Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elka Touitou | -- | 2005 | 2024-02-21 15:04:25 | | | |
| 2 | Jessie Wu | -3 word(s) | 2002 | 2024-02-22 04:04:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Touitou, E.; Natsheh, H. Nanovesicles for Enhanced Drug Delivery. Encyclopedia. Available online: https://encyclopedia.pub/entry/55308 (accessed on 08 February 2026).
Touitou E, Natsheh H. Nanovesicles for Enhanced Drug Delivery. Encyclopedia. Available at: https://encyclopedia.pub/entry/55308. Accessed February 08, 2026.
Touitou, Elka, Hiba Natsheh. "Nanovesicles for Enhanced Drug Delivery" Encyclopedia, https://encyclopedia.pub/entry/55308 (accessed February 08, 2026).
Touitou, E., & Natsheh, H. (2024, February 21). Nanovesicles for Enhanced Drug Delivery. In Encyclopedia. https://encyclopedia.pub/entry/55308
Touitou, Elka and Hiba Natsheh. "Nanovesicles for Enhanced Drug Delivery." Encyclopedia. Web. 21 February, 2024.
Copy Citation
Liposomes are the classic and first investigated phospholipid vesicles. These are rigid nanovesicles, and their ability to enhance drug permeability to the deeper skin layers is limited.
nanovesciles
soft
flexible
dermal/transdermal drug delivery
skin
ethosome
transfersome
niosome
glyecerosom
1. Introduction
Delivery of drugs and active molecules into and across the skin for both local and systemic effects is an attractive field of research. Topical administration of pharmaceutical and cosmeceutical agents for the treatment of skin disorders may allow effective treatment of various skin ailments, including skin cancer, autoimmune diseases, skin inflammation, acne vulgaris, skin pigmentation, skin aging, hair loss, and skin regeneration. Treatment of these conditions requires targeted drug delivery to various skin regions (Figure 1). Transdermal drug delivery to systemic circulation offers several advantages, including the avoidance of side effects in the gastrointestinal tract. Moreover, using this delivery method, the hepatic first-pass metabolism can be avoided, leading to improved treatment outcomes [1].
