Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carmen Lopez-Sanchez and Version 2 by Peter Tang.
Kaempferol, a flavonoid present in many food products, has chemical and cellular antioxidant properties that are beneficial for protection against the oxidative stress caused by reactive oxygen and nitrogen species. Kaempferol administration to model experimental animals can provide extensive protection against brain damage of the striatum and proximal cortical areas induced by transient brain cerebral ischemic stroke and by 3-nitropropionic acid.
- kaempferol
- flavonoids
- brain stroke
- ischemia reperfusion
- 3-nitropropionic acid
- Huntington’s disease
- brain neurodegeneration
- pharmacological implications
1. Introduction
Kaempferol is a polyphenol of the flavonol family of flavonoids. Therefore, kaempferol’s chemical structure (Figure 1) is closely related to other flavonols with beneficial chemotherapeutic properties, like quercetin [1][2][1,2]. Kaempferol, like other flavonoids, is largely present as a glycoside derivative in plants. Apart from the most abundant glycoside derivatives, kaempferol-3-O-glucoside (astragalin), kaempferol-3,7-dirhamnoside (kaempferitrin) and kaempferol-3-O-rutinoside (nicotiflorin) [3], many other bioactive kaempferol glycosides have been found in plants, like kaempferol 3-O-[(6-O-E-caffeoyl) β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside, kaempferol 3-O-[(6-O-E-p-coumaroyl)-β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside and kaempferol 3-O-[(6-O-E-feruloyl)-β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside [4][5][4,5].
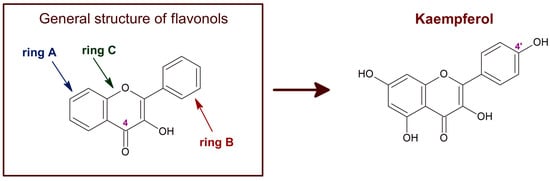
Figure 1. Chemical structure of flavonols in general and of kaempferol. The C4 position of the characteristic carbonyl group of flavonols is marked. Kaempferol, also known as 3,4′,5,7-tetrahydroxyflavone [chemical name: 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one], bears a single hydroxyl group in ring B, at position 4′.
Kaempferol and its glycoside derivatives can be found in a variety of plants and plant-derived food products, such as spinach, kale, cabbage, chives, lentils, tea, broccoli, apples, and Ginkgo biloba, among others [5][6][7][8][9][5,6,7,8,9]. The plasma concentrations of total flavonoid metabolites vary from 0 to 2.0 μM, with an intake of 50 mg aglycone equivalents in humans, although isoflavones can reach higher concentrations, as well as metabolites generated by the gut [10][11][10,11]. After food intake, bacterial enzymes and enterocyte β-glucosidases of the intestine hydrolyze flavonoid glycosides to their aglycones [12][13][12,13]. In addition, flavonoid aglycones undergo methylation and glucuronidation in the intestine [14] and their metabolites are further metabolized by the liver [12]. It should be noted that glial cells can also catalyze in vitro oxidation and conjugation of flavonoids with reduced glutathione (GSH) [15]. Although flavonoid metabolites are still bioactive as cellular antioxidants, most of these metabolites have been shown to have lower chemical antioxidant potency than the aglycone flavonoid [16][17][18][16,17,18], and this also seems to be the case of kaempferol [19]. Furthermore, the kaempferol glycosides most abundant in plants (astragalin, kaempferitrin, and nicotiflorin) have poorer antiproliferative activity than kaempferol [20]. Therefore, the medicinal use of kaempferol requires us to pay special attention to the administration route. Also, if the concentration of blood kaempferol needed in therapeutic treatments of brain damage is higher than 1–2 μM, instead of oral supplementation, other methods of administration must be used, i.e., intravenous (IV) or microencapsulation delivery.
