Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Coscarella | -- | 2956 | 2024-02-21 11:54:59 | | | |
| 2 | Sirius Huang | Meta information modification | 2956 | 2024-02-22 02:54:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Coscarella, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Characteristics and Extraction Capacity of Deep Eutectic Solvents. Encyclopedia. Available online: https://encyclopedia.pub/entry/55288 (accessed on 07 February 2026).
Coscarella M, Nardi M, Alipieva K, Bonacci S, Popova M, Procopio A, et al. Characteristics and Extraction Capacity of Deep Eutectic Solvents. Encyclopedia. Available at: https://encyclopedia.pub/entry/55288. Accessed February 07, 2026.
Coscarella, Mario, Monica Nardi, Kalina Alipieva, Sonia Bonacci, Milena Popova, Antonio Procopio, Rosa Scarpelli, Svilen Simeonov. "Characteristics and Extraction Capacity of Deep Eutectic Solvents" Encyclopedia, https://encyclopedia.pub/entry/55288 (accessed February 07, 2026).
Coscarella, M., Nardi, M., Alipieva, K., Bonacci, S., Popova, M., Procopio, A., Scarpelli, R., & Simeonov, S. (2024, February 21). Characteristics and Extraction Capacity of Deep Eutectic Solvents. In Encyclopedia. https://encyclopedia.pub/entry/55288
Coscarella, Mario, et al. "Characteristics and Extraction Capacity of Deep Eutectic Solvents." Encyclopedia. Web. 21 February, 2024.
Copy Citation
A renewed understanding of eco-friendly principles is moving the industrial sector toward a shift in the utilization of less harmful solvents as a main strategy to improve manufacturing. Green analytical chemistry (GAC) has definitely paved the way for this transition by presenting green solvents to a larger audience. Among the most promising, surely DESs (deep eutectic solvents), NaDESs (natural deep eutectic solvents), HDESs (hydrophobic deep eutectic solvents), and HNaDESs (hydrophobic natural deep eutectic solvents), with their unique features, manifest a wide-range of applications, including their use as a means for the extraction of small bioactive compounds.
bioactive compounds
environmental extraction
green chemistry
deep eutectic solvent
1. Introduction
As established by the development plan of the UN, there is a need for a more sustainable chemistry that looks after the environmental impact and sustainability of the methods employed. The new EU environmental policies and legislations for the period 2010–2050 [1] are calling, in fact, on a drastic reduction in solvents from non-renewable resources (e.g., fossil fuel): harmful volatile organic compounds’ use, although highly effective in many applications, must decrease [2][3][4][5][6][7][8][9][10][11][12]. High toxicity, high flammability, and non-biodegradability, being their main shortcomings, have exerted an intolerable pressure to the environment via unsustainable emissions. The green branch of chemistry, through green analytical chemistry (GAC), has investigated this challenge so far. Guided by 12 principles postulated in 2013 [13], GAC researchers have continuously strove to enhance the environmental performance from classical methods. As a result, greener options have emerged over petrochemicals. In particular, the new generation of ionic liquid (ILs), e.g., deep eutectic solvents (DESs) [14][15][16][17][18][19] and natural eutectic solvents (NaDESs) [20], are overtaking the way in many fields, as also witnessed in the scholar sector by an increasingly higher number of related publications each year. Indeed, DESs and their natural equivalents, NaDESs, have found a wide series of applications, including drug discovery [21][22][23][24] and drug delivery systems [25], employment as therapeutic deep eutectic solvents (THEDESs) [26][27], production of new nano-materials [28][29], desulfurization of fossil fuels [30], chromatography [31], organic synthesis [32], removal of environmental contaminants and separation of azeotropes [33], isolation and fractionation of compounds [34], and many more [35]. Likewise, in the food, cosmetic and pharmaceutical industry, following the view of the FAO for a circular economy based on zero waste, DESs and NaDESs are finding more and more space as a plausible means to extract bioactive compounds from several natural sources such as plants, vegetables, fruits and animals [36], as well as from different by-products and waste materials of the food and agri-food chain [37], with, to date, the majority of the studies on the discipline focused on the extraction of bioactive small molecules [38] and only a minority focused on the DESs’ extraction of bioactive biological macromolecules (e.g., proteins, carbohydrates, and lipids) [39].