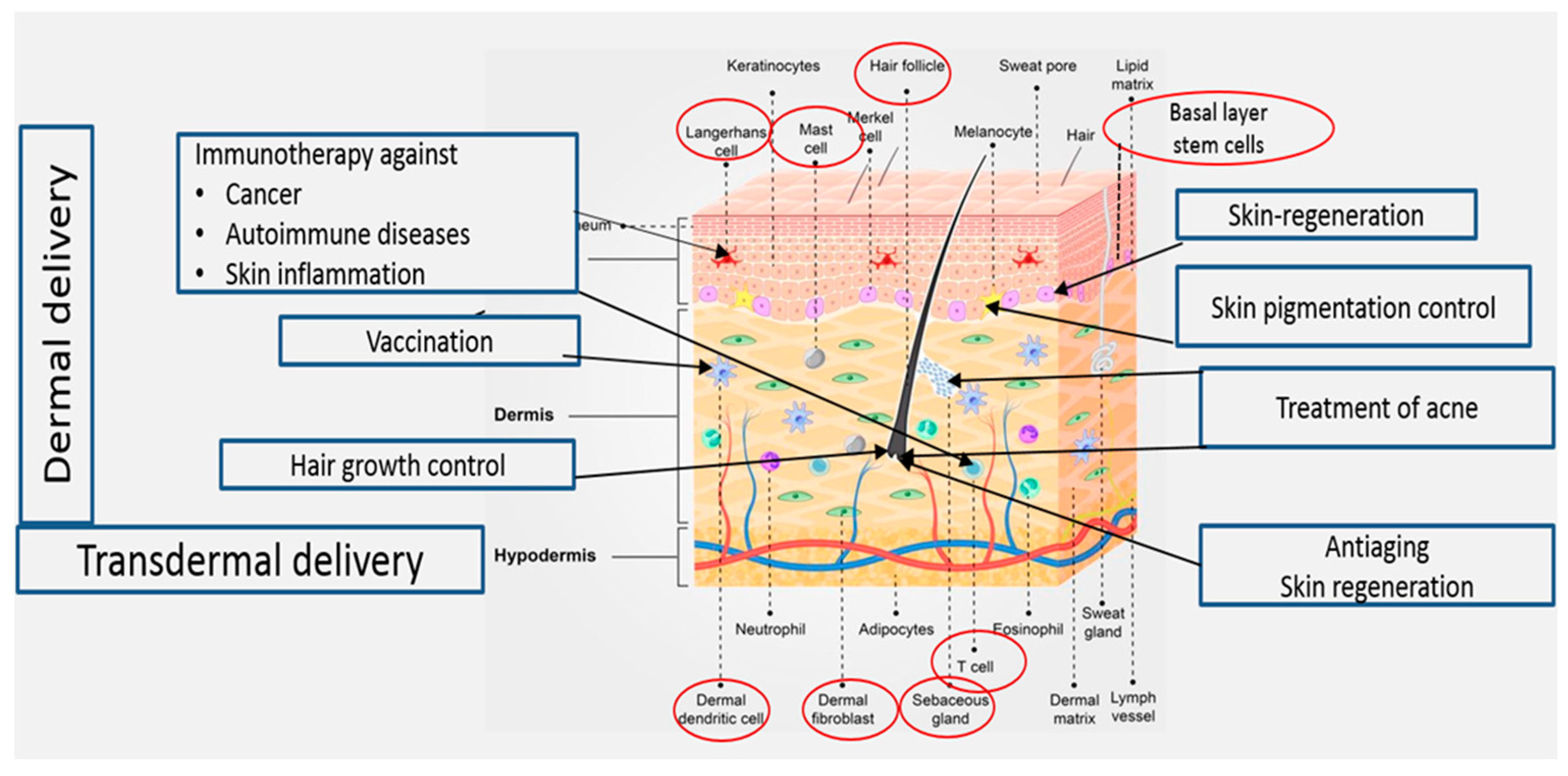
Figure 1. Treatments targeting cellular elements in the skin.
As is known, the impermeable nature of the skin limits access to therapeutics through this route. Many approaches have been employed to overcome the skin barrier and facilitate drug delivery into and across the skin. Active and passive methods are used for the enhancement of skin penetration. Active methods include microneedles, electrophoresis, and sonophoresis. On the other hand, passive approaches focus on carrier-mediated delivery such as chemical permeation enhancers and nanovesicular carriers. Dosage forms based on nanovesicular carriers based on amphiphilic compounds have distinct advantages over conventional ones. These carriers can be classified into two major categories: phospholipid and non-phospholipid-based nanovesicles [2][3]. Transfersomes and ethosomes are the most investigated phospholipid nanovesicles for enhanced drug delivery into and through the skin. The two carriers evolved from liposomes that are obtained by the incorporation of surfactant edge activators and high concentrations of ethanol, respectively. These systems can also be tailored to prolong the drug effect and provide a controlled release of the active substance [4][5]. Surface-modified liposomes have also been investigated. One example is the AS1411-aptamer conjugated liposome. These present a new generation of liposomes that have been designed for safe and convenient delivery of 5-fluorouracil to the tumor site [6]. Niosomes represent the non-phospholipid category of nanovesicular carriers.
2. Nanovesicles for Enhanced Drug Delivery into and across the Skin
Nanovesilcular carriers for the design of formulations for application on the skin include liposomes, ethosome, transfersome, transethosmes, glycerosomes, and nisomes. They can be divided into two main types: phospholipid and non-phospholipid nanovesicles [2][7][8][9]. Table 1 and Figure 2 illustrate the evolution of nanovesicular carriers investigated for drug delivery into and across the skin.
