Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samuele Notarbartolo | -- | 2152 | 2024-02-21 14:32:57 | | | |
| 2 | Jason Zhu | Meta information modification | 2152 | 2024-02-23 03:19:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manfrini, N.; Notarbartolo, S.; Grifantini, R.; Pesce, E. Innate Immunity and COVID-19 Vaccines. Encyclopedia. Available online: https://encyclopedia.pub/entry/55307 (accessed on 07 February 2026).
Manfrini N, Notarbartolo S, Grifantini R, Pesce E. Innate Immunity and COVID-19 Vaccines. Encyclopedia. Available at: https://encyclopedia.pub/entry/55307. Accessed February 07, 2026.
Manfrini, Nicola, Samuele Notarbartolo, Renata Grifantini, Elisa Pesce. "Innate Immunity and COVID-19 Vaccines" Encyclopedia, https://encyclopedia.pub/entry/55307 (accessed February 07, 2026).
Manfrini, N., Notarbartolo, S., Grifantini, R., & Pesce, E. (2024, February 21). Innate Immunity and COVID-19 Vaccines. In Encyclopedia. https://encyclopedia.pub/entry/55307
Manfrini, Nicola, et al. "Innate Immunity and COVID-19 Vaccines." Encyclopedia. Web. 21 February, 2024.
Copy Citation
The COVID-19 pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has led to almost seven million deaths worldwide. SARS-CoV-2 causes infection through respiratory transmission and can occur either without any symptoms or with clinical manifestations which can be mild, severe or, in some cases, even fatal. Innate immunity provides the initial defense against the virus by sensing pathogen-associated molecular patterns and triggering signaling pathways that activate the antiviral and inflammatory responses, which limit viral replication and help the identification and removal of infected cells.
COVID-19
SARS-CoV-2
innate immunity
vaccines
immune response
1. Introduction
To control the spread of the COVID-19 pandemic, various vaccines have been developed to produce neutralizing antibodies specific to the SARS-CoV-2 Spike (S) protein. Currently approved COVID-19 vaccines can be divided into four main categories: (1) RNA vaccines (e.g., Moderna’s Spikevax mRNA-1273 and Pfizer-BioNTech’s Comirnaty BNT162b2); (2) vector-based vaccines (e.g., AstraZeneca’s Vaxzevria, Covishield ChAdOx1, and Johnson & Johnson-Janssen’s Ad26.COV2.S); (3) protein subunit-based vaccines (e.g., Novavax’s Nuvaxovid and Covovax NVX-CoV2373); and (4) inactivated whole virus vaccines (e.g, Sinopharm’s Covilo, Sinovac’s CoronaVac, and Bharat Biotech’s Covaxin) (https://covid19.trackvaccines.org/agency/who/, accessed on 1 November 2023).
The first two categories include vaccines that do not physically contain the SARS-CoV-2 S protein but have the genetic information required for its production, such as mRNA and DNA vaccines. The third category is, instead, made of vaccines physically containing the SARS-CoV-2 S protein, which can be delivered in different forms and with added adjuvants. The fourth category consists of whole SARS-CoV-2 virus particles grown in cell culture that are chemically inactivated.
In addition to carrying the microbial antigen or the genetic information coding for it, which defines its specificity, a vaccine must be immunogenic, meaning that it has to be capable of efficiently activating the innate immune system to trigger an effective adaptive immune response and the consequent generation of immunological memory. The main difference between the four vaccine types in terms of their immunogenicity is that RNA vaccines and vector-based vaccines intrinsically and efficiently stimulate innate immune responses, and are indeed referred to as “self-adjuvanted” [1][2], while protein subunit-based and inactivated whole virus vaccines do not, and hence require additional adjuvant molecules.
All vaccine types are very efficient in preventing COVID-19, especially in protecting against severe disease and death (https://covid19.trackvaccines.org/agency/who/, accessed on 1 December 2023).
2. Differences in Innate Immune Responses Induced by Different Vaccines
COVID-19 mRNA vaccines contain a functional mRNA that is translated by the host translational machinery into the SARS-CoV-2 S protein. In this context, the genetic content of the vaccine provides both the information to produce the antigen and the adjuvant activity, since viral mRNA is recognized by the innate immune system and activates it. Rather, the mRNA contained in the vaccine has specific nucleotide modifications, namely cytosine, adenine, and uridine methylations, aimed at reducing the recognition by TLRs and RLRs to avoid an excessive innate immune response that, besides provoking reactogenicity to vaccination, could also restrict the translation of the encoded S protein [3][4], thus compromising the adaptive immune response to the antigen.
