Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | CARLOS TORRES-TORRES | -- | 2424 | 2024-02-20 19:48:07 | | | |
| 2 | Rita Xu | -7 word(s) | 2417 | 2024-02-21 03:03:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Medrano-Lopez, J.A.; Villalpando, I.; Salazar, M.I.; Torres-Torres, C. Hierarchical Nanobiosensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/55249 (accessed on 07 February 2026).
Medrano-Lopez JA, Villalpando I, Salazar MI, Torres-Torres C. Hierarchical Nanobiosensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/55249. Accessed February 07, 2026.
Medrano-Lopez, Jael Abigail, Isaela Villalpando, Ma Isabel Salazar, Carlos Torres-Torres. "Hierarchical Nanobiosensors" Encyclopedia, https://encyclopedia.pub/entry/55249 (accessed February 07, 2026).
Medrano-Lopez, J.A., Villalpando, I., Salazar, M.I., & Torres-Torres, C. (2024, February 20). Hierarchical Nanobiosensors. In Encyclopedia. https://encyclopedia.pub/entry/55249
Medrano-Lopez, Jael Abigail, et al. "Hierarchical Nanobiosensors." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Nanostructures have played a key role in the development of different techniques to attack severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Some applications include masks, vaccines, and biosensors. The latter are of great interest for detecting diseases since some of their features allowed us to find specific markers in secretion samples such as saliva, blood, and even tears.
SARS-CoV-2
hierarchical nanostructures
nanoparticles
1. Introduction
Sensors based on hierarchical nanostructures in the area of nanomedicine have been meticulously investigated in order to identify different enzymes and organisms such as bacteria or viruses. Biosensors are fascinating instruments that basically serve to detect biological or chemical parameters such as those related to molecules in tissues, microorganism cultures, and nucleic or acid chains [1]. The characteristics related to biodetection like selectivity, response speed, and stability depend on the morphology and structure of the sensing materials [2].
The main types of sensors used in biodetection are electrochemical [3], thermometric [4], piezoelectric [5], magnetic [6], and optical sensors (plasmonic [7], UV-Vis/infrared spectroscopy [8], Raman and SERS [9], or attenuated total reflection [10]). Biosensors that are developed using hierarchical nanostructures can be manufactured with different nanomaterials. For example, nanohybrids can be integrated into diverse materials such as noble metals [11], graphene [12], copper, titanium [13], zinc oxide [14], and bimetallic oxide [15], among others. The biosensors can be classified into three groups according to their mechanisms: the biocatalytic group that uses enzymes, bioaffinity group that involves antibodies and nucleic acids, and microorganism group that uses microbes [16].
A strong selective control of the manufacturing parameters of noble metals is possible [17], allowing their structure to be modified [18] to improve their physicochemical properties and adjust their shape [19]. There are multiple techniques for designing nanobiosensors, but the most common ones are based on electrochemical deposition [20], electroless deposition [21], electrocatalysts [22], and physicochemical methods [23].
Besides different processing routes that have been extensively explored to improve biosensing effects, the use of the LSPR phenomenon is very attractive [24], and the development of hierarchical nanostructured biosensors can promote exceptional optical, electrical, and chemical properties based on LSPR. Some of the special characteristics exhibited by hierarchical nanostructures are derived from their ultra-high specific surface area, high flexibility, light weight, high electrical conductivity, and bio-compatibility [25][26][27][28][29][30].
The hierarchical nanostructures are replacing conventional random hybrids in counterparts thanks to their physical characteristics, stability, and efficient transfer of electronic and ionic charges [31][32]. For example, their morphologies show a high surface area with adjustable porosity or packing density. Some hierarchical assemblies serve as programmable scaffolds that provide molecule-level control over the distribution of fluorophores and nanometer-scale control over their distance. Several strategies can be used to study imperfections and to stabilize various types of nanostructures, such as hollow ones [33] or cage frames to obtain a better performance [34].
