Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmen Lopez-Sanchez | -- | 2057 | 2024-02-21 12:10:04 | | | |
| 2 | Peter Tang | + 9 word(s) | 2066 | 2024-02-22 02:36:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
López-Sánchez, C.; Lagoa, R.; Poejo, J.; García-López, V.; García-Martínez, V.; Gutierrez-Merino, C. Kaempferol Protection against Brain Damage. Encyclopedia. Available online: https://encyclopedia.pub/entry/55293 (accessed on 08 February 2026).
López-Sánchez C, Lagoa R, Poejo J, García-López V, García-Martínez V, Gutierrez-Merino C. Kaempferol Protection against Brain Damage. Encyclopedia. Available at: https://encyclopedia.pub/entry/55293. Accessed February 08, 2026.
López-Sánchez, Carmen, Ricardo Lagoa, Joana Poejo, Virginio García-López, Virginio García-Martínez, Carlos Gutierrez-Merino. "Kaempferol Protection against Brain Damage" Encyclopedia, https://encyclopedia.pub/entry/55293 (accessed February 08, 2026).
López-Sánchez, C., Lagoa, R., Poejo, J., García-López, V., García-Martínez, V., & Gutierrez-Merino, C. (2024, February 21). Kaempferol Protection against Brain Damage. In Encyclopedia. https://encyclopedia.pub/entry/55293
López-Sánchez, Carmen, et al. "Kaempferol Protection against Brain Damage." Encyclopedia. Web. 21 February, 2024.
Copy Citation
Kaempferol, a flavonoid present in many food products, has chemical and cellular antioxidant properties that are beneficial for protection against the oxidative stress caused by reactive oxygen and nitrogen species. Kaempferol administration to model experimental animals can provide extensive protection against brain damage of the striatum and proximal cortical areas induced by transient brain cerebral ischemic stroke and by 3-nitropropionic acid.
kaempferol
flavonoids
brain stroke
ischemia reperfusion
3-nitropropionic acid
Huntington’s disease
brain neurodegeneration
pharmacological implications
1. Introduction
Kaempferol is a polyphenol of the flavonol family of flavonoids. Therefore, kaempferol’s chemical structure (Figure 1) is closely related to other flavonols with beneficial chemotherapeutic properties, like quercetin [1][2]. Kaempferol, like other flavonoids, is largely present as a glycoside derivative in plants. Apart from the most abundant glycoside derivatives, kaempferol-3-O-glucoside (astragalin), kaempferol-3,7-dirhamnoside (kaempferitrin) and kaempferol-3-O-rutinoside (nicotiflorin) [3], many other bioactive kaempferol glycosides have been found in plants, like kaempferol 3-O-[(6-O-E-caffeoyl) β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside, kaempferol 3-O-[(6-O-E-p-coumaroyl)-β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside and kaempferol 3-O-[(6-O-E-feruloyl)-β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside [4][5].
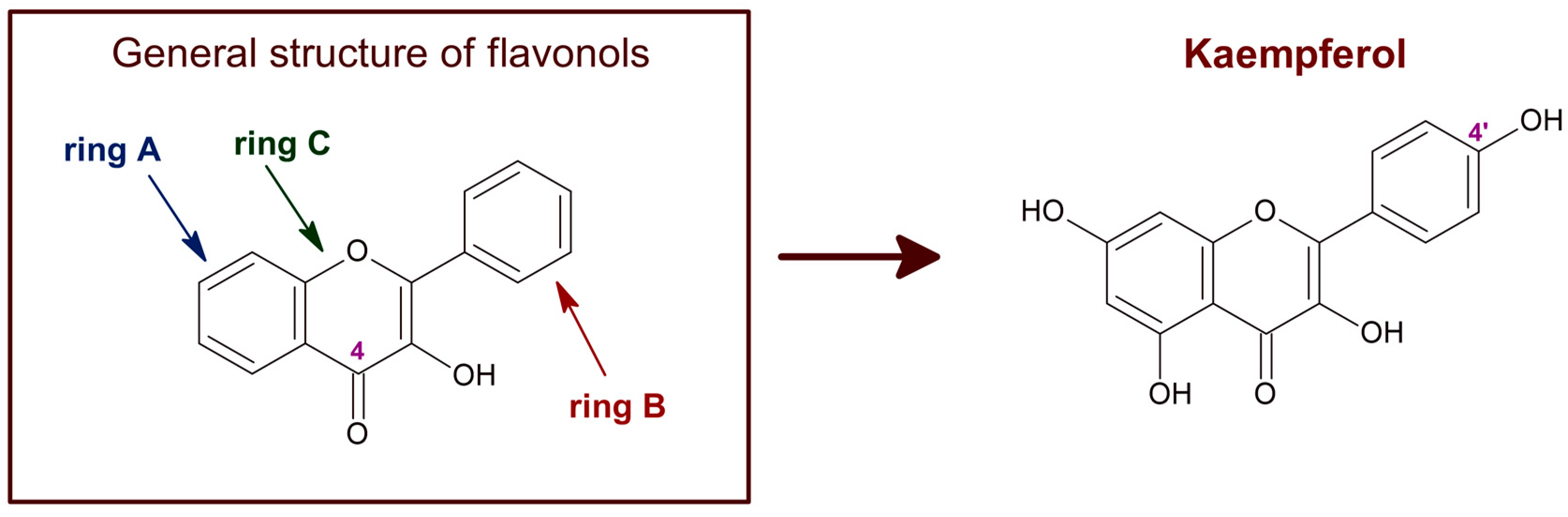
Figure 1. Chemical structure of flavonols in general and of kaempferol. The C4 position of the characteristic carbonyl group of flavonols is marked. Kaempferol, also known as 3,4′,5,7-tetrahydroxyflavone [chemical name: 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one], bears a single hydroxyl group in ring B, at position 4′.