Solvents designates as ‘green solvents’, such as subcritical water, supercritical fluids, ionic liquids, DESs and HDESs are all characterized by excellent properties, posing little to no toxicity to human health and to the environment, being more sustainable and bio-renewable in respect to the already existing hazardous [40]. The two latter however, which are often defined together with the term DESs [41] or sometimes also as deep eutectic liquids (DELs) [42] or as low-transition temperature mixtures (LTTMs), are the most promising and excel even over biomass-derived solvents [43]. Furthermore, their natural equivalents, i.e., NaDESs and HNaDESs, among many pros, for instance, also tend to more easily meet the GRAS (generally recognized as safe) requirements for solvents by the FDA [44], being that the chemical nature of NaDESs are fully compliant with the REACH Regulation. Moreover, it is accepted that DESs and NaDESs are less toxic than most organic solvents, and NaDESs are less toxic than DESs [45]. DESs have received increasing interest in a wide variety of chemical transformations [46][47]. In recent years, considerable attention has been paid to the application of DESs in the formation of amide bonds and in reactions for the protection of functional groups [15][16][17][18] (Scheme 1). Furthermore, the possibility of creating new DESs for specific tasks has aroused great interest both in the field of organic synthesis and metals processing [48] and in biomass [49][50][51][52][53].
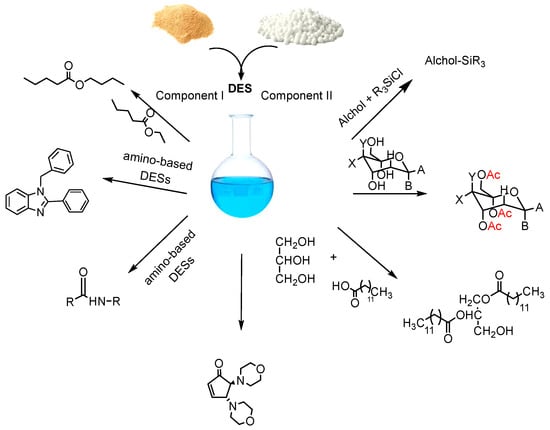
Scheme 1. Organic synthetic process using DESs.
The peculiar and tailorable features of DESs, their versatility and ease of preparation, have made them gain a lot of attention especially in the extraction field of valuable compounds: their unique super-molecular structure manifests high affinity towards various classes of molecules, resulting in high solubilization power, extraction efficiency, and stabilizing ability [20][54][55][56][57].
2. Definition of DESs
DESs have been a real breakthrough in the ground of green chemistry because of Abbott’s group publications [47][52][58][59]. The name ‘DESs’ takes its origin from ‘eutectic‘, namely as a mixture of compounds that, at a certain well-defined composition, displays a unique and minimum melting point in the phase diagram (Figure 1). The variation in the freezing point at the eutectic composition between a binary mixture of A and B and an ideal mixture can be quantified as ΔTf. This difference is directly influenced by the strength of the interaction between A and B. When the interaction is stronger, ΔTf will also be greater in magnitude. According to the most valued definition of the acronym DESs of Smith et al. [60], DESs (type I–IV) are salts formed by a eutectic mixture of Lewis or Bronsted acids and bases which can contain a variety of anionic and/or cationic species—type V of DESs are not necessarily made by salts but rather by molecular substances. Practically, DESs are formed by mixing, under certain optimal conditions (temperature and stirring time), two or three solid organic or inorganic compounds that do behave as hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) so that they liquefy (at a specific molar ratio) and form a stable eutectic. Depending on the type of the DES complexing agent, there are four/five types of DESs according to different authors: (I) quaternary ammonium salt with an anhydrous metal chloride; (II) quaternary salt with a metal chloride hydrate; (III) quaternary ammonium, sulfonium, or phosphonium salt (HBA) with an HBD compound; (IV) metal halide with an HBD; (V) ‘non-ionic DES‘ that are those in which both components are molecular substances [54][61]. In Figure 2, some common HBDs and HBAs in DES formation are reported; Table 1 summarizes instead the five mentioned DESs types.
