During the past decade, a growing body of literature demonstrated the significance of the extent of resection (EOR) as a pivotal prognostic factor in both low-grade and high-grade gliomas, documenting its positive impact in the achievement of higher percentages of overall survival (OS) and progression-free survival (PFS)
[1]. Indeed, these data confirmed the role of surgery as the first-choice treatment for primary intra-axial neoplasms and introduced the concept of “supramarginal” resection (i.e., beyond tumour boundaries) in both symptomatic and incidental tumours
[2]. Traditionally, the perception of supratentorial glioma corresponded to that of a growing mass compressing noble cortical sites, namely those designated to major neurological functions such as movement, language and vision. Consequently, the aggressiveness of the surgical cytoreduction has been conventionally guided by the orthodox subdivision into “eloquent” or “non-eloquent” brain areas in hopes of preserving the fragile equilibrium between the oncological and functional necessities, with few regards to the whole-brain circuitry
[3]. This topological philosophy influenced the whole surgical approach to glioma, resulting in acceptable oncological results and in the minimization of intraoperatively induced neurological deficiencies related to the major domains of language, motricity and visual function
[4][5]. Notwithstanding these advancements, nowadays patients continue to manifest subtle post-operative higher-order cognition impairments, preventing them from reintegration back into a canonical social life and workforce
[6]. Fortunately, past years have witnessed the birth and raising of a completely new scenario in neuroscience, the field of brain connectomics, thanks to the development of the Human Connectome Project (HCP) as of 2010. Indeed, this project paved the way for a profound reappraisal of the traditional concept of a modular brain architecture, starting with the parcellation of each hemisphere into 180 cortical regions, functionally intertwined in harmonious neural networks within a so-called “connectomal” architecture
[7]. In contrast to the conventional “localizationalist” dogma, where isolated cortical areas corresponded to specialized functions, brain connectomics favoured the introduction of a meta-networking theory of cerebral functions, in which adaptive behaviour and complex cognition are mediated by a perpetual succession of equilibrium states within and among constantly interacting delocalized neural circuits
[8]. Therefore, according to this innovative philosophy, the networks designated for cognition, conation, emotion and behaviour are structurally and functionally constituted by a mosaic of cortical areas organized in a fluid framework densely interconnected by subcortical bundles, in a way capable of achieving the minimal energetic dissipation and the maximal flux of information
[9]. Clearly, the actual meaning of this theory lies in the integration of the previous interpretation of brain architecture, founded on the presence of highly specialized and localized modular areas devoted to major neurological functions such as language, motricity and vision, with the idea that higher-order cognitive domains (i.e., cognition, conation, theory of mind, etc.) are instead highly delocalized and sustained by a brain-wide dialogue between sparse intercommunicating networks. Then, thanks to this new concept of brain structural and functional anatomy, neurosurgery is facing a new era of connectome-based tumoral resections, in which the core principle is still represented by the achievement of the ideal onco-functional balance, by maximizing the EOR while avoiding major neurological impairments, but with more attention on global circuitry and more awareness of the risks and benefits of the surgical procedure in a network-wide global perspective.
2. The Connectomal Architecture of Neural Anatomy: Beyond the Concept of “Eloquent” and “Non-Eloquent” Brain
2.1. The Development of a Novel Model of Brain Architecture
Since the seminal works of Broca and Wernicke in the definition of the core brain areas responsible for the articulation and perception of language and Jackson’s efforts to identify the primary motor cortex, the foundations of the traditional conception of brain network organization were established and represented as a fixed framework. In this model, major neurological (i.e., motricity, language and visuospatial organization) and higher-order functions (i.e., conation, cognition) were based on highly modular and specialized cortical regions, in which the occurrence of a lesion, either iatrogenic or pathological, would determine irreversible neurological impairments
[10][11][12]. The neuroscientific progress and the technological improvements contributed to evolve this dogmatic paradigm, proceeding to make this rather inflexible model more dynamic and fluid. Indeed, as demonstrated by Sawaya et al. in 1998, tumours closer to or inside eloquent areas tend to manifest higher rates of neurological complications, and gross tumour resection (GTR) can still be performed with an acceptable grade of functional impairment, proving that the sole eloquent location did not stand as an absolute contraindication to surgery
[13]. This suggests that major neurological functions, traditionally thought to be subserved by highly specialized and isolated brain areas, whose borders are not to be trespassed to avoid major functional impairments, could probably be the product of the dialogue between a more diffuse and sparse ensemble of subnetworks, located in close proximity or even far from the main cortical core (i.e., the frontal operculum—Broca’s area), interconnected by a dense web of subcortical bundles.