Kaempferol and its glycoside derivatives can be found in a variety of plants and plant-derived food products, such as spinach, kale, cabbage, chives, lentils, tea, broccoli, apples, and Ginkgo biloba, among others [5][6][7][8][9]. The plasma concentrations of total flavonoid metabolites vary from 0 to 2.0 μM, with an intake of 50 mg aglycone equivalents in humans, although isoflavones can reach higher concentrations, as well as metabolites generated by the gut [10][11]. After food intake, bacterial enzymes and enterocyte β-glucosidases of the intestine hydrolyze flavonoid glycosides to their aglycones [12][13]. In addition, flavonoid aglycones undergo methylation and glucuronidation in the intestine [14] and their metabolites are further metabolized by the liver [12]. It should be noted that glial cells can also catalyze in vitro oxidation and conjugation of flavonoids with reduced glutathione (GSH) [15]. Although flavonoid metabolites are still bioactive as cellular antioxidants, most of these metabolites have been shown to have lower chemical antioxidant potency than the aglycone flavonoid [16][17][18], and this also seems to be the case of kaempferol [19]. Furthermore, the kaempferol glycosides most abundant in plants (astragalin, kaempferitrin, and nicotiflorin) have poorer antiproliferative activity than kaempferol [20]. Therefore, the medicinal use of kaempferol requires us to pay special attention to the administration route. Also, if the concentration of blood kaempferol needed in therapeutic treatments of brain damage is higher than 1–2 μM, instead of oral supplementation, other methods of administration must be used, i.e., intravenous (IV) or microencapsulation delivery.
The redox potential of kaempferol, ~0.39 V [21][22], indicates that this compound is a chemical antioxidant. Furthermore, kaempferol, as well as other flavonoids, can form stable radicals that may act as reactive species scavengers [23]. The acceptance of flavonoids as cellular antioxidants is largely based upon their ability to scavenge hydroxyl radicals, superoxide anion, lipid peroxides, and peroxynitrite [23][24][25][26][27][28]. The number of hydroxyl groups in the B-ring and the presence of a carbonyl group in C4 of the C-ring are particularly relevant for the scavenging of these reactive oxygen species (ROS) [27]. Of note, the flavonoids that most efficiently scavenge hydroxyl radicals are flavonols [23], i.e., the family of flavonoids to which kaempferol belongs (Figure 1). Moreover, compared with other phenols and flavonoids, kaempferol has a relatively high peroxynitrite scavenger activity [29][30]. Another property of flavonoids relevant for their role as cellular antioxidants is their liposolubility. The inhibition of lipid peroxidation by flavonoids correlates with their partition coefficient between n-octanol and water, because flavonoids need to incorporate into the lipid bilayer to trap the species initiating lipid oxidation radical chains [31][32]. Indeed, flavonoids act as hydrogen donors in the reaction with the peroxyl radical produced in the oxidation of fatty acids [33]. It has been shown that the higher solubility in water can account for the lower potency of flavonoid glycosides as inhibitors of lipid peroxidation compared with their respective flavonoid aglycones [34][35]. In addition, the solubility of flavonoids in lipid bilayers favors its uptake by cells and tissues. Notably, kaempferol is one of the flavonoids with a higher lipid/water partition coefficient [36].
2. Kaempferol Administration Efficiently Prevents Brain Damage Induced by Ischemic Insults
The brain ischemic insult elicited by a stroke episode is a major health problem in humans, as they often cause death or chronic disability. Ischemia stroke is the most frequent type of cerebrovascular stroke [37][38]. The transient blockade of blood flow produces rapid tissue damage in the so-called ischemic core. The rapid metabolic energy falls, leading to the depolarization of cell membranes, which causes rapid necrosis in the ischemic core. Later, the brain damage extends into the peri-infarct region [39]. Restoration of blood flow (reperfusion) is necessary, but this can aggravate brain damage [40][41], as it leads to disability and a high morbidity in patients due to irreversible brain damage. The excessive production of ROS and reactive nitrogen species, excitotoxicity, and brain inflammation are implicated in the neuronal damage during a brain ischemia-reperfusion injury [42]. Of note, oxidative stress-mediated inflammation and apoptosis are considered to play a crucial role in brain damage associated with ischemia-reperfusion episodes [43][44]. Glutamate excitotoxicity [39][45][46], oxidative stress [39][47][48], edema and inflammation [39][46], activation of matrix metalloproteinases [49][50][51][52], and apoptosis [48][53][54] contribute to spreading the brain damage to the peri-infarct region. The slow time course of this phase opens a temporal window for neuroprotective interventions.
Protective effects of different flavonoids against the ischemic brain damage produced by cerebral ischemia have been shown in many studies with experimental animals, reviewed in [1]. As noted in [1], these studies showed that the efficiency of protection against brain damage in transient cerebral ischemia largely varies between different flavonoids and depends on the administration route and doses. For example, the neuroprotective action of Ginkgo biloba extract EGb761, which is rich in kaempferol, in brain ischemia models seems to be dependent on the expression of heme oxigenase-1 [55], an enzyme that is modulated by kaempferol in different cells [56][57]. Also, nicotiflorin (kaempferol-3-O-rutinoside) has neuroprotective effects in permanent [58] and transient [59] models of rat cerebral ischemia.
To study the effect of the IV administration of kaempferol on brain damage induced by ischemia-reperfusion, first the researchers setup a rat model of transient focal ischemia using a new surgical technique of middle cerebral artery occlusion (MCAO) by selective endovascular placement of a guide-wire [60]. The researchers' study published in 2007 [61] has been, to the best of the researchers' knowledge, the first work that reported on the role of kaempferol administration in the protection against the brain damage caused by transient cerebral ischemia. The main results and conclusions obtained in this work are summarized next (Figure 2 and Figure 3).