The redox potential of kaempferol, ~0.39 V [21][22][21,22], indicates that this compound is a chemical antioxidant. Furthermore, kaempferol, as well as other flavonoids, can form stable radicals that may act as reactive species scavengers [23]. The acceptance of flavonoids as cellular antioxidants is largely based upon their ability to scavenge hydroxyl radicals, superoxide anion, lipid peroxides, and peroxynitrite [23][24][25][26][27][28][23,24,25,26,27,28]. The number of hydroxyl groups in the B-ring and the presence of a carbonyl group in C4 of the C-ring are particularly relevant for the scavenging of these reactive oxygen species (ROS) [27]. Of note, the flavonoids that most efficiently scavenge hydroxyl radicals are flavonols [23], i.e., the family of flavonoids to which kaempferol belongs (Figure 1). Moreover, compared with other phenols and flavonoids, kaempferol has a relatively high peroxynitrite scavenger activity [29][30][29,30]. Another property of flavonoids relevant for their role as cellular antioxidants is their liposolubility. The inhibition of lipid peroxidation by flavonoids correlates with their partition coefficient between n-octanol and water, because flavonoids need to incorporate into the lipid bilayer to trap the species initiating lipid oxidation radical chains [31][32][31,32]. Indeed, flavonoids act as hydrogen donors in the reaction with the peroxyl radical produced in the oxidation of fatty acids [33]. It has been shown that the higher solubility in water can account for the lower potency of flavonoid glycosides as inhibitors of lipid peroxidation compared with their respective flavonoid aglycones [34][35][34,35]. In addition, the solubility of flavonoids in lipid bilayers favors its uptake by cells and tissues. Notably, kaempferol is one of the flavonoids with a higher lipid/water partition coefficient [36].
2. Kaempferol Administration Efficiently Prevents Brain Damage Induced by Ischemic Insults
The brain ischemic insult elicited by a stroke episode is a major health problem in humans, as they often cause death or chronic disability. Ischemia stroke is the most frequent type of cerebrovascular stroke [37][38][49,50]. The transient blockade of blood flow produces rapid tissue damage in the so-called ischemic core. The rapid metabolic energy falls, leading to the depolarization of cell membranes, which causes rapid necrosis in the ischemic core. Later, the brain damage extends into the peri-infarct region [39][51]. Restoration of blood flow (reperfusion) is necessary, but this can aggravate brain damage [40][41][52,53], as it leads to disability and a high morbidity in patients due to irreversible brain damage. The excessive production of ROS and reactive nitrogen species, excitotoxicity, and brain inflammation are implicated in the neuronal damage during a brain ischemia-reperfusion injury [42][54]. Of note, oxidative stress-mediated inflammation and apoptosis are considered to play a crucial role in brain damage associated with ischemia-reperfusion episodes [43][44][55,56]. Glutamate excitotoxicity [39][45][46][51,57,58], oxidative stress [39][47][48][51,59,60], edema and inflammation [39][46][51,58], activation of matrix metalloproteinases [49][50][51][52][61,62,63,64], and apoptosis [48][53][54][60,65,66] contribute to spreading the brain damage to the peri-infarct region. The slow time course of this phase opens a temporal window for neuroprotective interventions. Protective effects of different flavonoids against the ischemic brain damage produced by cerebral ischemia have been shown in many studies with experimental animals, reviewed in [1]. As noted in [1], these studies showed that the efficiency of protection against brain damage in transient cerebral ischemia largely varies between different flavonoids and depends on the administration route and doses. For example, the neuroprotective action of Ginkgo biloba extract EGb761, which is rich in kaempferol, in brain ischemia models seems to be dependent on the expression of heme oxigenase-1 [55][67], an enzyme that is modulated by kaempferol in different cells [56][57][68,69]. Also, nicotiflorin (kaempferol-3-O-rutinoside) has neuroprotective effects in permanent [58][70] and transient [59][71] models of rat cerebral ischemia. To study the effect of the IV administration of kaempferol on brain damage induced by ischemia-reperfusion, first thwe researchers sesetup a rat model of transient focal ischemia using a new surgical technique of middle cerebral artery occlusion (MCAO) by selective endovascular placement of a guide-wire [60][72]. The Ouresearchers' study published in 2007 [61][41] has been, to the best of the ouresearchers' knowledge, the first work that reported on the role of kaempferol administration in the protection against the brain damage caused by transient cerebral ischemia. The main results and conclusions obtained in this work are summarized next (Figure 2 and Figure 3).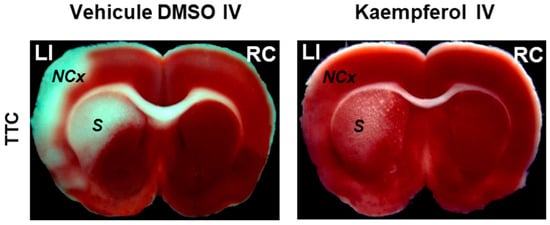
Figure 2. Kaempferol protects against ischemia/reperfusion-induced brain damage. The images of brain slices stained with 2,3,5-triphenyltetrazolium chloride (TTC) show the reduction of the damage extension in brain areas (NCx: neocortex and S: striatum) after kaempferol treatment in rats subjected to ischemia/reperfusion. LI and RC mean left ischemic and right control hemispheres, respectively. Meaning of other abbreviations used in this figure: DMSO (dimethyl sulfoxide) and IV (intravenous administration).