2.2. Implications in Epilepsy, Schizophrenia and Depression
Indeed, several conditions such as temporal-lobe epilepsy (TLE) related to mesiotemporal sclerosis and extra-temporal epilepsy (ETLE) due to malformations of cortical development were traditionally interpreted as localization-related diseases, but now have undergone a significative reconceptualization, putting the emphasis on network alterations both at a local and at a global level
[14][15]. In fact, network configuration is now recognized to have a preeminent role either in the generation and propagation of drug-resistant seizures or in the development of cognitive and emotional impairments
[16]. Apart from theoretical considerations, the connexional signature is being studied and investigated in patients destined to temporal lobectomies and extratemporal lesionectomies because of its potential role in surgical planning and in the estimation of the post-operative prognosis
[17][18]. In the case of schizophrenia, several studies proved that functional and structural connexional alterations have a central role in the genesis and natural history of the disease and in the definition of its clinical and cognitive subtypes, even if further evidence has to be documented to remodulate the clinical management of this condition
[19].
2.3. Implications in Neuro-Oncology
In neuro-oncology, as suggested by Duffau et al., the traditional conception of a modular brain organization, characterised by a fairly rigid subdivision of the brain into eloquent and non-eloquent areas, implied at least two major negative consequences in the surgical approach to slow-growing lesions such as DLGG
[3]. Specifically, if the tumour was situated in the presumed “eloquent” cortical regions, patients were less likely to be admitted to surgery, thereby causing a significative loss from an oncological point of view. On the contrary, if the tumour involved areas deemed “non-eloquent”, patients were more likely to be treated without the aid of brain mapping techniques, augmenting the risks of functional impairments.
However, as demonstrated by a sample study from the database of the Glioma Outcome Project published in 2005, the lateralization of the neoplasm, and therefore the involvement of either the dominant hemisphere (DH) or the non-dominant hemisphere (nDH), does not stand as an independent prognostic factor for the prediction of functional outcomes, except for language
[20]. Consequently, hemispheric dominance, for a long time closely related to the concept of eloquence, does not fully explain the complexity of neural architecture and the heterogeneity of neurocognitive deficiencies occurring both prior to and after the surgical treatment. With these premises agreed upon, the ground-breaking information gathered through the completion of the HCP paved the way for a complete reappraisal of the structural and functional organization of neural connectivity, offering an innovative and broader understanding of the wiring diagram of the brain. Particularly, a great amount of the current knowledge on neural architecture has been derived from graph-theoretical analyses of resting-state functional MRI (rsfMRI), which allowed for looking at neural circuitry from a fairly mathematical perspective, thereby representing cortical areas and their connections as a set of nodes interconnected by edges and demonstrating that the intrinsic cerebral connectivity is organized as a small-world network with significant modularity and populated by highly connected hub-regions
[21]. This network architecture can assume an infinite number of synchronization patterns by relying on an incessant succession of equilibrium states through a constant communication between functional systems, resulting in an adaptative behaviour tailored to environmental demands as well as in a pronounced neuroplastic potential responsive to physiological and pathological stimuli
[8]. Aside from more technical graph-theoretical observations, the information gathered from these analyses allowed the description of seven major networks participating in higher-order cognition, of which three constitute the so-called “main cognitive networks”: the default mode network (DMN), the central executive network (CEN) and the salience network (SN)
[22]. Predictably, recent evidence showed that the disruption or the abnormal connectivity in these major systems determines the impairments of higher-order functions and relates to neurocognitive decline and could also form the foundation of several psychiatric disorders such as schizophrenia, depression and anxiety
[23]. Therefore, the HCP and its corollary studies and analyses have provided a plethora of innovative information to further understand the complex nature behind human cognition and adaptive behaviour.
In conclusion, connectomics provides a reinterpretation of the concept of eloquence compared to the traditional topological philosophy. Indeed, rather than merely considering the tumour as an expansive process localized in a certain cortical area, but as a disturbing element plunged in a densely intercommunicating ensemble of networks, with which a mutual dialogue occurs (causing the actuation of both locoregional and global mechanisms of functional reallocation), it is possible to reinterpret the indications and the feasibility of a given surgical strategy and to predict the probability of post-operative neurological deficits from a perspective that takes into account a brain-wide web of neural connections among adjacent or distant brain areas, rather than from that of a rigid subdivision into “eloquent” or “non-eloquent” territories.