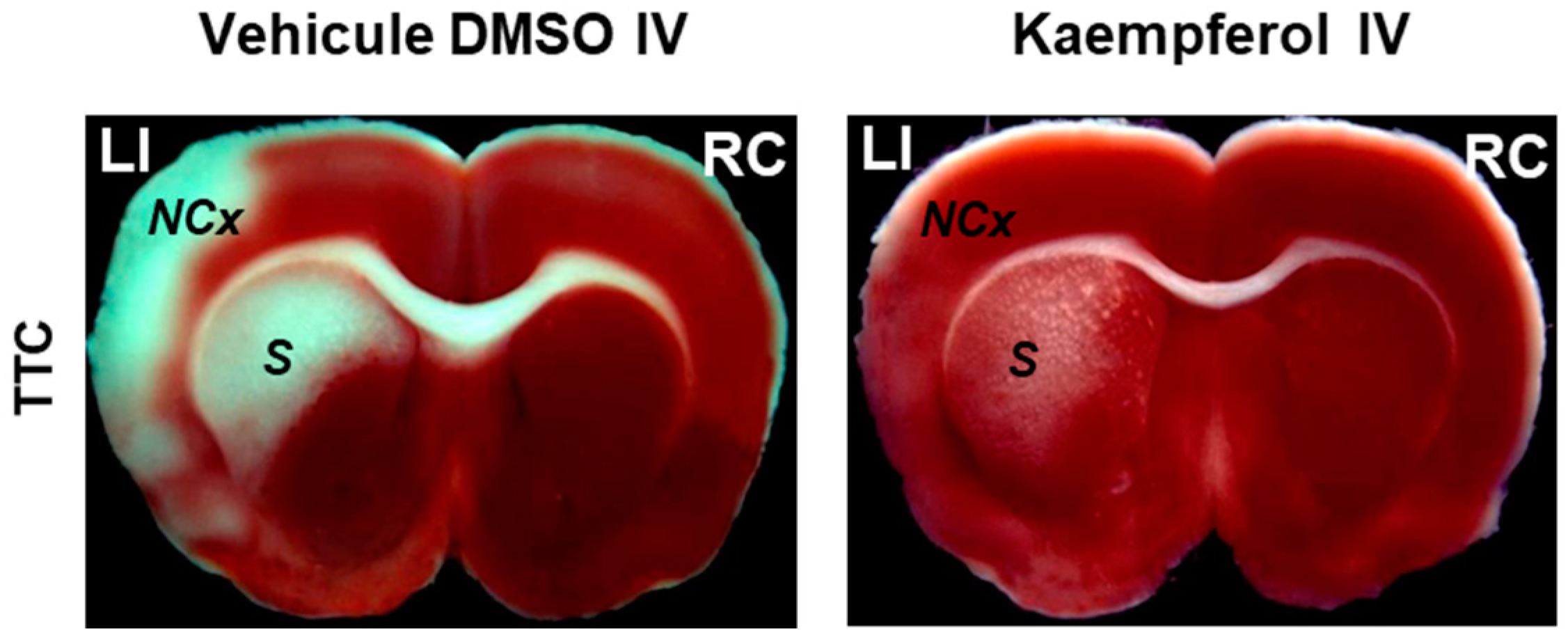
Figure 2. Kaempferol protects against ischemia/reperfusion-induced brain damage. The images of brain slices stained with 2,3,5-triphenyltetrazolium chloride (TTC) show the reduction of the damage extension in brain areas (NCx: neocortex and S: striatum) after kaempferol treatment in rats subjected to ischemia/reperfusion. LI and RC mean left ischemic and right control hemispheres, respectively. Meaning of other abbreviations used in this figure: DMSO (dimethyl sulfoxide) and IV (intravenous administration).
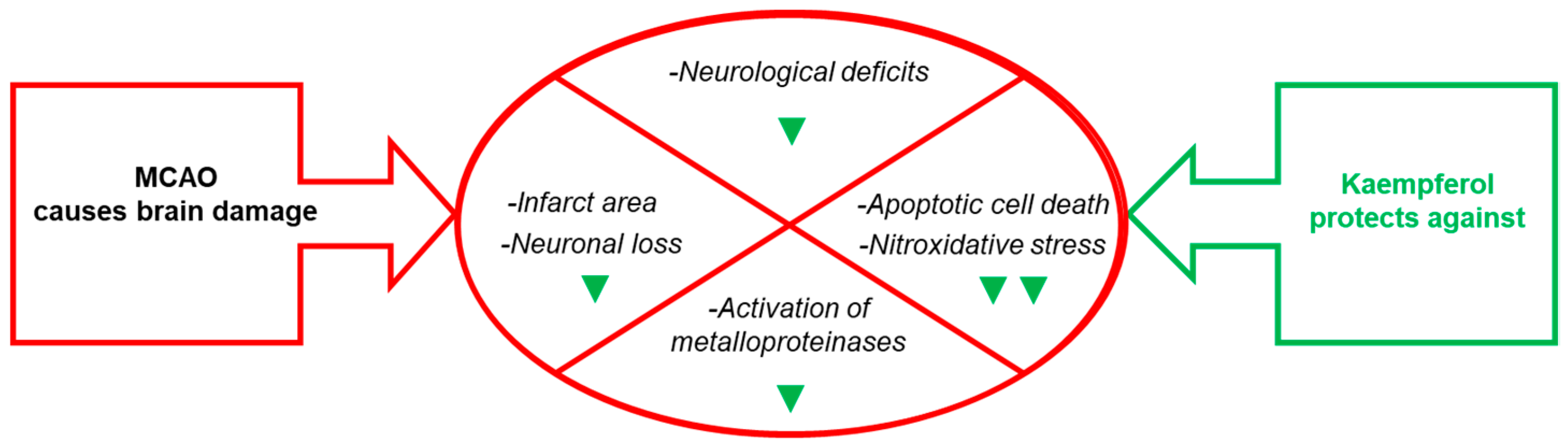
Figure 3. Effects of kaempferol administration highlighted in this section. Kaempferol reduces MCAO- induced brain damage in the infarct area, neurological deficits, apoptotic cell death and neuronal loss, as well as the levels of biomarkers of brain damage. The green symbols indicate attenuation by kaempferol: ▼ and ▼▼ mean partial and nearly complete attenuation, respectively.