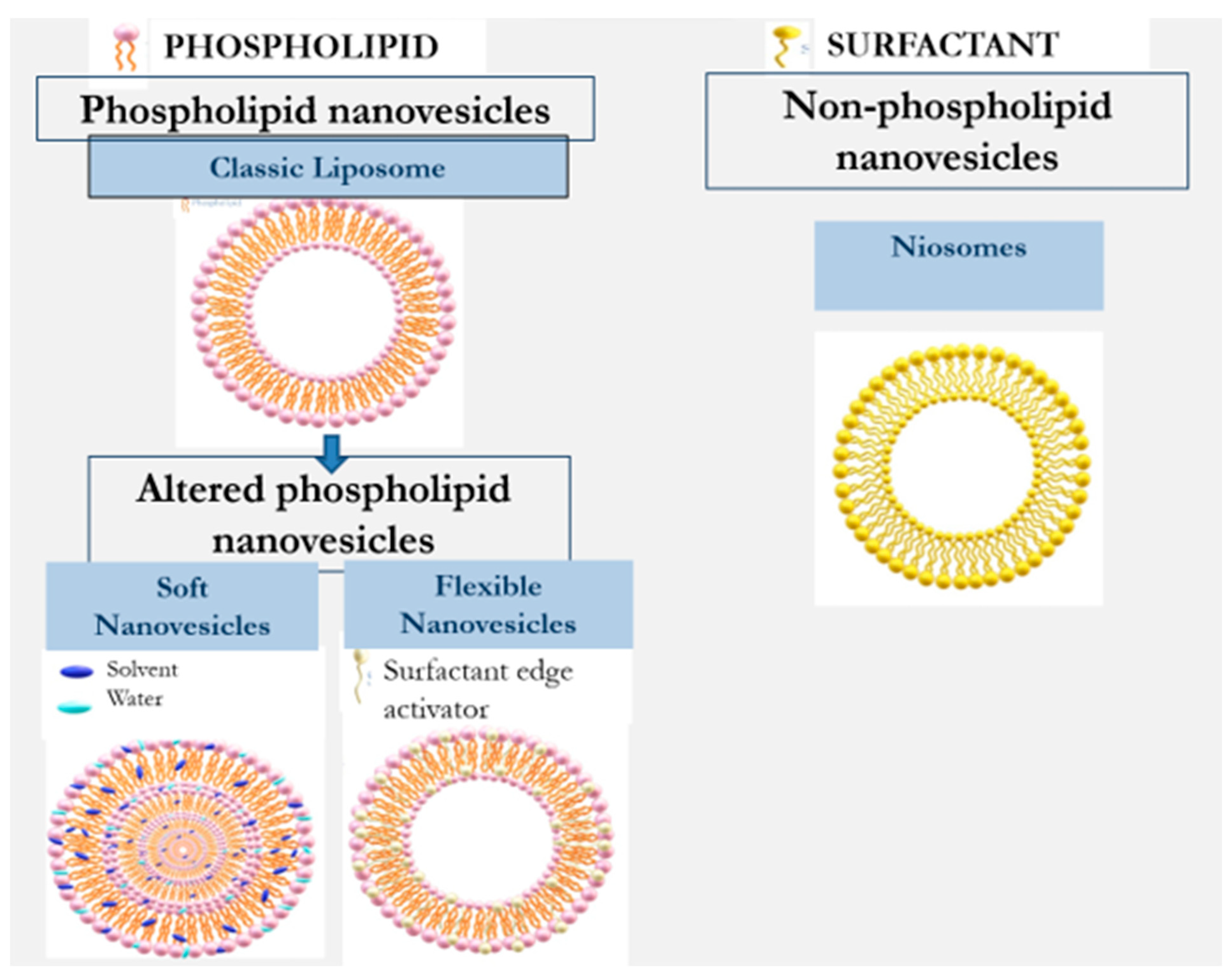
Figure 2. Evolution of nanovesicles for enhancement of dermal/transdermal drug delivery.
Table 1. Differences between various types of nanovesicles for drug delivery into and across the skin.
| Type | Vesicles Modifying Agent | Preparation Method | Year | Reference |
|---|---|---|---|---|
| Liposomes | -- | Film formation and hydration | 1964 | [10] |
| Phospholipid flexible nanovesicles | ||||
| Tranfersomes | Surfactants | Film formation and hydration | 1992 | [11] |
| Ultradeformable liposomes |
Surfactants | Film formation and hydration | 2003 | [5] |
| Phospholipid soft nanovesicles | ||||
| Ethosomes | Alcohol | Mixing method | 1998 | [8] |
| Glycerosomes | Glycerin | Film formation and hydration | 2013 | [12] |
| Tranethosomes | Surfactants and alcohol | Mixing method | 2012 | [13] |
| Plurethosomes | Copolymer Pluronic |
Mixing method | 2021 | [14] |
| Non-phospholipid vesicles | ||||
| Niosomes | Non-ionic surfactants | Mixing method; Film forming and hydration | 1975 | [15] |
3. Phospholipid-Based Nanovesicles for Dermal/Transdermal Drug Delivery
Several studies have indicated that these vesicles can carry drugs and active molecules only to the upper skin strata [7][8]. Further developed phospholipid carriers containing altered nanovesicles have been shown to overcome the permeability barrier in the skin [3][16]. The literature describes several approaches for altering the properties of conventional phospholipid vesicles to improve their penetration behavior. Transfersomes and ethosomes are the most investigated penetration-enhancing phospholipid nanovesicles. These nanovesicles are flexible and soft nanovesicles which are obtained by adding surfactant edge activators and alcohols, respectively [2]. These developments were followed by more recently designed transethosmes and glycerosomes. Niosomes are a non-phospholipid type of nanovesicles consisting mainly of non-ionic surfactants [9].