In addition to its genetic content, lipid nanoparticles (LNPs), which are used as a vaccine carrier, also contribute to the immunogenicity of mRNA vaccines. LNPs protect the mRNA, help with its entry into cells, and allow it to be delivered efficiently to the lymphatic system, specifically to the lymph nodes (LNs). LNPs play a role in eliciting inflammatory responses triggered by vaccines and serve as adjuvants to enhance adaptive immune responses [2].
Upon entry into host cells, mRNA is detected by innate sensors, including TLR3, TLR7, and TLR8 in the endosome, and RIG-I and MDA-5 in the cytosol, leading to type-I IFN production [2] (Figure 1).
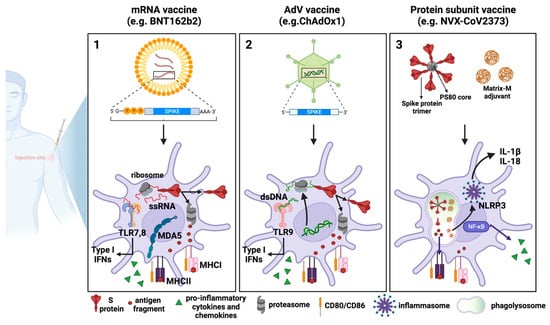
Figure 1. COVID-19 vaccines: processing by dendritic cells and innate immune response activation. Schematic representation of the mechanisms of action of the three major vaccine platforms against SARS-CoV-2: (1) mRNA-based vaccines, in which an mRNA molecule encoding for the SARS-CoV-2 S protein is encapsulated in lipid nanoparticles; (2) AdV vaccines, in which the SARS-CoV-2 S protein is encoded by a DNA molecule embedded in an AdV capsid; and (3) recombinant protein-based vaccines in which SARS-CoV-2 S proteins are assembled around nanoparticles to resemble the actual virus structure.
Compared to mRNA-based vaccines, Adenovirus Vector (AdV)-based DNA vaccines require a more complex pathway to reach the synthesis of functional mRNA and the production of the native S protein. An intranuclear passage of adenoviral DNA is required for transcription and RNA processing. This added complexity, combined with the nature of the vaccine, can lead to heterogeneous immune reactions, which can be attributed, for example, to the adenovirus type or the vector-specific genetic modifications.
The strategy used for current AdV-based COVID-19 vaccines is the replacement of the early region 1 (E1) adenoviral gene with the full-length SARS-CoV-2 S gene and the deletion of the E3 region. The deletion of the E1 gene, in addition to enabling the insertion of the S gene, is essential to abolish the replication of the viral vector, making the vaccine safe for the recipient. Deletion of the E3 gene, which is involved in evading host immunity but is not essential for virus production, is planned, along with that of the E1 region (E1/E3 deletion), to accommodate larger recombinant genes, such as SARS-CoV-2 S [5][6].
Innate immune responses triggered by AdV-based vaccines are different from those induced by mRNA vaccines since the DNA is detected by different PRRs. In particular, AdVs expose PAMPs sensed by TLR2 and TLR4 at the plasma membrane level, and by TLR9, which is located endosomally. Furthermore, the viral DNA can be detected following endosomal rupture via cytosolic DNA sensors like cGAS and the inflammasome, which promote the secretion of type-I IFNs [7] (Figure 1).
Among the approved SARS-CoV-2 recombinant protein subunit-based vaccines, the most widely used is NVX-CoV2373. It comprises the trimeric full-length SARS-CoV-2 S, which is generated as a recombinant protein in Sf9 cells through a baculovirus expression system. The SARS-CoV-2 S trimer is arranged around a polysorbate 80 (PS80)-based detergent mixed with the saponin-based adjuvant Matrix M [8]. At the site of injection and the draining lymph nodes (dLNs), Matrix M triggers rapid activation of innate immune cells, including APCs. The consequent cytokine release from APCs recruits additional innate immune cells, causing a cascading local immune response. Matrix M also induces the activation of NLRP3 inflammasome, which leads to the release of IL-1β and IL-18 and the production and secretion of other proinflammatory cytokines [9][10] (Figure 1).