3. Kaempferol Administration Efficiently Prevents Brain Damage Induced by 3-Nitropropionic Acid
The 3-nitropropionic acid (NPA) is a neurotoxin for cattle and humans and is produced by some fungi and plants [62][63]. This neurotoxin can induce brain striatum degeneration and neurological disturbances that mimic some aspects of Huntington’s disease (HD) when administered systemically to rodents and non-human primates [64][65][66][67]. The systemic administration of NPA also produces metabolic alterations in cortical areas adjacent to the striatum, as well as in the hippocampus and the cerebellum [68][69], which can account for neurological alterations seen in pre-motor stages of HD, like cognitive dysfunction, visuospatial deficits, memory loss, and difficulty in learning new skills [70][71].
NPA is a suicide inhibitor of succinate dehydrogenase [72][73][74] and causes the rapid loss of ATP in cultured neurons in vitro [75][76]. The enhanced generation of ROS that activate cell death pathways due to the impairment of mitochondrial function has been accepted to play a major role in the neurotoxicity of NPA [66][76][77][78][79][80][81]. The relevance of NPA-induced striatal degeneration models as animal models of HD is further supported by reported anomalies in mitochondrial function and oxidative stress in the brain degeneration of HD patients [78][82].
To the best of the researchers' knowledge, the researchers' work [83] was the first report of kaempferol protection against NPA-induced brain damage and associated neurological dysfunctions in an animal model. In this work the researchers used chronic treatment of Wistar rats with intraperitoneal (IP) injections of 25 mg of NPA/kg of body weight (BW) in normal saline (0.9% w/v NaCl) every 12 h during several days. The researchers assessed that this treatment induced neurological disturbances (hypoactivity, dystonic movements of hind limbs, and an abnormal gait) and striatal degeneration similar to those reported in previous publications of other investigators [64][67]. The researchers showed that IP injections of 21 mg of kaempferol/kg BW 48 h before the first NPA treatment and every day 30 min prior to the morning NPA injection largely attenuated NPA-induced neurological disturbances [83][84]. Also, the results pointed out an NPA-induced lesion highly localized in the striatum, because all the histological markers used (TTC, hematoxylin-eosin and TUNEL staining) did not show a significant increase in the vicinal brain cortical area, and IP daily doses of 21 mg of kaempferol/kg BW afforded an almost complete blockade of the striatal lesion (Figure 4).
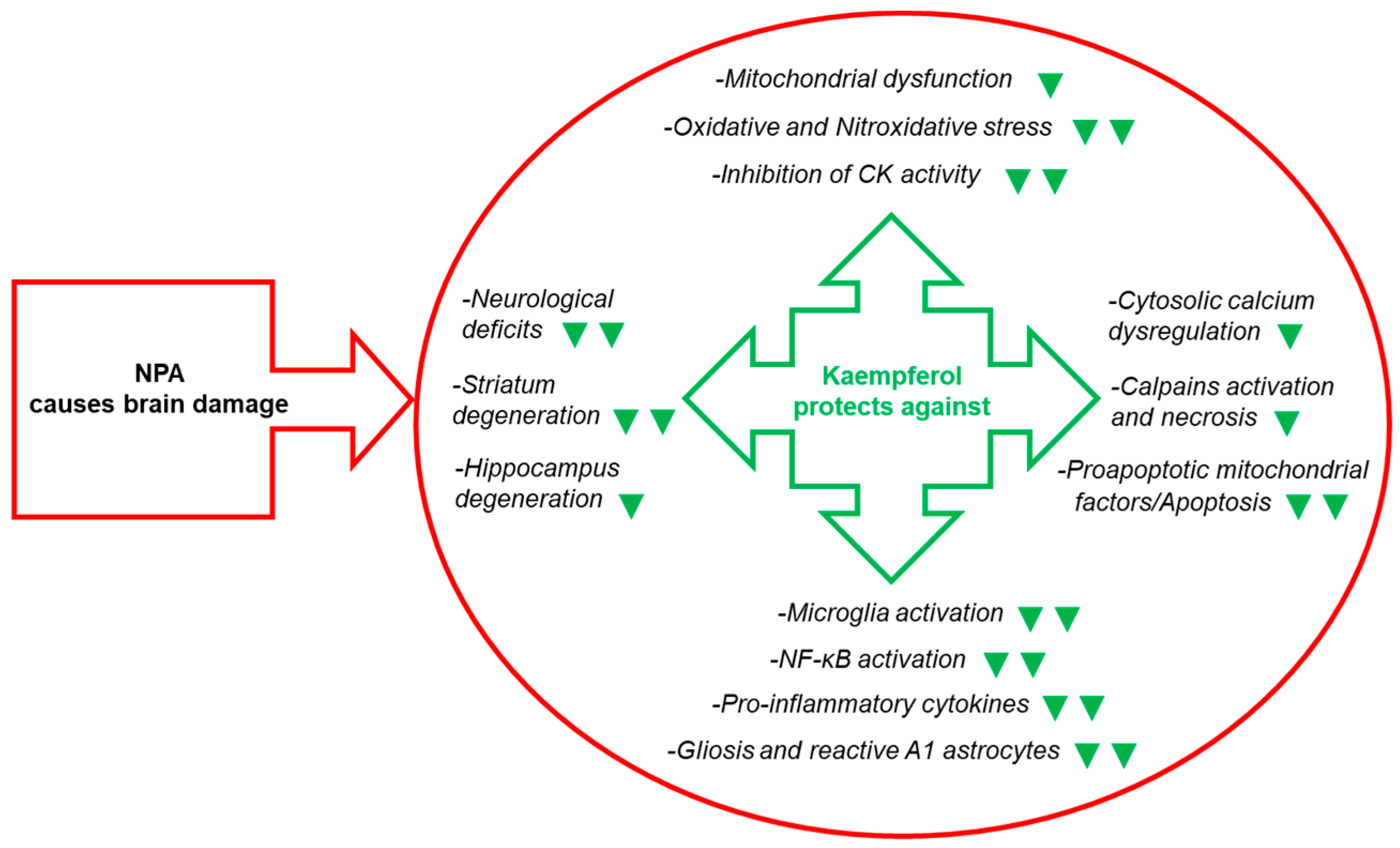
Figure 4. Kaempferol reduces NPA-induced brain lesion, neurological dysfunctions, and, also, the levels of many biochemical and cellular biomarkers of brain degeneration. The green symbols indicate attenuation by kaempferol of the NPA-induced change: ▼ and ▼▼ mean partial and nearly complete attenuation, respectively. Abbreviations used in this figure: CK (creatine kinase) and NF-κB (nuclear factor kappa light-chain enhancer of activated B cells).