It is worth noting that hierarchical metamaterials have been reported for the development of virus-based light learning systems, in plasmonic structures for application in high-performance metamaterials, and in binary nanoparticle networks and liquid crystal arrays for sensing technologies and imaging [35]. With these procedures, diverse techniques have been demonstrated strong fluorescence intensity and mild levels of enhancement, which allows them to manipulate photonic excitation and photoemission [36].
Hierarchical nanostructures represent a potential key to the next generation of new nanomaterials. For example, a controlled structure in the agglomeration between nanoparticles can increase plasmonic effects while the stacking distance between other nanoparticles decreases; all of this can be used to develop new and effective detection methods. Some of the representative hierarchically structured shapes are nanopillars [37], nanocones [38], nanoholes [39], and gecko pillars [40], among others.
Hierarchical nanostructures can be fabricated using techniques such as nanosphere lithography [41] with multiple patterns [42], electron beam lithography [43], pattern transfer [44], and focused ionization [45].
The characterization of the morphology, structure, and stability of hierarchical nanostructures can be explored by different methods. The typical characterization techniques for hierarchical nanostructures are X-ray diffraction [46], electrical effects [47], TEM [48], energy dispersive spectroscopy (EDX) [49], AFM [50], optical interactions [51], PL [52], Brunauer–Emmett–Teller surface area analysis [53], UV–visible absorption spectroscopy [54], photovoltaic performance [55], photocatalytic processes [56], Raman spectroscopy [57], and magnetic phenomena [58].
A hierarchy in nanostructures can be developed through in situ plasmon-driven syntheses [59] or through amino acids [60] to easily detect analytes at trace levels, such as pesticides, heavy metals, explosives, proteins, pathogens, and other chemical and biological contaminants [61]. It is clear that nanomaterial sciences are essential for developing biosensors with high reliability and speed using innovative technology [62][63][64][65].
In the last two years, diverse experiments have been carried out in the development of biosensors using different hierarchical nanostructures. It is worth highlighting some examples that have been very useful in the commitment to developing biosensors with better properties.
It has been pointed out that biosensors can be used to see the effectiveness of the vaccines in healthy, convalescent, or vaccinated people [66]. They can be used to monitor diseases, observe how many antibodies exist in people’s fluids, as well as determine whether the vaccines are effective for the test subjects [67]. In the faster biosensors, it takes approximately 20 min to obtain the result. The research has sought to develop biosensors with these nanomaterials to achieve a relatively rapid response, achieving a response time of 15 min.
It has been observed that current biosensors also have some disadvantages such as not being capable of detecting analytes in samples when there are external stimuli. This has to be addressed with the development of different biosensors with the properties of nanomaterials, such as different probes, including plasmonic [68] and incorporated ones [69]. Biosensors capable of detecting pathogens with very little genetic material compared to other assays have also been developed [70]. Additionally, calorimetric strips for smartphones aimed at antibodies or antigens to combat the rapid spread of these diseases have been considered since wearable biosensors can constantly monitor patients [71].
With this motivation, different aspects of the cutting-edge biosensors in the detection of SARS-CoV-2, focusing in those based on hierarchical and hybrid nanoparticles. Figure 1 shows the main characteristics.