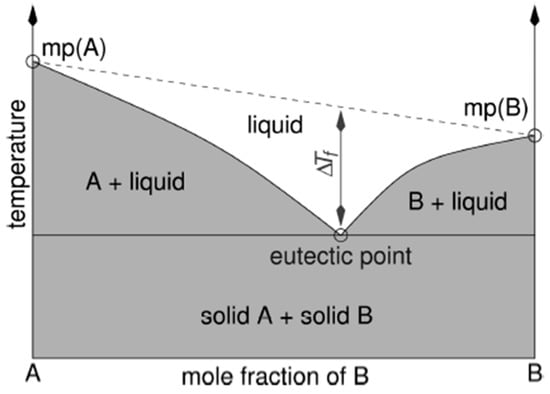
Figure 1. Schematic representation of a eutectic point on a two-component phase diagram [60].
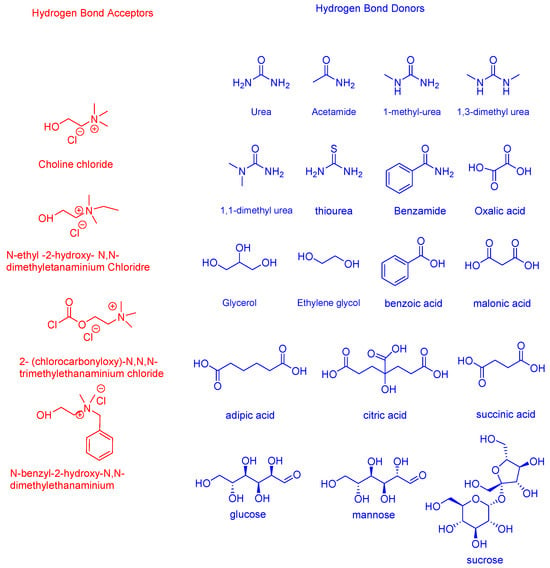
Figure 2. Structures of some halide salts (HBAs in red) and (HBDs in blue) for DESs formation.
Table 1. Classification of the five DESs ty [45].
| Type | General Formula | Terms |
|---|---|---|
| I | Cat+X− zMClx | M = Zn, Sn, Fe, Al, Ga, In |
| II | Cat+X− zMClx yH2O | M = Cr, Co, Cu, Ni, Fe |
| III | Cat+X− zRZ | Z = CONH2, COOH, OH |
| IV | MClx RZ | M = Al, Zn and Z = CONH2, COOH |
| V | Non-ionic DESs | Composed only of molecular substances |
With that being said, the most common type of DESs is type III, in which choline chloride (ChCl) constitutes the quaternary ammonium salt, acting as HBA and either urea, polyalcohols, sugars, amides, organic acids, or PCs are the HBD species. DESs type I and II are used to synthesize hydrophilic DESs, whilst DESs type III and IV are used for hydrophobic DESs [37]. ‘Natural‘ DESs or NaDESs are instead considered as being DES derivatives [41]. The term was coined to distinguish such a liquid made by primary or secondary metabolites of cells; this means that NaDESs are solvents prepared using natural components from cell metabolism. Over 135 primary metabolites based on NaDESs have been found, characterized by high polarity and hydrophilicity [62][63]. This has led to the hypothesis that the existence of natural DESs in living organisms might play an important role as a liquid phase for solubilizing, storing, or transporting non-water-soluble metabolites in living cells and organisms. Recently, another class of eutectic solvents has emerged for the extraction of phenolic compounds; these solvents are known as hydrophobic deep eutectic solvents (HDESs) and hydrophobic natural deep eutectic solvents (HNaDESs) [64][65][66], which are based respectively on, as HBA species, either quaternary ammonium salts with long alkyl chains or hydrophobic natural compounds, both coupled to hydrophobic HBDs such as carboxylic acids or alcohols with long alkyl chains, giving the solvent hydrophobic character. HDESs can be useful for the extraction of some PCs with nonpolar characteristics such as tocopherols [67]; HNaDESs have been employed for the purification of OMWW from endogenous phenol [66][68].
3. Brief Resumé on the Advantages of DESs over ILs
Research on DESs blew up in the early 2000s to attempt to overcoming the at least questionable and weak green character of ILs. In fact, although over conventional solvents, ILs have been exhibited several pros (such as negligible vapor pressure, good thermal properties, wide liquid range, wide solubility and miscibility range, suitability for chemical reactions, and good recycling properties), studies have also highlighted many cons (high preparation costs for large-scale applications that in some cases are ten times higher than for conventional organic solvents; similar or higher toxicity than organic solvents and low biodegradability) [69]. On the other hand, DESs are more inclined towards large scale-up processing, having much easier preparation and greater availability from relatively inexpensive raw material that does not require the tedious and costly dual-step synthesis of ILs that is not even devoid of by-products, while also exhibiting superior biodegradability, lower toxicity, chemical inertness with water, and fine tailorable properties. For these reasons, DESs are more properly considered a class of entirely newly generated fluids [70] rather than a subtype of ILs, as for many, the differences between the two outweigh the similarities. In fact, although DESs and ILs share similar physical properties, such as low volatility, high viscosity, chemical and thermal stability and non-flammability, they differ in the nature of the constituents, in the methods for the formation, and in the type of dominant intermolecular forces involved [71]. More detailed information on ILs can be found in the literature [72].