4. Molecular and Cellular Mechanisms That Contribute to Kaempferol Protection against the Brain Damage Produced by Ischemia-Reperfusion and by NPA Administration
A mitochondrial energetics failure is the initial event in the brain degeneration induced by both ischemic injury (oxygen supply shortage) and NPA administration (inhibition of mitochondrial succinate dehydrogenase) (Figure 5). Notably, in vitro studies have shown that flavonoids can afford an effective protection in the 1–10 μM range in cell death models where mitochondrial dysfunction and apoptosis are implicated [85][86][87][88][89]. More recently, it has been noted that the beneficial effects of kaempferol after traumatic injury in the rats developing brain (a model system used for understanding the molecular mechanisms underlying the distinct neuropathological consequences of traumatic brain injury in children) is through protection of mitochondrial function, oxidative metabolism, and neural viability [90]. As shown in [91], kaempferol was one of the most potent flavonoids as an inhibitor of the rate of H2O2 production by brain mitochondria, which reaches nearly 100% inhibition with 10 μM flavonoid with 50% inhibition concentration of 1.8 μM. However, up to 10 μM kaempferol did not significantly affect the oxygen consumption rate of brain mitochondria [87][91]. The major systems involved in ROS production by respiring mitochondria are mitochondrial respiratory complexes I and III [92][93]. In [91], the researchers identified the mitochondrial respiratory complex I as the molecular target of kaempferol for the inhibition of mitochondrial ROS production, and found kinetic competition between CoQ and kaempferol modulation of the activity of complex I, suggesting that kaempferol binds to complex I at a site, at least, partially overlapping with the quinone-inhibitor binding pocket. It should be recalled here that mitochondrial respiratory complex I has been proposed to be the source of ROS in models of heart failure and, also, the initial site of ischemia-elicited damage to mitochondria in the heart [94]. In addition, an impaired function of the mitochondrial respiratory complex I is well documented in several neurodegenerative diseases [92]. Notably, oral kaempferol administration decreases mitochondrial fission and helps to preserve mitochondrial functional integrity and morphology in a C57BL/6 mice MCAO model of ischemic stroke [95].
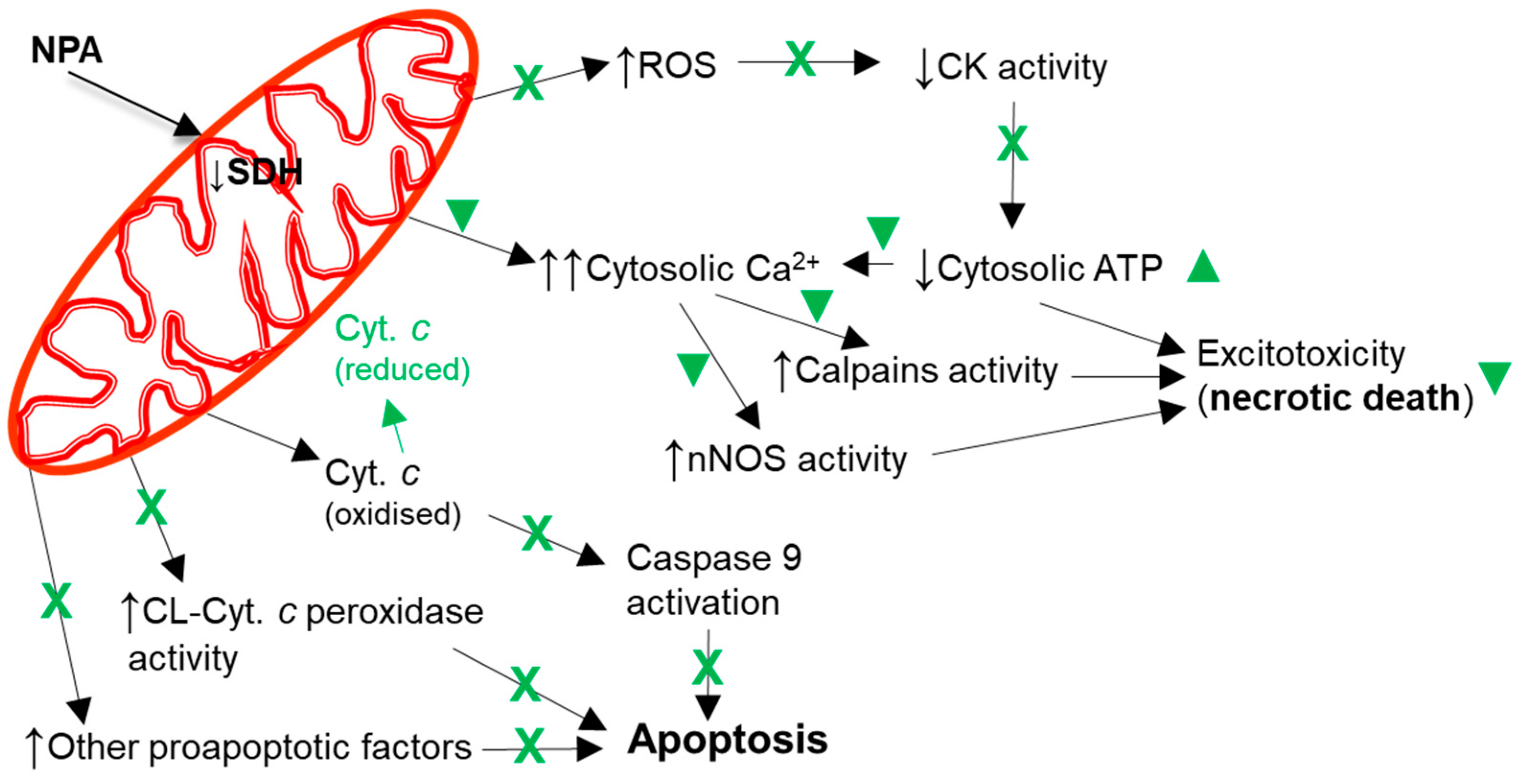
Figure 5. Kaempferol protects against mitochondrial dysfunctions leading to cellular bioenergetics crisis and apoptosis. Black arrows indicate the effects of NPA administration. The reduction of cytochrome c by kaempferol is highlighted in green. A green cross (X) means almost complete protection and a green symbol (▼) means partial protection. Abbreviations used in this figure: CK (creatine kinase), CL-Cyt. c peroxidase (cardiolipin-induced cytochrome c peroxidase activity), Cyt. c (cytochrome c), and SDH (succinate dehydrogenase).