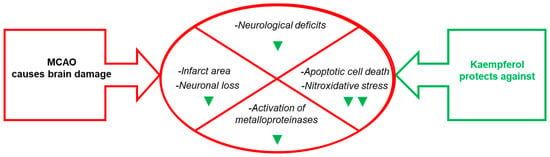
Figure 3. Effects of kaempferol administration highlighted in this section. Kaempferol reduces MCAO- induced brain damage in the infarct area, neurological deficits, apoptotic cell death and neuronal loss, as well as the levels of biomarkers of brain damage. The green symbols indicate attenuation by kaempferol: ▼ and ▼▼ mean partial and nearly complete attenuation, respectively.
3. Kaempferol Administration Efficiently Prevents Brain Damage Induced by 3-Nitropropionic Acid
The 3-nitropropionic acid (NPA) is a neurotoxin for cattle and humans and is produced by some fungi and plants [62][63][90,91]. This neurotoxin can induce brain striatum degeneration and neurological disturbances that mimic some aspects of Huntington’s disease (HD) when administered systemically to rodents and non-human primates [64][65][66][67][92,93,94,95]. The systemic administration of NPA also produces metabolic alterations in cortical areas adjacent to the striatum, as well as in the hippocampus and the cerebellum [68][69][96,97], which can account for neurological alterations seen in pre-motor stages of HD, like cognitive dysfunction, visuospatial deficits, memory loss, and difficulty in learning new skills [70][71][98,99].
NPA is a suicide inhibitor of succinate dehydrogenase [72][73][74][100,101,102] and causes the rapid loss of ATP in cultured neurons in vitro [75][76][103,104]. The enhanced generation of ROS that activate cell death pathways due to the impairment of mitochondrial function has been accepted to play a major role in the neurotoxicity of NPA [66][76][77][78][79][80][81][94,104,105,106,107,108,109]. The relevance of NPA-induced striatal degeneration models as animal models of HD is further supported by reported anomalies in mitochondrial function and oxidative stress in the brain degeneration of HD patients [78][82][106,110].
To the best of the ouresearchers' knowledge, the researchers'our work [83][43] was the first report of kaempferol protection against NPA-induced brain damage and associated neurological dysfunctions in an animal model. In this work thwe researchers used chronic treatment of Wistar rats with intraperitoneal (IP) injections of 25 mg of NPA/kg of body weight (BW) in normal saline (0.9% w/v NaCl) every 12 h during several days. ThWe researchers assessed that this treatment induced neurological disturbances (hypoactivity, dystonic movements of hind limbs, and an abnormal gait) and striatal degeneration similar to those reported in previous publications of other investigators [64][67][92,95]. ThWe researchers shshowed that IP injections of 21 mg of kaempferol/kg BW 48 h before the first NPA treatment and every day 30 min prior to the morning NPA injection largely attenuated NPA-induced neurological disturbances [83][84][43,44]. Also, theour results pointed out an NPA-induced lesion highly localized in the striatum, because all the histological markers used (TTC, hematoxylin-eosin and TUNEL staining) did not show a significant increase in the vicinal brain cortical area, and IP daily doses of 21 mg of kaempferol/kg BW afforded an almost complete blockade of the striatal lesion (Figure 4).