4. Green Characteristics of DESs
DESs and NaDESs share very similar physicochemical properties (strong ability to dissolve protic molecules, low vapor pressure, and miscibility with water, among others). As said, the most common DESs are type III formed by ChCl with cheap and safe HBDs, with the most popular ones being urea, ethylene glycol, and glycerol, but other alcohols, amino acids, carboxylic acids and sugars have also been quite often used [38]. DESs are characterized by a well-defined composition, which displays a significantly lower melting point in the solid–liquid phase diagram in respect to those of the pure compounds. The strong decrease in freezing point can span up to 200 °C, as in the case of the choline chloride:urea DES prepared at a molar ratio of 1:2 that reaches 12 °C, whilst the corresponding melting points are 302 °C (choline chloride) and 133 °C (urea) [47][52][58][59]. This interesting phenomenon of pure solid compounds that become liquid (at room temperature or at a temperature below 70 °C) by mixing them in a certain molar ratio under mild heat, according to the available instrumental analysis so far [73], is attributed to a decreased lattice energy due to hydrogen-bonding and van der Waals interactions formation [25][49]. For instance, in the case of the mentioned choline chloride:urea DES, the chloride ion of the HBA and the OH group of the HBD strongly link through hydrogen bonds—this interaction could explain the weakening of the lattice energy of the system from which the measured marked depression in melting point arises [74]. Having a solvent that is liquid at room temperature is a plus applicable to an extraction solvent. Another highlight is that the HBA-HBD molar ratio can influence the melting point. For instance, the choline chloride:urea DES at a molar ratio of 1:1 exhibits a melting point > 50 °C [34]. Also, the choice of the HBD partner has an important effect on the resulting melting point. For instance, the use of citric acid, malonic acid, oxalic acid, glycerol, ethylene glycol, and xylitol as HBDs resulted in the formation of DESs with melting temperatures of 69 °C, 10 °C, 34 °C, −40 °C, −66 °C, and room temperature, respectively [39]. More examples reporting the melting point of different combinations of DESs can be found in the review performed by Ling and Hadinoto [39]. For what concerns the production of DESs, this process is relatively straightforward and inexpensive and does not pose any significant post-purification or disposal problems. DESs can be conventionally obtained either by heating-stirring, grinding, evaporation, or freeze-drying. Usually, the components are put in a closed bottle and heated at 60 °C under stirring until a clear liquid appears. In the case of carboxylic acids as HBDs with ChCl, however, it is preferable to choose the grinding option over the heating one, or otherwise by-products (e.g., esters) from the reaction of the two species might form. So, with carboxylic acids, the DES components are rather pounded with a mortar to form the liquid to obtain a purer DES. The evaporation method, then, implies the dissolution of the DES components in water followed by evaporation at 50 °C with the rotary evaporator. The obtained liquid is put in a desiccator with silica gel until it reaches a constant weight [75]. Finally, the freeze-drying approach is less frequently used [76]. Apart from conventional ways, some greener preparation approaches have recently been introduced. Gomez et al. [77] prepared a microwave-assisted method that only needs 20 s of synthesis time to make the DES, while Santana et al. have presented an ultrasound-assisted method [78].
5. The Two Main Characteristics of DESs at the Basis of Their Extraction Performance: Viscosity and Polarity
The extraction performance of DESs is ruled by two principal properties: viscosity and polarity [79]. These two features can be tailored by the addition of water and changes in the extraction conditions (e.g., temperature, solid-to-solvent ratio, choice of the HBD species) [40][80].