References
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212.
- Lagoa, R.; Samhan-Arias, A.K.; Gutierrez-Merino, C. Correlation between the potency of flavonoids for cytochrome c reduction and inhibition of cardiolipin-induced peroxidase activity. BioFactors 2017, 43, 451–468.
- Harborne, J.B. Nature, distribution and function of plant flavonoids. Prog. Clin. Biol. Res. 1986, 213, 15–24.
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139.
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol and its glycoside derivatives as modulators of etoposide activity in HL-60 cells. Int. J. Mol. Sci. 2021, 22, 3520.
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807.
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arsha, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S.
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837.
- Kuhnle, G.; Spencer, J.P.E.; Schroeter, H.; Shenoy, B.; Debnam, E.S.; Srai, S.K.S.; Rice-Evans, C.; Hahn, U. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem. Biophys. Res. Commun. 2000, 277, 507–512.
- Moon, J.H.; Nakata, R.; Oshima, S.; Inakuma, T.; Terao, J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R461–R467.
- Vafeiadou, K.; Vauzour, D.; Rodriguez-Mateos, A.; Whiteman, M.; Williams, R.J.; Spencer, J.P.E. Glial metabolism of quercetin reduces its neurotoxic potential. Arch. Biochem. Biophys. 2008, 478, 195–200.
- Manach, C.; Morand, C.; Crespy, V.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336.
- Yamamoto, N.; Moon, J.H.; Tsushida, T.; Nagao, A.; Terao, J. Inhibitory effect of quercetin metabolites and their related derivatives on copper ion induced lipid peroxidation in human low-density lipoprotein. Arch. Biochem. Biophys. 1999, 372, 347–354.
- Moon, J.H.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285.
- Shafek, R.E.; Shafik, N.H.; Michael, H.N. Antibacterial and antioxidant activities of two new kaempferol glycosides isolated from Solenostemma argel stem extract. Asian J. Plant Sci. 2012, 11, 143–147.
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563.
- Bors, W.; Michel, C.; Schikora, S. Interactions of flavonoids with ascorbate and determination of their univalent redox potentials: A pulse radiolysis study. Free Radic. Biol. Med. 1995, 19, 45–52.
- JØrgensen, L.V.; Skibsted, L.H. Flavonoid deactivation of ferrylmyoglobin in relation to ease of oxidation as determined by cyclic voltammetry. Free Rad. Res. 1998, 28, 335–351.
- Husain, S.R.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491.
- Sichel, G.; Corsaro, C.; Scalia, M.; Di Bilio, A.J.; Bonomo, R.P. In vitro scavenger activity of some flavonoids and melanins against O2−. Free Radic. Biol. Med. 1991, 11, 1–8.
- Bors, W.; Michel, C.; Saran, M. Flavonoid antioxidants: Rate constants for reactions with oxygen radicals. Methods Enzymol. 1994, 234, 420–429.
- Pannala, A.S.; Rice-Evans, C.A.; Halliwell, B.; Singh, S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Commun. 1997, 232, 164–168.
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 1998, 24, 1355–1363.
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. Vitr. 2001, 15, 3–6.
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Vekemans, J.A.J.M.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206.
- Santos, M.R.; Mira, L. Protection by flavonoids against the peroxynitrite-mediated oxidation of dihydrorhodamine. Free Radic. Res. 2004, 38, 1011–1018.
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486.
- Jovanovic, S.V.; Steenken, S.; Simic, M.G.; Hara, H. Flavonoids in Health and Disease; Rice-Evans, C., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 137–161.
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects and safety. Ann. Rev. Nutr. 2002, 22, 19–34.
- Fukumoto, L.; Mazza, G. Assessing antioxidant and prooxidant activity of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604.
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2008, 25, 555–611.
- Rothwell, J.A.; Day, A.J.; Morgan, M.R. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360.
- Broussalis, E.; Killer, M.; McCoy, M.; Harrer, A.; Trinka, E.; Kraus, J. Current therapies in ischemic stroke. Part, A. Recent developments in acute stroke treatment and in stroke prevention. Drug Discov. Today 2012, 17, 296–309.
- Varghese, C.; Oyere, O.; Cowan, M.; Davis, S.; Norrving, B. World Health Organization. Stroke 2016, 47, e210.
- Iadecola, C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997, 20, 132–139.
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C.K. Protective effect of flavonoid-rich extract from Rosa laevigataMichx on cerebral ischemia- reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532.
- Zhou, X.; Wang, H.Y.; Wu, B.; Cheng, C.Y.; Xiao, W.; Wang, Z.Z.; Yang, Y.Y.; Li, P.; Yang, H. Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3beta-dependent increases in mitochondrial membrane permeability. Oncotarget 2017, 8, 44682–44693.
- Li, Z.; Yulei, J.; Yaqing, J.; Jinmin, Z.; Xinyong, L.; Jing, G.; Min, L. Protective effects of tetramethylpyrazine analogue Z-11 on cerebral ischemia reperfusion injury. Eur. J. Pharmacol. 2019, 844, 156–164.
