
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Mariel Berosich | -- | 796 | 2024-02-21 06:52:01 | | | |
| 2 | Vivi Li | Meta information modification | 796 | 2024-02-21 07:08:40 | | |
Video Upload Options
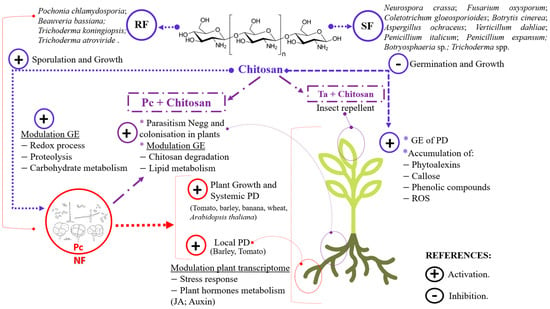
Nematophagous fungi (NFs), which are responsible for soil suppression of plant-parasitic nematodes, are multitrophic biocontrol agents. This raises the question of the transition between lifestyles (e.g., endophytism vs. egg parasitism). The NF Pochonia chlamydosporia colonises food crops and promotes their growth and yield. When colonising the plant, P. chlamydosporia induces the plant immunity (PI). However, it also evades the PI. To do this, both endophytic NF and pathogenic fungi (PF) secrete LysM effectors (LysM-effs). LysM effectors have been shown to have diverse functions in different organisms, including the protection of fungal chitin from plant chitinases. P. chlamydosporia is resistant to chitosan, which modulates gene expression in fungi and plants and has antimicrobial properties. P. chlamydosporia chitin deacetylases (CDA) and chitosanases (CSN) also help P. chlamydosporia evade plant immunity, resist exogenous chitosan, and are induced during fungal infection of nematode eggs. NF-chitosan formulations are new biomanagement tools against plant parasitic nematodes, fungal wilt pathogens and insect pests that currently threaten food security crops. Furthermore, omics techniques are useful tools to elucidate the role of CDAs, CSNs, LysM-effs, adhesion proteins and carbohydrate-active enzymes in pathogen–BCA–plant interactions, adhesion and infection to nematode eggs and their modulation by chitosan.
References
- Bushley, K.E.; Raja, R.; Jaiswal, P.; Cumbie, J.S.; Nonogaki, M.; Boyd, A.E.; Owensby, C.A.; Knaus, B.J.; Elser, J.; Miller, D.; et al. The Genome of Tolypocladium inflatum: Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster. PLoS Genet. 2013, 9, e1003496.
- Sung, G.-H.; Poinar, G.O.; Spatafora, J.W. The oldest fossil evidence of animal parasitism by fungi supports a Cretaceous diversification of fungal–arthropod symbioses. Mol. Phylogenet. Evol. 2008, 49, 495–502.
- Bent, E.; Loffredo, A.; McKenry, M.V.; Becker, J.O.; Borneman, J. Detection and Investigation of Soil Biological Activity against Meloidogyne incognita. J. Nematol. 2008, 40, 109–118.
- Lamovsek, J.; Urek, G.; Trdan, S. Biological control of root-knot nematodes (Meloidogyne spp.): Microbes against the pests. Acta Agron. Slov. 2013, 101, 263–275.
- Bordallo, J.J.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J.; Persmark, L.; Asensio, L. Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol. 2002, 154, 491–499.
- Singh, S.; Sharma, A. Fungi as Biological Control Agents. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, The Netherlands, 2019; Volume 55.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332.
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin Deacetylases: Structures, Specificities, and Biotech Applications. Polymer 2018, 10, 352.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol. 2016, 120, 572–585.
- Zare, R.; Gams, W.; Evans, H.C. A revision of Verticillium section Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwig. 2001, 73, 51–86.
- Berlemont, R. Distribution and diversity of enzymes for polysaccharide degradation in fungi. Sci. Rep. 2017, 7, 222.
- Andreou, A.; Giastas, P.; Christoforides, E.; Eliopoulos, E.E. Structural and Evolutionary Insights within the Polysaccharide Deacetylase Gene Family of Bacillus anthracis and Bacillus cereus. Genes 2018, 9, 386.
- Sun, H.; Gao, L.; Xue, C.; Mao, X. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796.
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867.
- Geoghegan, I.A.; Gurr, S.J. Chitosan Mediates Germling Adhesion in Magnaporthe oryzae and Is Required for Surface Sensing and Germling Morphogenesis. PLoS Pathog. 2016, 12, e1005703.
- Christodoulidou, A.; Bouriotis, V.; Thireos, G. Two Sporulation-specific Chitin Deacetylase-encoding Genes Are Required for the Ascospore Wall Rigidity of Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 31420–31425.
- Sánchez-Vallet, A.; Mesters, J.R.; Thomma, B.P. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol. Rev. 2015, 39, 171–183.
- White, S.; McIntyre, M.; Berry, D.R.; McNeil, B. The autolysis of industrial filamentous fungi. Crit. Rev. Biotechnol. 2002, 22, 1–14.
- Zhao, Y.; Park, R.-D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46.
- Cord-Landwehr, S.; Melcher, R.L.; Kolkenbrock, S.; Moerschbacher, B.M. A chitin deacetylase from the endophytic fungus Pestalotiopsis sp. efficiently inactivates the elicitor activity of chitin oligomers in rice cells. Sci. Rep. 2016, 6, 38018.
- Liu, T.; Chen, Y.; Tian, S.; Li, B. Crucial Roles of Effectors in Interactions between Horticultural Crops and Pathogens. Horticulturae 2023, 9, 250.
- Lopez-Llorca, L.V.; Gómez-Vidal, S.; Monfort, E.; Larriba, E.; Casado-Vela, J.; Elortza, F.; Jansson, H.B.; Salinas, J.; Martín-Nieto, J. Expression of serine proteases in egg-parasitic nematophagous fungi during barley root colonization. Fungal Genet. Biol. 2010, 47, 342–351.
- Larriba, E.; Jaime, M.D.; Carbonell-Caballero, J.; Conesa, A.; Dopazo, J.; Nislow, C.; Martín-Nieto, J.; Lopez-Llorca, L.V. Sequencing and functional analysis of the genome of a nematode egg-parasitic fungus, Pochonia chlamydosporia. Fungal Genet. Biol. 2014, 65, 69–80.
- Aranda-Martinez, A.; Lenfant, N.; Escudero, N.; Zavala-Gonzalez, E.A.; Henrissat, B.; Lopez-Llorca, L.V. CAZyme content of Pochonia chlamydosporia reflects that chitin and chitosan modification are involved in nematode parasitism. Environ. Microbiol. 2016, 18, 4200–4215.
- Aranda-Martinez, A.; Grifoll-Romero, L.; Aragunde, H.; Sancho-Vaello, E.; Biarnés, X.; Lopez- Llorca, L.V.; Planas, A. Expression and specificity of a chitin deacetylase from the nematophagous fungus Pochonia chlamydosporia potentially involved in pathogenicity. Sci. Rep. 2018, 8, 2170.
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environ. Microbiol. 2021, 23, 4980–4997.
- Suarez-Fernandez, M.; Aragon-Perez, A.; Lopez-Llorca, L.V.; Lopez-Moya, F. Putative LysM Effectors Contribute to Fungal Lifestyle. Int. J. Mol. Sci. 2021, 22, 3147.