The vast majority of DESs shows a higher viscosity at room temperature than many conventional solvents, but they are similar to ILs (>100 cP). The high viscosity of DESs is primarily due to the extensive hydrogen bonding type of interactions between each component of the system but, also, to a lesser extent, to van der Waals and electrostatic interactions [81]. This high parameter can be very beneficial when processing single drop micro-extraction but it limits the extraction applications of DESs in most settings, since it hampers the mass transfer rate between the sample and the extraction phase. Considering the inverse correlation between viscosity and extraction efficiency, it is more appropriate to choose low-viscosity DESs [39][82]. As a rule of thumb for DESs, the higher the number of OH groups present in the system, the more enhanced the hydrogen bond network and the higher the deriving viscosity. This results in limited mass transfer, thus reducing the extraction yield.
The inverse proportion between viscosity and temperature, on the other hand, can be useful in promoting extraction efficiency. In fact, since viscosity decreases significantly when the temperature increases, by raising the temperature up to a certain limit, the internal resistance of molecules decreases, causing the molecules to flow more easily [67]. For instance, the viscosity of glucose:ChCl:water is decreased by 2/3 when the temperature increases from 20 °C to 40 °C. With that being said, a too elevated raise in temperature levels might also impact the chemical bonds and structure of the targeted compounds, leading to thermal degradation and/or oxidation of targeted phenolics, consequently reducing the extraction efficiency [83].
Another option for lowering the viscosity of DESs is represented by playing with the HBD component. For instance, DESs have higher viscosity when sugars and carboxylic acids are employed as an HBD partner, whilst ethylene glycol, glycerol, and phenol as HBD species result in less viscous DESs. As already explained, the higher the number of OHs, the stronger the hydrogen bonding interaction, the higher the viscosity, and the worse the extraction efficiency.
Research has also discovered that adding water to DESs is able to diminish their viscosity. Upon water addition, the weakening of the hydrogen bonding and the increase in osmotic pressure enhance the mass transfer, thus lowering viscosity. This effect stays optimal when the water content is generally in between 20% and 30% (v/v). Above that value, i.e., if the water content goes above 50% (v/v), the hydrogen bonding between the species is so weakened that it even disappears and the DES loses its stability and exists only as a liquid with individual and separated HBA and HBD [84][85].
Aside from viscosity, the other strategic factor for improving the extraction efficiency of DESs is polarity [86]. Indeed, having a solvent with a polarity close to the one of the desired compounds to extract favors solubilization, ultimately empowering the extraction efficiency [87]. The polarity of DESs increases with an increasing proportion of water. This effect was already visible in one of the first studies of Choi et al. [20]. Also, the polarity of DESs varies when different HBD components are used. Organic acid-based DESs are reported to be the most polar (44.81 kcal/mol) and both sugar- and polyalcohol-based DESs are less polar, with a polarity value closer to that of methanol (51.89 kcal/mol) [88]. It is also possible to adjust the polarity of a DES by tailoring the molar ratio of the DES components.
A more comprehensive and detailed list of studies in which the extraction efficiency of DESs is correlated to important analytical parameters can be found in the study of Ali Redha [80] and in the study of Ling et al. [40]. Together with the findings they present, it is possible to draw some conclusions. The viscosity of DESs should be low, otherwise the solubility of the targeted compounds might be not so favorable, resulting in poor extraction efficiency. Change in water content and mild temperature increase can hamper the high viscosity of DESs and facilitate the extraction process. The HBD component can influence the physicochemical properties of DESs. For these reasons, an evaluation of the properties is therefore necessary to ensure optimized extraction performance. Nonetheless, extraction process variables, such as extraction temperature, time, and liquid-to-solid ratio, also play critical roles in the extraction efficiency of target compounds. Lastly, advanced technologies like microwave and ultrasound can boost the extraction performance.
References
- European Environment Agency. Towards a Green Economy in Europe. In EU Environmental Policy Targets and Objectives 2010–2050; European Environment Agency: Copenhagen, Denmark, 2013.
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Romeo, G. Mild and efficient method for the cleavage of benzylidene acetals by using erbium (III) triflate. Org. Biomol. Chem. 2005, 3, 4129–4133.
- Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. An Erbium-Based Bifuctional Heterogeneous Catalyst Erbium-Based: A Cooperative Route Towards C-C Bond Formation. Molecules 2014, 19, 10218–10229.
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A Mesoporous Er(III)-MCM-41 Catalyst for the Cyanosilylation of Aldehydes and Ketones under Solvent-free Conditions. Chem. Sustain. Chem. 2008, 1, 916–919.