- Xie, L.; Wang, Z.; Li, C.; Yang, K.; Liang, Y. Protective effect of nicotinamide adenine dinucleotide (NAD+) against spinal cord ischemia-reperfusion injury via reducing oxidative stress-induced neuronal apoptosis. J. Clin. Neurosci. 2017, 36, 114–119.
- Fu, J.; Sun, H.; Zhang, Y.; Xu, W.; Wang, C.; Fang, Y.; Zhao, J. Neuroprotective effects of luteolin against spinal cord ischemia-reperfusion injury by attenuation of oxidative stress, inflammation, and apoptosis. J. Med. Food 2018, 21, 13–20.
- Hartings, J.A.; Rolli, M.L.; Lu, X.C.M.; Tortella, F.C. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: Relation to infarct growth and neuroprotection. J. Neurosci. 2003, 23, 11602–11610.
- Hossmann, K.A. Pathophysiology and therapy of experimental stroke. Cell. Mol. Neurobiol. 2006, 26, 1057–1083.
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14.
- Saito, A.; Hayashi, T.; Okuno, S.; Nishi, T.; Chan, P.H. Oxidative stress is associated with XIAP and Smac/DIABLO signaling pathways in mouse brains after transient focal cerebral ischemia. Stroke 2004, 35, 1443–1448.
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 9, 1020–1030.
- Heo, J.H.; Lucero, J.; Abumiya, T.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 624–633.
- Asahi, M.; Asahi, K.; Jung, J.C.; del Zoppo, G.J.; Fini, M.E.; Lo, E.H. Role for matrix metalloproteinase 9 after focal cerebral ischemia. Effects of gene knockout and enzyme inhibition with BB-94. J. Cereb. Blood Flow Metab. 2000, 20, 1681–1689.
- Gu, Z.Z.; Cui, J.; Brown, S.; Fridman, R.; Mobashery, S.; Strongin, A.Y.; Lipton, S.A. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. 2005, 25, 6401–6408.
- Krajewski, S.; Krajewska, M.; Ellerby, L.M.; Welsh, K.; Xie, Z.H.; Deveraux, Q.L.; Salvesen, G.S.; Bredesen, D.E.; Rosenthal, R.E.; Fiskum, G.; et al. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. USA 1999, 96, 5752–5757.
- Cho, S.; Liu, D.; Gonzales, C.; Zaleska, M.M.; Wood, A. Temporal assessment of caspase activation in experimental models of focal and global ischemia. Brain Res. 2003, 982, 146–155.
- Saleem, S.; Zhuang, H.; Biswal, S.; Christen, Y.; Doré, S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke 2008, 39, 3389–3396.
- Hong, J.T.; Yen, J.H.; Wang, L.; Lo, Y.H.; Chen, Z.T.; Wu, M.J. Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells. Toxicol. Appl. Pharmacol. 2009, 237, 59–68.
- Gao, S.S.; Choi, B.M.; Chen, X.Y.; Zhu, R.Z.; Kim, Y.; So, H.; Park, R.; Sung, M.; Kim, B.R. Kaempferol suppresses cisplatin-induced apoptosis via inductions of heme oxygenase-1 and glutamate-cysteine ligase catalytic subunit in HEI-OC1 cells. Pharm. Res. 2010, 27, 235–245.
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F.; Li, Q. Neuroprotection of nicotiflorin in permanent focal cerebral ischemia and in neuronal cultures. Biol. Pharm. Bull. 2006, 29, 1868–1872.
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F.; Li, Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J. Ethnopharmacol. 2006, 107, 143–150.
- Sun, F.; Lopez-Sanchez, C.; Martin-Romero, F.J.; Luis, L.; Gutierrez-Merino, C.; Garcia-Martinez, V. Transfemoral selective “intraluminal wiring” technique for transient middle cerebral artery occlusion in rats. J. Neurosci. Methods. 2005, 149, 82–89.
- Lopez-Sanchez, C.; Martin-Romero, F.J.; Sun, F.; Luis, L.; Samhan-Arias, A.K.; Garcia-Martinez, V.; Gutierrez-Merino, C. Blood micromolar concentrations of kaempferol afford protection against ischemia/reperfusion induced damage in rat brain. Brain Res. 2007, 1182, 123–137.
- Ludolph, A.C.; He, F.; Spencer, P.S.; Hammerstad, J.; Sabri, M. 3-Nitropropionic acid—Exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991, 18, 492–498.
- He, F.; Zhang, S.; Qian, F.; Zhang, C. Delayed dystonia with striatal CT lucencies induced by a mycotoxin (3-nitropropionic acid). Neurology 1995, 45, 2178–2183.
- Beal, M.F.; Brouillet, E.; Jenkins, B.G.; Ferrante, R.J.; Kowall, N.W.; Miller, J.M.; Storey, E.; Srivastava, R.; Rosen, B.R.; Hyman, B.T. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 1993, 13, 4181–4192.
- Brouillet, E.; Jenkins, B.G.; Hyman, B.T.; Ferrante, R.J.; Kowall, N.W.; Srivastava, R.; Roy, D.S.; Rosen, B.R.; Beal, M.F. Age dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J. Neurochem. 1993, 60, 356–359.
- Brouillet, E.; Hantraye, P.; Ferrante, R.J.; Dolan, R.; Leroy-Willig, A.; Kowall, N.W.; Beal, M.F. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc. Natl. Acad. Sci. USA 1995, 92, 7105–7109.
- Brouillet, E.; Conde, F.; Beal, M.F.; Hantraye, P. Replicating Huntington’s disease phenotype in experimental animals. Prog. Neurobiol. 1999, 59, 427–468.
- Tsang, T.M.; Haselden, J.N.; Holmes, E. Metabolomic characterization of the 3-nitropropionic acid rat model of Huntington’s disease. Neurochem. Res. 2009, 34, 1261–1271.