Cevc [11] introduced the transfersome as a modified liposome with flexible and elastic properties. Having a stress-responsive characteristic, these nanovesicles can penetrate skin openings much smaller than their own size. Their elasticity is imparted by the presence of an edge activator, generally a single-chain surfactant with a high radius of curvature. Once inserted between the phospholipids, the edge activator destabilizes the membrane bilayers, increasing their deformability. Sodium cholate, bile salts, oleic acid, Span 80, Tween 20, dipotassium glycyrrhizinate, and Tween 80 are examples of surfactants used for the preparation of transfersomes. Cevc used surfactants in transfersomal systems at a concentration range of 0.02–10% [17][18][19].
Deformable liposomes modified with edge activators or vegetable oils for improved dermal delivery of methotrexate have been investigated by Trotta et al. [20]. Epikuron 200 (phospholipid containing 95% phosphatidylcholine, PC) or hydrogenated lecithin (PL100H)-based liposomes were modified by adding the natural surface-acting agent dipotassium glycyrrhizinate (KG). These modified liposomes exhibited high deformability and elasticity, allowing them to pass through barriers with pores smaller than their own diameter by a factor of about three. The authors reported a similar size of these deformable liposomes before and after passing through 100 nm pores. Nanovesicles with a mean diameter of 352 ± 28 nm displayed a value of 345 ± 20 nm after the filtration, indicating their high degree of elasticity. The effect of these deformable liposomes on the skin permeation of methotrexate was evaluated in vitro on porcine ear skin. The cumulative methotrexate amounts permeated through the skin during 24 h from the deformable vesicles containing PC and PL100H were 23.55 ± 4.3 and 16.8 ± 4.0 µg, respectively. On the other hand, the rigid liposome and aqueous solution of the drug had lower permeation profiles of 5.7 µg. Zheng et al. [21] studied the morphology of soybean lecithin transfersomes modified with sodium deoxycholate containing itraconazole as an active compound model. Stable nanovesicles, composed of 6 mmol/L phospholipids, 6 mmol/L sodium deoxycholate, and 1.23 mmol/L itraconazole, were prepared using the thin layer evaporation method. The reported mean size of the obtained nanovesicles was 100 nm. Transmission microscopic examination indicated that these nanovesicles are spherical, uni-lamellar hollow associates.
The development of soft vesicles presents an interesting evolution of phospholipid vesicles. This type of nanovesicle is obtained owing to the presence of solvents such as alcohols. The first and most investigated soft vesicle is the ethosome, introduced by Touitou [8]. This nanovesicular carrier is composed of phospholipid, ethanol (20–50% w/w) with or without glycols, water, and the active molecule. Generally, ethosomes are multilamellar nanovesicles containing bilayers from wall to wall. The ethosomal phospholipid vesicles contain short-chain alcohols like ethanol or isopropyl alcohol with an optional addition of glycols such as Transcutol® or propylene glycol. Phosphorous (31P) NMR data indicated that the high alcohol concentration in ethosomes imparts fluidity to the phospholipid bilayers and organizes them in a spherical lamellar-closed shape. This notable fluidity of the lipid bilayers in ethosomal vesicles, compared to liposome, was further confirmed by the results of fluorescent anisotropy measurements of 9-antrivinyl labeled analogue of PC (AVPC) and differential scanning calorimetry (DSC) data. A reduction of up to 30 °C was reported in the main Tm of the phospholipid in ethosomal nanovesicles when compared with the corresponding liposomes [7]. The changes in the Tm values ethosomes and other phospholipid nanovesicles are presented in Figure 3.