Inactivated vaccines are derived from the complete SARS-CoV-2 virus, which has been rendered inactive through exposure to physical or chemical inactivating agents like UV rays, formalin, or formaldehyde administration [11]. Like SARS-CoV-2 recombinant protein subunit-based vaccines, inactivated whole virus vaccines must be combined with adjuvants to be effective and able to boost their immunogenicity. For example, the adjuvant used in Covaxin is a TLR7/8 agonist. TLR7 and TLR8 agonists potentiate the Th1 but suppress the Th2 immune response, an event which is beneficial for COVID-19 vaccines. TLR recognition in the innate cell population has also been linked to the generation of early type-I IFN, which promotes viral clearance and pro-inflammatory cytokine generation [12].
In contrast to the initial three vaccine categories where the S protein serves as the sole immunogen, inactivated vaccines elicit more extensive immune responses because of the presence of additional immunogenic proteins, such as the M, N, and E proteins. This results in a lower S-specific T cell response but is accompanied by a broader polyclonal T cell response, with T cells also being specific for other viral epitopes [13].
3. Innate and Adaptive Immune Response Cross-Talk upon COVID-19 Vaccination
Although the initial phase of the immune response to a vaccine involves the innate immune system, the effective immunization of the host requires the proper stimulation of the humoral and cellular adaptive immune system to induce the production of neutralizing antibodies on the one side and the differentiation of memory B cells and T cells on the other.
Professional APCs, such as DCs, bridge the two arms of the immune system by presenting the vaccine-associated antigen to naïve CD4+ and CD8+ T cells onto MHC-II and MHC-I molecules, respectively. Migratory DCs, which capture the antigen in the periphery and then migrate to the LNs, and LN-resident DCs, which uptake soluble antigen arrived into the LNs from the afferent lymphatics, induce protective immunity by priming antigen-specific naïve CD4+ helper T cells and CD8+ cytotoxic T cells. Certain CD4+ T cells with specificity for the antigen undergo differentiation into follicular T helper cells (Tfh). These Tfh cells play a role in facilitating the differentiation of B cells into high-affinity antibody-secreting plasma cells and memory B cells. This process leads to the production of specific neutralizing antibodies against the virus, establishing immune memory. Consequently, this protection helps prevent the individual from developing the disease and, in some instances, from acquiring the infection [14].
Both mRNA and AdV vaccines require multiple doses to gain optimal protection. More pronounced vaccine-associated inflammatory states have been associated with vaccine boosters compared to the first doses. This is a result of short-term “trained immunity” of innate cells (e.g., macrophages) and of the activation of memory T and B cells generated by the primary immune response [15]. The resulting production of type-I IFN amplifies T cell memory and promotes B cell differentiation and survival, contributing to the generation of long-lasting immunological memory.
In contrast to mRNA vaccines, during an AdV-based vaccination, pre-existing immunity to the vector may limit the ability to deliver genetic material to host cells, thus reducing the vaccine’s efficacy [16]. To overcome this issue, vectors from alternative AdV serotypes with low prevalence in the population have been developed, and different AdV vectors can be used for the first and the second doses of the vaccine. This was the strategy used, for example, in the original vaccination protocol with the Sputnik V vaccine that consisted of a two-dose regimen with two different human AdVs, namely AdV26 and AdV5. To mitigate the issue of vector-specific pre-existing immunity, AdV from other species such as chimpanzees, cattle, and pigs have been also used as candidates for vaccine development [17].
In contrast to mRNA and AdV vaccines, which lead to the intracellular production of the antigen, in protein subunit-based vaccines the antigen is taken up by APCs from the extracellular space. In this context, the activation of CD8+ T cells mostly relies on the cross-presentation capacity of APCs; this is the process by which an extracellular antigen, which would be normally loaded onto MHC-II, is presented by MHC-I molecules. Therefore, while antibody production and CD4+ T cell responses induced by protein-subunit-based vaccines are comparable to those of mRNA and AdV vaccines, the frequency of S-specific CD8+ T cells is lower [18]. Nonetheless, the orchestrated humoral and cellular immune response elicited by protein subunit-based vaccines is sufficient to efficiently protect individuals from COVID-19, especially from its severe forms [19].