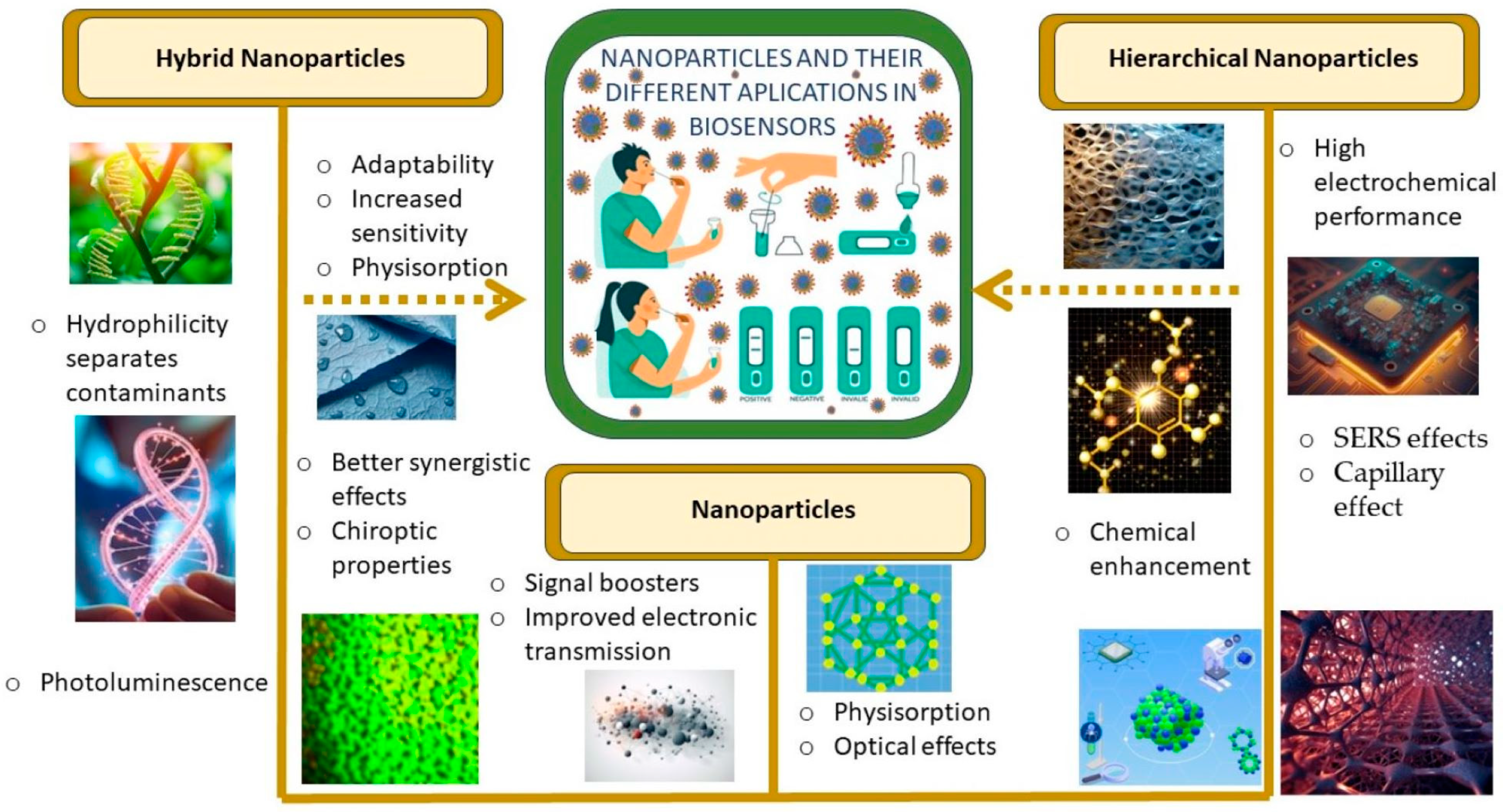
Figure 1. Representative characteristics exhibited by different nanostructures in biosensing applications.