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Nardi, M.; Oliverio, M.; Russo, B. Er(OTf)3 as new efficient catalyst for the stereoselective synthesis of C-pseudoglycals. Synthesis 2006, 15, 2608–2612.
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773.
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An Eco-Sustainable Erbium (III)-Catalysed Method for Formation/Cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443.
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Romeo, R. MW-assisted Er(OTf)3-catalyzed mild cleavage of isopropylidene acetals in Tricky substrates. Tetrahedron Lett. 2008, 49, 1961–1964.
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135.
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760.
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269.
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5541.
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84.
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7.
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives. Molecules 2018, 23, 1891.
- Di Gioia, M.L.; Cassano, R.; Costanzo, P.; Herrera Cano, N.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules 2019, 24, 2885.
- Devi, B.K.; Naraparaju, S.; Soujanya, C.; Gupta, S.D. Green Chemistry and Green Solvents: An Overview. Curr. Green Chem. 2020, 7, 314–325.
- Nardi, M.; De Luca, G.; Novelli, P.; Oliverio, M.; Romano, S.; Procopio, A. Amine protection by in situ formation of choline chloride-based deep eutectic solvents. Green Chem. 2023, 25, 3208–3213.
- Romano, S.; Rescifina, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Procopio, A. New Insights on Choline Chloride Role in Synthesis: The Case of Direct Amidation. ACS Sustain. Chem. Eng. 2023, 11, 11668–11680.
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta. 2013, 766, 61–68.
- Voci, S.; Gagliardi, A.; Molinaro, R.; Fresta, M.; Cosco, D. Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels. Gels 2021, 7, 33.
- Gagliardi, A.; Voci, S.; Giuliano, E.; Salvatici, M.C.; Celano, M.; Fresta, M.; Cosco, D. Phospholipid/zein hybrid nanoparticles as promising carriers for the protection and delivery of all-trans retinoic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 128112331.
- Silvia Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Ascorbic acid-loaded gliadin nanoparticles as a novel nutraceutical formulation. Food Res. Int. 2022, 161, 111869.
- Gagliardi, A.; Voci, S.; Ambrosio, N.; Fresta, M.; Duranti, A.; Cosco, D. Characterization and Preliminary In Vitro Antioxidant Activity of a New Multidrug Formulation Based on the Co-Encapsulation of Rutin and the α-Acylamino-β-Lactone NAAA Inhibitor URB894 within PLGA Nanoparticles. Antioxidants 2023, 12, 305.
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195.
- Duarte, A.R.; Ferreira, A.S.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304.
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007.
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50.
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.H.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567.
- Majid, M.F.; Zaid, H.F.M.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J. Mol. Liq. 2020, 306, 112870.
- Cai, T.; Qiu, H. Application of deep eutectic solvents in chromatography: A review. TrAC Trends. Anal. Chem. 2019, 120, 115623.
- Mota-Morales, J.D.; Sánchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153.
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. ChemSusChem 2014, 7, 1784–1800.
- Abdul Hadi, N.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of choline-based deep eutectic solvents in the extraction of tocols from crude palm oil. J. Am. Oil Chem. Soc. 2015, 92, 1709–1716.
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33.
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23.
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897.
- Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381.
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312.
- Durand, E.; Lecomte, J.; Villeneuve, P. From green chemistry to nature: The versatile role of low transition temperature mixtures. Biochimie 2015, 120, 119–123.
- Kurtulbaş, E.; Yazar, S.; Ortaboy, S.; Atun, G.; Şahin, S. Evaluation of the phenolic antioxidants of olive (Olea europaea) leaf extract obtained by a green approach: Use of reduced graphene oxide for electrochemical analysis. Chem. Eng. Commun. 2020, 51, 422–429.
- Gevorgyan, A.; Hopmann, K.H.; Bayer, A. Exploration of New Biomass-Derived Solvents: Application to Carboxylation Reactions. ChemSusChem 2020, 13, 2080–2088.
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.F. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146.
- Della Posta, S.; Gallo, V.; Gentili, A.; Fanali, C. Strategies for the recovery of bioactive molecules from deep eutectic solvents extracts. TrAC Trends Anal. Chem. 2022, 157, 116798.
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 2006, 51, 1280–1282.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Chen, Y.; Zhang, M.; Zhang, S.; Hao, Z.; Zhang, Z. Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes. Green Chem. 2022, 24, 4071–4081.