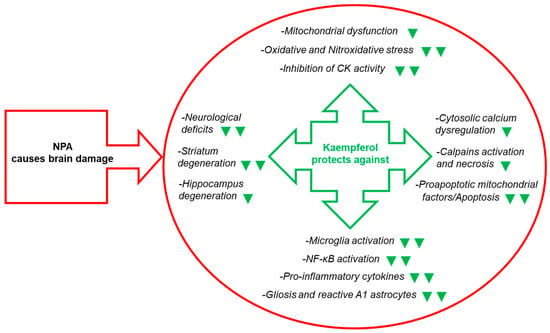

Figure 4. Kaempferol reduces NPA-induced brain lesion, neurological dysfunctions, and, also, the levels of many biochemical and cellular biomarkers of brain degeneration. The green symbols indicate attenuation by kaempferol of the NPA-induced change: ▼ and ▼▼ mean partial and nearly complete attenuation, respectively. Abbreviations used in this figure: CK (creatine kinase) and NF-κB (nuclear factor kappa light-chain enhancer of activated B cells).
4. Molecular and Cellular Mechanisms That Contribute to Kaempferol Protection against the Brain Damage Produced by Ischemia-Reperfusion and by NPA Administration
A mitochondrial energetics failure is the initial event in the brain degeneration induced by both ischemic injury (oxygen supply shortage) and NPA administration (inhibition of mitochondrial succinate dehydrogenase) (Figure 5). Notably, in vitro studies have shown that flavonoids can afford an effective protection in the 1–10 μM range in cell death models where mitochondrial dysfunction and apoptosis are implicated [85][86][87][88][89][40,152,153,154,155]. More recently, it has been noted that the beneficial effects of kaempferol after traumatic injury in the rats developing brain (a model system used for understanding the molecular mechanisms underlying the distinct neuropathological consequences of traumatic brain injury in children) is through protection of mitochondrial function, oxidative metabolism, and neural viability [90][156]. As shown in [91][45], kaempferol was one of the most potent flavonoids as an inhibitor of the rate of H2O2 production by brain mitochondria, which reaches nearly 100% inhibition with 10 μM flavonoid with 50% inhibition concentration of 1.8 μM. However, up to 10 μM kaempferol did not significantly affect the oxygen consumption rate of brain mitochondria [87][91][45,153]. The major systems involved in ROS production by respiring mitochondria are mitochondrial respiratory complexes I and III [92][93][157,158]. In [91][45], thwe researchers identified the mitochondrial respiratory complex I as the molecular target of kaempferol for the inhibition of mitochondrial ROS production, and found kinetic competition between CoQ and kaempferol modulation of the activity of complex I, suggesting that kaempferol binds to complex I at a site, at least, partially overlapping with the quinone-inhibitor binding pocket. It should be recalled here that mitochondrial respiratory complex I has been proposed to be the source of ROS in models of heart failure and, also, the initial site of ischemia-elicited damage to mitochondria in the heart [94][159]. In addition, an impaired function of the mitochondrial respiratory complex I is well documented in several neurodegenerative diseases [92][157]. Notably, oral kaempferol administration decreases mitochondrial fission and helps to preserve mitochondrial functional integrity and morphology in a C57BL/6 mice MCAO model of ischemic stroke [95][77].
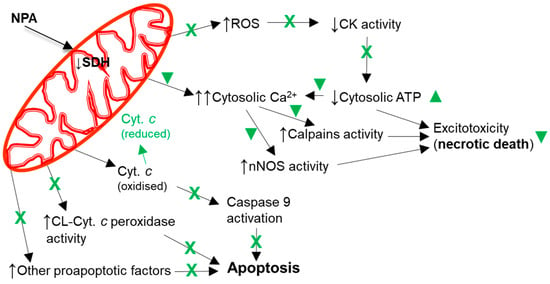

Figure 5. Kaempferol protects against mitochondrial dysfunctions leading to cellular bioenergetics crisis and apoptosis. Black arrows indicate the effects of NPA administration. The reduction of cytochrome c by kaempferol is highlighted in green. A green cross (X) means almost complete protection and a green symbol (▼) means partial protection. Abbreviations used in this figure: CK (creatine kinase), CL-Cyt. c peroxidase (cardiolipin-induced cytochrome c peroxidase activity), Cyt. c (cytochrome c), and SDH (succinate dehydrogenase).