2. Synthesis of Hierarchical Nanostructures and Multicomponent Assemblies for Biosensors
Materials with hierarchical nanostructures have excellent mechanical properties due to the functional adaptation of their structures into different hierarchical levels. Hierarchical structures can be observed in nature, such as in bones, wood, cork, and plant stems, or in glass sponges [72]. Hierarchical nanomaterials show different architectural designs that are ordered at multiple length scales. They are grouped according to their main characteristics; in the case of porous materials, they contain interconnected pores with at least two levels of pore hierarchy from molecular (1–100 A), nano (10–100 nm), and meso (1–100 μm), to macropores [73]. It should be noted that the construction of hierarchical nanostructures requires knowledge of particular principles to avoid limitations on their properties [74]. Hierarchical materials can mimic the mechanical properties of their biological counterparts. Smart hierarchical materials can exhibit specific stimulus-response properties [75], such as self-healing and self-regeneration [76] in order to improve fracture resistance and increase strength [77]. Arrays can be constructed using proteins and microscale mechanical constraints can be used to form ordered networks within macroscopic structures [78]. The synthesis at different orders of magnitude from nanoscale to macroscale can be used to acquire outstanding characteristics through interacting with different analytes of different sizes [79], from small proteins to living cells. Different networks can be designed according to the geometry of the templates used [80]. The nanoclusters can be protected by ligands that can be prepared with atomic precision, exhibiting well-defined structures and resulting in versatile building blocks to manufacture excellent structures capable of performing certain functions [81]. For instance, nanofibers are used to construct multifunctional walkways with up to five levels of organization (depending on the method used). In the first level, there is a composite nanofiber; in the second level, a layer of composite material coated on the composite nanofiber that will result in the third level. The fourth level organizes the nanofibers to form an assembly and finally, in the last level, an assembly of nanofibers can be encapsulated within a matrix to form a massive structure by default [82]. Nanotubes are commonly used for the manufacture of hierarchical materials since they consist of molecular blocks, whose characteristics can be related to an anisotropic supramolecular self-assembly behavior at a personalized nanoscale, which allows for the creation of a percolation network at the mesoscale. They are regulated by dynamic self-assembly into four hierarchical levels of self-organization [83]. Nanosheets are composed of 2D building blocks, which have atomic or molecular thicknesses and they are considered the thinnest functional nanomaterials. They can be organized into various nanostructures or combined with a variety of materials at the nanoscale. Thanks to this, wide-range assemblies such as organic molecules, polymer gels, and inorganic nanoparticles can be designed [84].
Although hierarchical nanomaterials can be considered hybrid materials [85], nanohybrids are composed in a different way. Hybrid materials can have a variety of complex architectures with or without hierarchy. Their size varies from nanometers to several micrometers and several millimeters. Hybrid nanomaterials are combined through the synergistic mixture of two or more nanomaterials, which can be either inorganic or organic [86], that create a single material with properties that go beyond their properties as individual elements. They consist of groups of blocks with similar properties and structures with groups that cross-link the polymer into chains [87]. Their properties are determined by a combination of structure and composition at each length scale [88]. As a result, their properties are expressed in molecular length scale structures [89]. This indicates that the new mixture has superior properties compared to the original mixture. The properties to look at are the advantages derived from nanomaterials at a macroscopic level, such as energy absorption performance. The lightweight structure maximizes its functionality and improves the efficiency of the material [90][91].
There are different forms of nanohybrids such as sandwich structures, foams, reticular structures, segmented structures, zero expansion [92], and meso-structured thin films [93]. These structures serve different purposes, especially in integrated refractive and diffractive optical devices. Since these nanomaterials have a large thermal stability and better compatibility, they are typically used in the production of semiconductive devices [94]. In order to characterize organic–inorganic materials, techniques such as FTIR, Raman spectroscopy, LSPR, and various techniques based on MS are used [95].
Hybrid nanomaterials are good candidates for developing nanomaterials in the fight against bacteria and viruses thanks to their high sensitivity, good stability, and selectivity. In particular, they can detect antigens in plasma since their good electrochemical activity helps in the immobilization of the chains of different aptamers [96].
Nanomedicine, based on hybrid and hierarchical nanomaterials, has achieved great progress in the field of biosensors for the diagnosis, prevention, detection [97], and treatment of diseases in the post-pandemic period [98]. Compared to bulk materials, nanostructures are more precise, more reliable, less invasive, and easier to carry according to their chemical elements [99]. The effectiveness of nanomaterials has advanced to detect diseases at a very early stage using new technologies based on nanobiosensors [100], whose physical principles at the nanoscale level allow the biological receptors to be highly sensitive [101]. Nanobiosensors can be tailored by using different types of nanomaterials and structures [102].