Regarding the cell-mediated adaptive immune response, in the case of NVX-CoV2373, the MatrixM adjuvant has been shown to induce a preferential polarization of CD4+ T cells toward a Th1 response [20], preventing the immune response from veering toward Th2-like responses, which is undesirable for host defense against SARS-CoV-2 [21][22].
All three types of vaccines work similarly at the cellular level, as they all are endocytically internalized by DCs either at the site of injection or following trafficking to the dLNs. The vaccine-derived SARS-CoV-2 S protein is then degraded by the immunoproteasome into peptides that will be presented as antigens by the MHC class I. Furthermore, antigens released extracellularly can be phagocytosed by professional APCs, processed, and presented by MHC class II. What differs between vaccines is how the SARS-CoV-2 S protein is produced or processed. (1) In mRNA vaccines, after endosomal escape, mRNA can enter the cytosol and be immediately translated by the host cell translational machinery, in turn producing high levels of S protein. (2) Once the AdV escapes the endosome, the partially disassembled AdV capsids can reach the nucleus where the S transgene can be transcribed. After being exported into the cytoplasm, SARS-CoV-2 S mRNA can be translated into protein. (3) In the case of recombinant protein-based vaccines, for example, in NVX-CoV2373, the MatrixM adjuvant and SARS-CoV-2 S protein are internalized by host cells and are directly processed by phagolysosomes and by proteasome for antigen presentation. MatrixM induces the upregulation of co-stimulatory and MHC molecules, thus promoting the presentation of antigenic peptides. Due to their intrinsic adjuvant activity, mRNA and AdV vaccines are also able to activate innate sensors, which produce type-I IFNs, pro-inflammatory cytokines, and chemokines. Such sensors comprise TLR7, TLR8, and MDA5, which detect RNA, and TLR9, which detects double-stranded DNA. Concerning recombinant protein-based vaccines, the addition of the MatrixM adjuvant in the formulation facilitates both the recruitment and activation of innate immune cells at the site of injection and the likely activation of the NLRP3 inflammasome, which mediates the maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18 through caspase-1 activation.
References
- Kowalczyk, A.; Doener, F.; Zanzinger, K.; Noth, J.; Baumhof, P.; Fotin-Mleczek, M.; Heidenreich, R. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine 2016, 34, 3882–3893.
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200.
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20.
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91.
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582.
- Kovesdi, I.; Hedley, S.J. Adenoviral Producer Cells. Viruses 2010, 2, 1681–1703.
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094.
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-MTM adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccin. Immunother. 2023, 19, 2189885.
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543.
- Tirelli, C.; De Amici, M.; Albrici, C.; Mira, S.; Nalesso, G.; Re, B.; Corsico, A.G.; Mondoni, M.; Centanni, S. Exploring the Role of Immune System and Inflammatory Cytokines in SARS-CoV-2 Induced Lung Disease: A Narrative Review. Biology 2023, 12, 177.
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646.
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis. 2022, 22, 1435–1443.
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197.
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 2018, 175, 1634–1650.e17.
- McCoy, K.; Tatsis, N.; Korioth-Schmitz, B.; Lasaro, M.O.; Hensley, S.E.; Lin, S.-W.; Li, Y.; Giles-Davis, W.; Cun, A.; Zhou, D.; et al. Effect of Preexisting Immunity to Adenovirus Human Serotype 5 Antigens on the Immune Responses of Nonhuman Primates to Vaccine Regimens Based on Human- or Chimpanzee-Derived Adenovirus Vectors. J. Virol. 2007, 81, 6594–6604.
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97.
- Rydyznski Moderbacher, C.; Kim, C.; Mateus, J.; Plested, J.; Zhu, M.; Cloney-Clark, S.; Weiskopf, D.; Sette, A.; Fries, L.; Glenn, G.; et al. NVX-CoV2373 vaccination induces functional SARS-CoV-2–specific CD4+ and CD8+ T cell responses. J. Clin. Investig. 2022, 132, 19.
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318.
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332.
- Diamond, M.S.; Pierson, T.C. The Challenges of Vaccine Development against a New Virus during a Pandemic. Cell Host Microbe 2020, 27, 699–703.
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
830
Revisions:
2 times
(View History)
Update Date:
23 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




