin C2 (annexin I)
[125]. Both can bind phosphatidylserine in a Ca
2+-dependent manner
[126][127]. Only annexin C1 accumulates at the wound site immediately after wounding. Wounded annexin C1-null cells exhibit irregular curves with multiple peaks in PI influx, Ca
2+ influx, and actin dynamics. Additionally, annexin C1-null cells have a significantly reduced survival rate following injury, suggesting that annexin C1 partially contributes to wound repair in
Dictyostelium cells
[48][58].
6.2. ESCRT Complexes
The endosomal sorting complex required for transport (ESCRT) is categorized into five protein complexes (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and Vps4). These complexes play integral roles in various cellular processes, including endosomal budding transport, virus budding, and cytokinesis. The ESCRT complex constricts and severs narrow necks during membrane budding processes
[44][128][129][130][131][132][133][134][135][136]. Additionally, ESCRT complexes have been implicated in shedding wounded membranes as extracellular vesicles
[44][105][136]. Upon injury, ESCRT complexes promptly accumulate at the wound site, protrude the wounded membrane as a bud or bleb, and subsequently cut it off to release extracellular vesicles. This ESCRT-mediated abscission of the wounded membrane appears to limit smaller-sized wounds (<100 nm in diameter)
[137][138].
Interestingly, ESCRT complexes also participate in repairing damaged membranes of intracellular organelles, such as the nuclear envelope and lysosomes
[139][140][141]. Furthermore, ESCRT complexes mediate the sealing of holes in the nascent nuclear envelope and nascent autophagosome
[142].
While ESCRT complexes themselves are not sensitive to Ca
2+, Ca
2+-sensitive proteins like ALG-2 and calmodulin confer Ca
2+ sensitivity on ESCRT complexes
[10][136][143]. Recently, it has been reported that annexin A6 also plays a similar role in the secretion of exosomes
[144].
In
Dictyostelium cells, components of the ESCRT complexes accumulate at the wound site immediately upon injury, depending on the influx of Ca
2+ [51]. However, in ESCRT null cells, PI influx ceases normally, and actin dynamics are observed, similar to wild-type cells. This suggests that ESCRT complexes are not essential for wound repair in
Dictyostelium cells
[48].
During cytokinesis in animal cells, ESCRT complexes and annexins accumulate at the cleavage furrow and/or midbody and are considered to play a role in cytokinesis
[145][146][147][148][149][150].
Dictyostelium cells lack a midbody and undergo division through physical cutting via the constriction of the contractile ring and the traction force of the two daughter fragments migrating in opposite directions
[151][152]. This suggests that this abscission might be a form of ‘physiological wound’. However, preliminary observations indicate that neither ESCRT components nor annexins accumulate at the torn edges, suggesting the existence of a novel mechanism for cytokinetic abscission in
Dictyostelium cells.
6.3. Synaptotagmin
Synaptotagmin comprises a family of Ca
2+-binding and membrane-trafficking proteins, with particular emphasis on its well-characterized role in the release of synaptic vesicles in neurons, where it regulates Ca
2+-dependent exocytosis
[153]. Synaptotagmin 7, specifically, participates in the repair of wounded membranes through Ca
2+-dependent lysosome exocytosis
[83][154]. Knockdown mice lacking synaptotagmin 7 exhibit defects in wound repair
[155]. While
Dictyostelium possesses a synaptotagmin-like protein, there is currently no available information regarding its role in wound repair.
6.4. Dysferlin
Dysferlin, a membrane protein within the Ferlin family involved in vesicle fusion, is notably abundant in skeletal and cardiac muscle
[156]. Dysferlin binds to vesicles containing acid phospholipids, such as phosphatidylserine, through the dysferlin C2 domain, relying on Ca
2+ [157]. Mice deficient in dysferlin display defects in wound repair in muscle cells and develop muscular dystrophy
[12]. Dysferlin is proposed to generate a membrane patch by recruiting and fusing vesicles in wounded skeletal and cardiac muscle cells
[87][158]. Dysferlin organizes vesicle fusion with the assistance of binding partners like S100A10, annexin A2, AHNAK, caveolin-3, and TRIM72 (MG53)
[13][66][93][106][159]. TRIM72, also known as MG53, which is highly expressed in muscle cells, assembles into a higher-ordered structure on the phosphatidylserine-enriched membranes. This assembly and association with the membrane depend on its oligomeric assembly and ubiquitination activity, facilitating vesicle transport to the wound site
[160]. Notably,
Dictyostelium cells lack a dysferlin homolog.