Depending on their interactions, nanobiosensors can be classified into two different groups called biocatalytic or biophilic. These two groups can be classified according to recognition factors, for example, cells, organelles, tissues, enzymes, receptors, antibodies, nucleic acids, MIPs, PNAs, or aptamers.
Nanostructures are capable of obtaining information through molecular interactions in real time, and in normal and pathological biological states which provides an effective and relatively fast result. For example, in a drop of blood, an enzyme such as glucose oxidase, glucose dehydrogenase, or hexokinase can cause a reaction, which can be measure by a low-dimensional detector in a glucometer (biosensor) [103].
Because the manufacturing of biosensors has several drawbacks, efforts have been made to develop improvements in manufacturing [104][105][106][107][108]. Characteristics like adhesion ability, strong adsorption capacity, chemical catalytic efficiency, and corrosion and oxidation resistance facilitate the fabrication [109], chemical stability, and electron transfer kinetics [110]. The challenges for optimizing highly selectivity binding properties are continuously being overcome to analyze nanoscale elements of biomolecules [111].
High crystallinity with insignificant structural defects can be relevant to detecting different samples such as glucose, proteins, and nucleic acids [112]. The other main advantages of nanohybrid materials are the specific binding sites that generate a selective sensor signal, which also improves its magnitude and composition. The high surface-to-volume ratio of nanofibers can also improve the capture efficiency and it provides some surface area-related phenomena, including ion exchange and catalysis [113][114].
Heterounions in hierarchical nanomaterials can promote the selective formation of specialized structures and sensitive responses not found in other sensors [115][116][117][118]. Nanomaterials produced through molecular printing can create selectivity for specific enzymes. This method can be worked with 3D nanostructures and it is used to manufacture versatile materials for the construction of sensors to detect various analytes [119]. It has been demonstrated that these nanostructures can eliminate pathogens and better detect enzymes compared to other nanomaterials [120].
The existing improvements found when assembling nanostructures are versatile, and they open new methods for different technologies to control their structure and combine physicochemical properties [121][122][123][124][125][126][127][128][129][130][131]. On the other hand, some nanostructured systems based on organic polymers have been proposed [132], and applications for spectrochemical biosensing have been demonstrated. Biosensors based on RNA hybridization can be considered for several biological reactions and for generating analytical signals that are easily detected by different electrochemical aptasensors [133], electrochemical luminescence sensors [134], and optical transducers, among others [135]. It has been pointed out that RT-PCR [136] can be used to amplify cDNA from virus RNA [137]. This is of great interest in the studies that have to do with inhibitors that target the enzyme helicase since it is known to participate in the processes of duplication and cell reproduction [138].
Photonic nanobiosensors have been also highlighted with respect to their potential use against SARS-CoV-2. In addition to monoclonal antibody pairs, which are rapid antigen tests [139], it is important to look for more efficient ways to detect pathogens [140]. In this direction, biosensors using some promising plasmonic nanoparticles are the most powerful tools employed for the detection of viruses [141]. Moreover, polystyrene nanoparticles, graphene, and carbon nanotubes [142] present different advantages such as selectivity towards particular molecular expressions corresponding to an important challenge that requires high specificity, sensitivity, and a multiplex detection capability to offer good virus detection. The design of POC testing arrangements [143], such as LFIAs, should be mentioned as they offer fast and easy-to-use methods, as well as reliability. Each synthesis procedure can be functional, but inherent limitations in the quantitative analysis of the virus in the application of biosensors should be noted [144].
In summary, hierarchical nanostructures are formed by hybrid nanoparticles, which are good candidates for the development of nanobiosensors for pathogen detection. These nanoparticles are prepared through different synthesis methods, as it is illustrated in Figure 2.

Figure 2. Representative processing routes for the synthesis of hierarchical nanostructures used in the development of biosensors.
References
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554.
- Huang, T.; Cao, W.; Elsayed-Ali, H.E.; Xu, X.-H.N. High-throughput ultrasensitive characterization of chemical, structural and plasmonic properties of EBL-fabricated single silver nanoparticles. Nanoscale 2012, 4, 380–385.