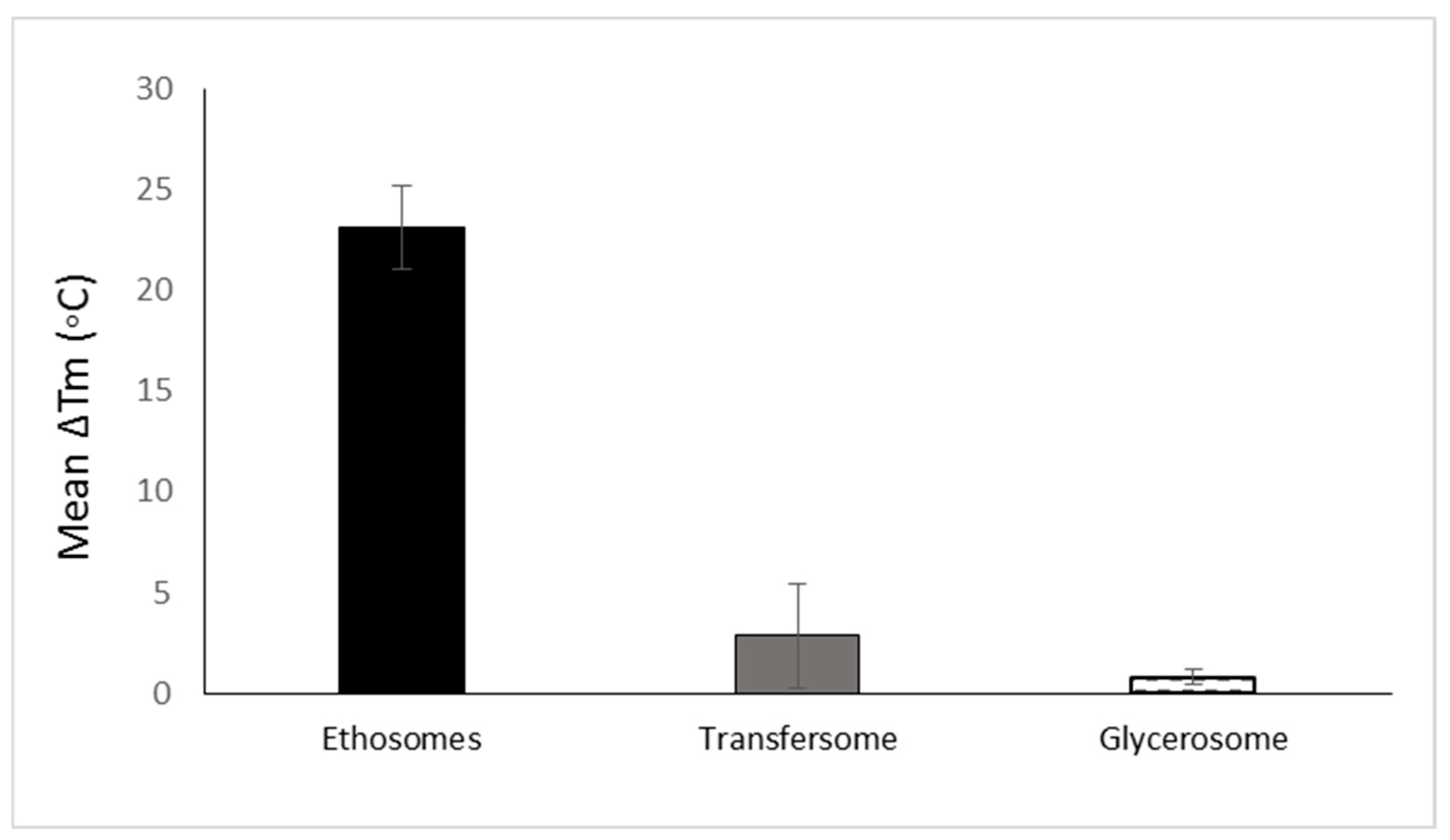
Figure 3. The mean reduction of Tm of various phospholipid vesicular carriers for trans-mucosal drug delivery dermal/transdermal as compared to the classic liposome based on data collected and published by Natsheh and Touitou [2].
When comparing the effect of ethanol on the vesicle fluidity with that of surfactant edge activators, a significant difference can be observed. El Maghraby et al. showed that the addition of edge activators such as oleic acid, Tween 80, and Span 80 to dipalmitoylglycerophosphatidylcholine (DPPC) nanovesicles reduced the Tm values by 2.1, 0.84, and 7.3 °C, respectively. Trotta et al. [20] showed that PL100H vesicles containing 1% w/w KG had a transition temperature (Tm) value reduced by 0.9 °C in comparison with liposomes. It can be noted that the effect of solvents on vesicle fluidity is more significant than that of edge activators. This behavior is supported by the reduction in Tm values for nasally administered phospholipid nanovesicles modified by ethanol and propylene glycol, where the Tm was reduced by up to 16 °C (Figure 3).