- Menze, E.; Esmat, A.; Tadros, M.G.; Abdel-Naim, A.B.; Khalifa, A.E. Genistein improves 3-NPA-induced memory impairment in ovariectomized rats: Impact of its antioxidant, anti-inflammatory and acetylcholinesterase modulatory properties. PLoS ONE 2015, 10, e0117223.
- Ho, A.K.; Sahakian, B.J.; Brown, R.G.; Barker, R.A.; Hodges, J.R.; Ané, M.N.; Snowden, J.; Thompson, J.; Esmonde, T.; Gentry, R.; et al. Profile of cognitive progression in early Huntington’s disease. Neurology 2003, 61, 1702–1706.
- Phillips, W.; Shannon, K.M.; Barker, R.A. The current clinical management of Huntington’s disease. Mov. Disord. 2008, 23, 1491–1504.
- Hylin, J.W.; Matsumoto, H. Inhibition of succinic dehydrogenase by 3-nitropropanoate. Toxicol. Appl. Pharmacol. 1964, 6, 168–171.
- Alston, T.A.; Mela, L.; Bright, H.J. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc. Natl. Acad. Sci. USA 1977, 74, 3767–3771.
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-Nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006, 281, 5965–5972.
- Zeevalk, G.D.; Derr-Yellin, E.; Nicklas, W.J. NMDA receptor involvement in toxicity to dopamine neurons in vitro caused by the succinate dehydrogenase inhibitor 3-nitropropionic acid. J. Neurochem. 1995, 64, 455–458.
- Nasr, P.; Gursahani, H.I.; Pang, Z.; Bondada, V.; Lee, J.; Hadley, R.W.; Geddes, J.W. Influence of cytosolic and mitochondrial Ca2+, ATP, mitochondrial membrane potential, and calpain activity on the mechanism of neuron death induced by 3-nitropropionic acid. Neurochem. Int. 2003, 43, 89–99.
- Beal, M.F.; Ferrante, R.J.; Henshaw, R.; Matthews, R.T.; Chan, P.H.; Kowall, N.W.; Epstein, C.J.; Schulz, J.B. 3-Nitropropionic acid neurotoxicity is attenuated in copper/zinc superoxide dismutase transgenic mice. J. Neurochem. 1995, 65, 919–922.
- Brouillet, E.; Jacquard, C.; Bizat, N.; Blum, D. 3-Nitropropionic acid: A mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 2005, 95, 1521–1540.
- Schultz, J.B.; Henshaw, D.R.; MacGarvey, U.A.; Beal, M.F. Involvement of oxidative stress in 3-nitropropionic acid neurotoxicity. Neurochem. Int. 1996, 29, 167–171.
- Kim, G.W.; Copin, J.C.; Kawase, M.; Chen, S.F.; Sato, S.; Gobbel, G.T.; Chan, P.H. Excitotoxicity is required for induction of oxidative stress and apoptosis in mouse striatum by the mitochondrial toxin, 3-nitropropionic acid. J. Cereb. Blood Flow Metab. 2000, 20, 119–129.
- Rosenstock, T.R.; Carvalho, A.C.P.; Jurkiewicz, A.; Frussa-Filho, R.; Smaili, S.S. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J. Neurochem. 2004, 88, 1220–1228.
- Gil, J.M.; Rego, A.C. Mechanisms of neurodegeneration in Huntington’s disease. Eur. J. Neurosci. 2008, 27, 2803–2820.
- Lagoa, R.; Lopez-Sanchez, C.; Samhan-Arias, A.K.; Gañán, C.M.; García-Martínez, V.; Gutierrez-Merino, C. Kaempferol protects against rat striatal degeneration induced by 3-nitropropionic acid. J. Neurochem. 2009, 111, 473–487.
- Lagoa, R.; Lopez-Sanchez, C.; Samhan-Arias, A.K.; Gañán, C.M.; García-Martínez, V.; Gutierrez-Merino, C. Neuroprotective effects of kaempferol in the 3-nitropropionic acid model of Huntington’s disease. In Free Radicals, Health and Lifestyle (Proceedings of the SFFR-Europe 2009); Caporossi, D., Pigozzi, F., Sabatini, S., Eds.; Medimond, S.r.l.: Bologna, Italy, 2009; pp. 85–88. ISBN 978-88-7587-515-2.
- Samhan-Arias, A.K.; Martín-Romero, F.J.; Gutiérrez-Merino, C. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive species production at the plasma membrane in the commitment to apoptosis. Free Radic. Biol. Med. 2004, 37, 48–61.
- Schroeter, H.; Spencer, J.P.E.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557.
- Silva, B.; Oliveira, P.J.; Dias, A.; Malva, J.O. Quercetin, kaempferol and biapigenin from hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox. Res. 2008, 13, 265–279.
- Schroeder, E.K.; Kelsey, N.A.; Doyle, J.; Breed, E.; Bouchard, R.J.; Loucks, F.A.; Harbison, R.A.; Linseman, D.A. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antoxid. Redox Signal. 2009, 11, 469–480.
- Nichols, M.; Zhang, J.; Polster, B.; Elustondo, P.; Thirumaran, A.; Pavlov, E.V.; Robertson, G.S. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 2015, 308, 75–94.
- Chitturi, J.; Santhakumar, V.; Kannurpatti, S.S. Beneficial effects of kaempferol after developmental traumatic brain injury is through protection of mitochondrial function, oxidative metabolism, and neural viability. J. Neurotrauma 2019, 36, 1264–1278.
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. BBA Bioenerg. 2011, 1807, 1562–1572.
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795.
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13.
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial dysfunction in cardiac disease: Ischemia–reperfusion, aging, and heart failure, J. Mol. Cell. Cardiol. 2001, 33, 1065–1089.
- Wu, B.; Luo, H.; Zhou, X.; Cheng, C.Y.; Lin, L.; Liu, B.L.; Liu, K.; Li, P.; Yang, H. Succinate induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2307–2318.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
457
Revisions:
2 times
(View History)
Update Date:
22 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