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516.
- Yi, D.; Wei, Z.; Zheng, W.; Pan, Y.; Long, Y.; Zheng, H. Glucose detection based on the photothermal effect of OxTMB using a thermometer. Sens. Actuators B Chem. 2020, 323, 128691.
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448.
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing using magnetic particle detection Techniques. Sensors 2017, 17, 2300.
- Divya, J.; Selvendran, S.; Raja, A.S.; Sivasubramanian, A. Surface plasmon based plasmonic sensors: A review on their past, present and future. Biosens. Bioelectron. X 2022, 11, 100175.
- Tahseen, A.Q.; Noshin, F.; Khasan, S.K.; Adib, I.M. A novel and stable ultraviolet and infrared intensity sensor in impedance/capacitance modes fabricated from degraded CH3NH3PbI3-xClx perovskite materials. J. Mater. Res. Technol. 2020, 9, 12795–12803.
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134.
- Wang, T.-J.; Cheng, C.-C.; Yang, S.-C. Surface plasmon resonance biosensing by electro-optically modulated attenuated total reflection. Appl. Phys. B Laser Opt. 2010, 103, 701–706.
- He, J.; Kunitake, T.; Nakao, A. Facile in Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406.
- Zhang, M.; Meng, J.; Wang, D.; Tang, Q.; Chen, T.; Rong, S.; Liu, J.; Wu, Y. Biomimetic Synthesis of hierarchical 3D Ag Butterfly wing scales arrays/graphene composites as ultrasensitive SERS substrates for efficient trace chemical detection. J. Mater. Chem. C 2018, 6, 1933–1943.
- Ran, Y.; Lu, H.; Zhao, S.; Jia, L.; Guo, Q.; Gao, C.; Jiang, Z.; Wang, Z. Structural and plasmonic properties of Ti Sr N ternary nitride thin films. Appl. Surf. Sci. 2019, 476, 560–568.
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289.
- Zhang, L.; Cai, W.; Ren, J.; Tang, Y. Cu-Co bimetal oxide hierarchical nanostructures as high-performance electrocatalyst for oxygen evolution reaction. Mater. Today Energy 2021, 21, 100703.
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Creaniofacial Res. 2016, 6, 153–159.
- Marques-Hueso, J.; Morton, J.A.S.; Wang, X.; Bertran-Serra, E.; Desmulliez, M.P.Y. Photolithographic nano seeding method for selective synthesis of metal catalysed nanostructures. Nanotechnology 2018, 30, 015302.
- Amendola, V.; Scaraamuzza, S.; Agnoli, S.; Granozzi, G.; Meneghetti, M.; Campo, G.; Bonanni, V.; Pineider, F.; Sangregorio, C.; Ghihna, P.; et al. Laser generation of iron-doped silver nano truffles with magnetic and plasmonic properties. Nano Res. 2015, 8, 4007–4023.
- Hou, P.; Liu, H.; Li, J.; Yang, J. One-pot synthesis of noble metal nanoparticles with a core-shell construction. CrystEngComm 2015, 17, 1826–1832.
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of nanomaterials for electrochemical sensing. Sens. J. 2019, 19, 1186.
- Münch, F. Electroless plating of metal nanomaterials. Chemelectrochem 2021, 8, 2993–3012.
- Lee, J.; Takemura, K.; Park, E.Y. Plasmonic nanomaterial-based Optical biosensing platforms for virus Detection. Sensors 2017, 17, 2332.
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726.
- Zhao, Y.; Tong, R.-J.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505.
- Marzana, M.; Morsada, Z.; Faruk, O.; Ahmed, A.; Khan, M.A.; Jalil, M.A.; Hossain, M.; Rahman, M.M. Nanostructured Carbons: Towards Soft-Bioelectronics, Biosensing and Therapeutic Applications. Spec. Issue Recent Adv. Chem./Mater. 2022, 22, e202100319.