Another distinguishing property of ethosome is the multilamellar structure from wall-to-wall that has been seen by electron microscopic (EM) micrographs. This structure of ethosomes was retained in drug delivery systems containing many drugs, such as minoxidil and testosterone [7][22]. On the other hand, the incorporation of other molecules such as buspirone and erythromycin yielded uni-lamellar ethosomes [4][23]. The reported dynamic light scattering data indicated a mean size distribution of 150 ± 4 nm for drug-free ethosomes composed of 2% phospholipid and 30% ethanol. Increasing the ethanol concentration from 20 to 45% led to a vesicular size reduction to 103 nm. It is worth mentioning that the corresponding liposomes, prepared by the conventional film-forming method, had a larger average size of 677 ± 31 nm [7].
The content and structure of the vesicles affect their properties, such as their capacity to incorporate the active molecule. As an example, the classic liposome vesicle contains an aqueous core surrounded by phospholipid lamella. The incorporated molecule, if soluble in water, will be dissolved in the core of the liposome. On the other hand, lipophilic molecules will be incorporated between the phospholipid bilayers. Different from the above structure, the ethosome contains phospholipid bilayers from wall-to-wall incorporating the ethanol and water between them. This unique structure enables the incorporation of both lipophilic and hydrophilic molecules in the whole vesicles.
Published data on ethosomes indicates their efficiency in delivering active molecules into and across the skin, in terms of quantity and depth, as compared to classic liposomes or a hydroalcoholic solution. This property was reported from an in vitro experiment using Franz diffusion cells and confocal laser scanning (CLS) microscopic examination, where the ethosomal system containing the fluorescence probe, calcein, penetrated mouse skin into a depth of 160 µm. This is in comparison with a hydroethanolic solution and liposome, which carried the molecule only to depths of 80 and 60 µm, respectively [7].
The extensive research published on transfersomes and ethosomes led to the development of a new generation of nanovesicular carriers including transethosomes, glycerosomes, and plurethosomes. Transethosomes are phospholipid nanovesicular carriers combining the properties of ethosomes and transfersomes by consisting of high ethanol content (≥20%) together with edge activators. Song et al. [13] prepared voriconazole transethosomes by adding the edge activators Tween 80, sodium taurocholate, and oleic acid at a concentration of 0.53% w/w, to ethosomes containing 30% ethanol. TE microscopic examination indicated the presence of irregular-shaped vesicles with a mean size in a range of 146 to 192 nm. These irregular morphological features could be a result of combining ethanol and edge activators that may rearrange the lipid bilayers of this type of vesicle. Rady et al. [24] prepared a Fe-chlorophyllin transethosomal system based on phosphatidylcholine, the edge activators Tween 80, Tween 20, Span-20 or Cremophor-A25, and 20% w/v ethanol. The reported mean vesicular size ranged between 455 to 686 nm when these surface-active agents were used at the 0.1% concentration. The values decreased to 305–385 nm when a higher concentration of edge activators was used (0.3% w/v). The authors also reported that vesicles containing 0.1% w/v Cremophor A25 exhibited a deformability index of 26.2 ± 3.8 mL/s.
Glycerosomes are based on another modification of phospholipid vesicles, containing glycerol at a concertation of 10–30% v/v [12]. The authors reported that this trihydric alcohol increases the fluidity of the vesicles’ bilayer, thus improving the ability of the carrier to enhance molecule penetration into the skin. However, the effect of glycerol on Tm values is considered low when compared to that of ethanol in ethosomes. The reduction in Tm due to the presence of glycerol in glycerosomes is quite small and does not exceed 0.9 °C. To understand the reason behind this minimal effect of glycerol on the vesicles fluidity, it should be noted that glycerosomes were prepared using DPPC, hydrogenated soy phosphatidyl choline (PL90H) and 1,2-dimyristoyl-sn-glycero-3 phosphatidylcholine (DMPC) [12][25][26]. In addition, these vesicles contain cholesterol, a membrane stabilizer, contributing to the vesicular rigidity [27]. TEM examination revealed that glycerosomes are multilamellar spherical nanovesicles with a mean diameter of 105–130 nm. The authors reported that the vesicle elasticity improved as glycerol content increased; empty and drug-loaded glycerosomes with 30% glycerol were two-fold more deformable than the control. Glycerosomal systems containing 20% and 30% glycerol were more efficient in delivering diclofenac into the skin compared to systems containing 10% glycerol or conventional liposomes.