3. Application in Cancer Neuroscience: Connectomics for a Gentler and Safer Neurosurgical Act
3.1. Connectome-Based Brain Mapping and Graph-Theoretical Analysis
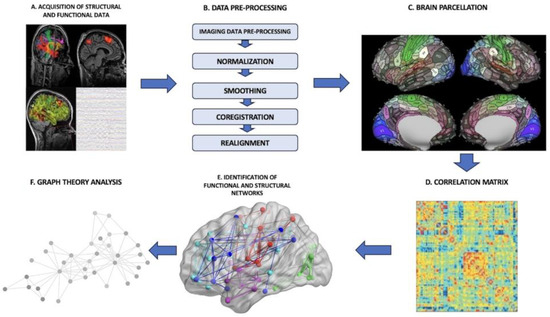
In the pre-operative setting, one of the possible applications of a connectome analysis is to derive an individualized connectomic map for each patient from rsfMRI sequences. The process requires us to follow a specific pipeline constituted by several phases (
Figure 1): (I) There is the acquisition of the imaging data, that is, functional data from rsfMRI or MEG, to construct a functional connectome and, vice versa, DTI or anatomical MPRAGE sequences to build a structural connectome. Depending on the source used to construct the connectome, either functional or structural, the final provided information is different. In the functional connectome, brain areas are paired by defining metabolic or electric connections. In the structural connectome, the final result reflects physical connections among brain areas made by subcortical tracts and white matter bundles. (II) The brain is split into distinct regions through a process known as parcellation, where each parcel represents a so-called “node”. (III) In the case of functional connectomes, the connections among the several nodes are established by extracting the blood-oxygen-level-dependent (BOLD) time series from each parcel and compared with the time series from all other parcels. (IV) The connectedness among the various cortical areas is eventually displayed as a correlation matrix. (V) Further analyses are processed through the application of graph-theoretical methodologies to investigate the properties of individual regions, the links between these regions or the effect of lesioning or perturbation of specific networks
[24].
Figure 1. General connectome-analysis pipeline. (A) Acquisition of structural or functional data; (B) data pre-processing phase; (C) brain parcellation phase; (D) construction of a correlation matrix to establish the connectedness among the various parcels; (E) identification of functional brain networks; (F) application of graph-theoretical analyses to define the properties of networks.
Graph theory is a branch of mathematics that studies networks of connected elements and it is frequently applied in the analysis of complex systems. A graph is defined by point-like components, nodes or vertices, and by the connections between them, edges or vertices. Therefore, neural networks can be represented as a set of nodes (single neurons, neuronal populations, macroscopic brain areas) and edges (structural and functional interactions between them)
[25][26].
3.2. An Insight into the Application of QuicktomeTM for the Identification of Brain Networks in Patients Affected by Primary Intra-Axial Neoplasms
As already mentioned, the main goal of neuro-oncological surgery is to enhance the EOR while providing the best possible neurological outcome. However, the equilibrium between the effectiveness of the surgical act and the occurrence of QoL-impairing post-operative neurological deficits is fragile. Moreover, if we consider that QoL can be severely affected by subtle deficiencies regarding higher-order neurological domains such as personality, memory, executive and semantic functions or metacognition, it is evident that a deeper knowledge of the “non-traditional” eloquent areas is needed to provide an all-around evaluation of every single-case to ensure the best treatment to the individual patient. Then, the traditional concept of a modular subdivision of the brain into eloquent or non-eloquent areas seems to not fully encompass its complexity and wide-scale interactions anymore. On the other hand, the integration of this traditional philosophy with the innovative perspective provided by the discipline of connectomics could allow the definition of a brain mapping paradigm in which the outcome, that is, a given neurological function, is the product of a choral dialogue among both adjacent and distant networks and subnetworks, instead that of highly modular and isolated noble cortical sites.
Several imaging techniques are routinely used in the pre-operative neurosurgical setting to identify the relationship between the tumour and eloquent cortical sites. fMRI, DTI and repetitive navigated transcranial magnetic stimulation (rTMS) are effective methodologies that, apart from intrinsic technical limitations, require expert personnel, and are time-consuming, expensive and often not available at many institutions.
Quicktome
TM (Ominiscient Neurotechnology; Sydney, Australia) is a Food and Drug Administration (FDA)-approved novel software based on the HCP that uses machine learning and reparcellation techniques to accurately identify the brain network and that can be integrated into conventional neuronavigation systems. The main advantage of this cloud-based software is that it provides an individual version of the HCP Multi-Model parcellation version 1 atlas (HCP-MMP1 atlas)
[7] based on DTI-structural connectivity and not on anatomy-based methods to overcome the limitations induced by a distorted neuroanatomy
[27]. This system was created firstly by training a machine learning model on 200 normal adult brains according to a specific processing pipeline of DTI and T1 sequences
[27]. Secondly, the HCP-MMP1 atlas in Montreal Neurologic Institute (MNI) space was warped onto each brain to establish structural connectivity between each pair of cortical parcels, eight subcortical regions per hemisphere and the brainstem.
4. Conclusions
The ground-breaking innovations introduced by the discipline of connectomics paved the way for a better understanding of the complex functional and structural architecture of the brain. The interpretation of the substrata underpinning higher-order cognition and complex behaviour has switched from the traditional localizationalist dogma to a new meta-networking theory of brain functions. The analysis of brain connectivity through graph-theoretical measures could improve the understanding and interpretation of the neurological substrata of complex cognition and adaptative behaviour. The application of a connectome-based approach through the implementation of novel technologies based on machine learning offers new perspectives in the setting of intracerebral surgery for primary intra-axial neoplasms.