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Hu, Y.L.; Liu, S.; Lei, T.; Lin, L. Green Processing of Lignocellulosic Biomass and Its Derivatives in Deep Eutectic Solvents. ChemSusChem 2017, 10, 2696–2710.
- Galehassadi, M.; Pourreza, S. Base and Catalyst-Free Preparation of Silyl Ethers in the Choline Chloride/Urea Deep Eutectic Solvent (DES). J. Inorg. Organomet. Polym. Mater. 2019, 29, 541–549.
- Sunitha, S.; Kanjilal, S.; Reddy, P.S.; Prasad, R.B.B. Liquid–liquid biphasic synthesis of long chain wax esters using the Lewis acidic ionic liquid choline chloride· 2ZnCl2. Tetrahedron Lett. 2007, 48, 6962–6965.
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. O-Acetylation of cellulose and monosaccharides using a zinc based ionic liquid. Green Chem. 2005, 7, 705–707.
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237.
- Bart, H.J. Extraction of Natural Products from Plants-An Introduction; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011.
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239.
- Fernández, M.L.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural Deep Eutectic Solvents-Mediated Extractions: The Way Forward for Sustainable Analytical Developments. Anal. Chim. Acta 2018, 1038, 1–10.
- Choi, Y.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 19, 2010–2011.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082.
- Mannu, A.; Blangetti, M.; Baldino, S.; Prandi, C. Promising technological and industrial applications of deep eutectic systems. Materials 2021, 14, 2494.
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705.
- Tao-Xiang, Y.; Li-Qing, Z.; Juan, W.; Guo-Li, S.; Hai-Min, L.; Hui, C.; Zhen, Y. Improving Whole-Cell Biocatalysis by Addition of Deep Eutectic Solvents and Natural Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722.
- van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521.
- Ribeiro, B.D.; Florindo, C.; Iff, L.; Coelho, M.A.Z.; Marrucho, M.I. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. 2015, 3, 2469–2477.
- Buldo, M.; Cicci, A.; Sed, G.; Sapone, V.; Bravi, M. Detoxification of Olive Oil Mill Wastewaters by Liquid-liquid Extraction with Natural Deep Eutectic Solvents. Chem. Eng. Trans. 2019, 74, 1495–1500.
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Hydrophobic Eutectic Solvents for Extraction of Natural Phenolic Antioxidants from Winery Wastewater. Sep. Purif. Technol. 2021, 254, 117590.
- Carmona, I.; Aguirre, I.; Griffith, D.M.; García-Borrego, A. Towards a circular economy in virgin olive oil production: Valorization of the olive mill waste (OMW) “alpeorujo” through polyphenol recovery with natural deep eutectic solvents (NADESs) and vermicomposting. Sci. Total Environ. 2023, 872, 162198.
- Meksi, N.; Moussa, A. A review of progress in the ecological application of ionic liquids in textile processes. J. Clean. Prod. 2017, 161, 105–126.
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs. ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539.
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038.
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706.
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428.
- Santos, L.B.; Assis, R.S.; Barreto, J.A.; Bezerra, M.A.; Novaes, C.G.; Lemos, V.A. Deep eutectic solvents in liquid-phase microextraction: Contribution to green chemistry. TrAC Trends Anal. Chem. 2021, 146, 116478.
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2021, 65, 3591–3601.
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515.
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A greener approach to prepare natural deep eutectic solvents. Chem. Select 2018, 3, 6122.
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452.
- Tolmachev, D.; Lukasheva, N.; Ramazanov, R.; Nazarychev, V.; Borzdun, N.; Volgin, I.; Andreeva, M.; Glova, A.; Melnikova, S.; Dobrovskiy, A.; et al. Computer simulations of deep eutectic solvents: Challenges, solutions, and perspectives. Int. J. Mol. Sci. 2022, 23, 645.
- Redha, A.A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912.
- Tang, B.; Ho, K. Recent developments in deep eutectic solvents in chemical sciences. Monatshefte Für Chem.-Chem. Mon 2013, 144, 1427–1454.
- Espino, M.; de los Ángeles, F.M.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136.
- Chemat, F.; Anjum, H.; Shariff, M.D.; Kumar, P.; Murugesan, T. Thermal and physical properties of (Choline chloride + urea + L-arginine) deep eutectic solvents. J. Mol. Liq. 2016, 218, 301–308.
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166.
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19.
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028.
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970.
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