References
- Touitou, E.; Natsheh, H. Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129.
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959.
- Sahin, N.O. Niosomes as nanocarrier systems. In Nanomaterials and Nanosystems for Biomedical Applications; Springer: Amsterdam, The Netherlands, 2007; pp. 67–81.
- Shumilov, M.; Touitou, E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int. J. Pharm. 2010, 387, 26–33.
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474.
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Mihai, C.T.; Bacaita, S.E.; Popa, M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics 2021, 13, 866.
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes- novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418.
- Touitou, E. Compositions for Applying Active Substances to or through the Skin. U.S. Patent 5,716,638, 10 February 1998.
- Carafa, M.; Santucci, E.; Alhaique, F.; Coviello, T.; Murtas, E.; Riccieri, F.M.; Lucania, G.; Torrisi, M.R. Preparation and properties of new unilamellar non-ionic/ionic surfactant vesicles. Int. J. Pharm. 1998, 160, 51–59.
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Boil. 1964, 8, 660-IN10.
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta 1992, 1104, 226–232.
- Manca, M.L.; Manconi, M.; Zaru, M.; Valenti, D.; Peris, J.E.; Matricardi, P.; Maccioni, A.M.; Fadda, A.M. Glycerosomes: Investigation of role of 1,2-dimyristoyl-sn-glycero-3-phosphatidycholine (DMPC) on the assembling and skin delivery performances. Int. J. Pharm. 2017, 532, 401–407.
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304.
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as vesicular system for cutaneous administration of mangiferin: Formulative study and 3D skin tissue evaluation. Pharmaceutics 2021, 13, 1124.
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206.
- Lai, F.; Caddeo, C.; Manca, M.L.; Manconi, M.; Sinico, C.; Fadda, A.M. What’s new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 2020, 583, 119398.
- Schätzlein, A.; Cevc, G. Non-uniform cellular packing of the stratum corneum and permeability barrier function of intact skin: A high-resolution confocal laser scanning microscopy study using highly deformable vesicles (Transfersomes). Br. J. Dermatol. 1998, 138, 583–592.
- Cevc, G.; Gebauer, D.; Stieber, J.; Schätzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta 1998, 1368, 201–215.
- Cevc, G. Preparation for the Application of Agents in Mini-Droplets. U.S. Patent 6,165,500, 8 April 1992.
- Trotta, M.; Peira, E.; Carlotti, M.E.; Gallarate, M. Deformable liposomes for dermal administration of methotrexate. Int. J. Pharm. 2004, 270, 119–125.
- Zheng, W.S.; Fang, X.Q.; Wang, L.L.; Zhang, Y.J. Preparation and quality assessment of itraconazole transfersomes. Int. J. Pharm. 2012, 436, 291–298.
- Ainbinder, D.; Touitou, E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2005, 12, 297–303.
- Godin, B.; Touitou, E. Erythromycin ethosomal systems: Physicochemical characterization and enhanced antibacterial activity. Curr. Drug Deliv. 2005, 2, 269–275.
- Rady, M.; Gomaa, I.; Afifi, N.; Abdel-Kader, M. Dermal delivery of Fe-chlorophyllin via ultradeformable nanovesicles for photodynamic therapy in melanoma animal model. Int. J. Pharm. 2018, 548, 480–490.
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74.
- Manca, M.L.; Cencetti, C.; Matricardi, P.; Castangia, I.; Zaru, M.; Sales, O.D.; Nacher, A.; Valenti, D.; Maccioni, A.M.; Fadda, A.M.; et al. Glycerosomes: Use of hydrogenated soy phosphatidylcholine mixture and its effect on vesicle features and diclofenac skin penetration. Int. J. Pharm. 2016, 511, 198–204.
- El Maghraby, G.M.; Williams, A.C.; Barry, B.W. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
681
Revisions:
2 times
(View History)
Update Date:
22 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