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Wang, S.; Zhang, S.; Liu, Q.; Wang, X.; Wang, G.; Zhang, Y.; et al. Carbon nanotubes: A powerful bridge for conductivity and flexibility in electrochemical glucose sensors. J. Nanobiotechnol. 2023, 21, 320.
- He, Y.; Hu, C.; Li, Z.; Wu, C.; Zeng, Y.; Peng, C. Multifunctional carbon nanomaterials for diagnostic applications in infectious diseases and tumors. Mater. Today Bio 2022, 14, 100231.
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in Nanomaterials-Based Electrochemical Biosensors for Foodborne Pathogen Detection. Nanomaterials 2021, 11, 2700.
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. All-carbon multi-scale and hierarchical fibers and related structural composites: A review. Compos. Sci. Technol. 2020, 186, 107932.
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946.
- Cajigas, S.; Alzate, D.; Orozco, J. Gold nanoparticle/DNA-based nanobioconjugate for electrochemical detection of Zika virus. Microchim. Acta 2020, 187, 594.
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063.
- Ding, M.; Xie, N.; Wang, C.; Kou, X.; Zhang, H.; Guo, L.; Sun, Y.; Chuai, X.; Gao, Y.; Liu, F.; et al. Enhanced NO2 gas sensing properties by Ag-doped hollow urchin-like In2O3 hierarchical nanostructures. Sens. Actuators B Chem. 2017, 252, 418–427.
- Mandal, D.; Biswas, S.; Chowdhury, A.; De, D.; Tiwary, C.S.; Gupta, A.N.; Singh, T.; Chandra, A. Hierarchical cage-frame type nanostructure of CeO2 for bio sensing applications: From glucose to protein detection. Nanotechnology 2020, 32, 2.
- Wen, A.M.; Podgornik, R.; Strangi, G.; Steinmetz, N.F. Photonics and plasmonics go viral: Self-assembly of hierarchical metamaterials. Rend. Lincei 2015, 26, 129–141.
- Wang, D.; Capehart, S.L.; Pal, S.; Liu, M.; Zhang, L.; Schuck, P.J.; Liu, Y.; Yan, H.; Francis, M.B.; De Yoreo, J.J. Hierarchical Assembly of Plasmonic Nanostructures Using Virus Capsid Scaffolds on DNA Origami Templates. ACS Nano 2014, 8, 7896–7904.
- Greer, J.R.; Kim, J.-Y.; Burek, M.J. The In-situ Mechanical testing of nanoscale single-crystalline nanopillars. Nanomech. Test. 2009, 61, 19–25.
- Bae, J.; Hong, J.-I.; Han, W.H.; Choi, Y.J.; Snyder, R.L. Superior field emission properties of ZnO nanocones synthesized by pulsed laser deposition. Chem. Phys. Lett. 2009, 475, 260–263.
- Kijima, T.; Nagatomo, Y.; Takemoto, H.; Uota, M.; Fujikawa, D.; Sekiya, Y.; Kishishita, T.; Shimoda, M.; Yoshimura, T.; Kawasaki, H.; et al. Synthesis of Nanohole-Structured Single-Crystalline platinum nanosheets using surfactant-liquid-crystals and their electrochemical characterization. Adv. Funct. Mater. 2009, 19, 545–553.
- Zhang, M.; Ma, L.; Wang, Q.; Hao, P.; Zheng, X. Wettability behavior of nanodroplets on copper surfaces with hierarchical nanostructures. Colloids Surf. A 2020, 604, 125291–125297.
- Cheung, C.L.; Nikolić, R.J.; Reinhardt, C.E.; Wang, T.F. Fabrication of nanopillars by nanosphere lithography. IOP Sci. 2006, 17, 1339.
- Xu, X.; Yang, Q.; Wattanatorn, N.; Zhao, C.; Chiang, N.; Jonas, S.J.; Weiss, P.S. Multiple-Patterning Nanosphere Lithograhy for Fabricating periodic Three-Dimensional Hierechical Nanostrutures. ACS Nano 2017, 11, 10384–10391.
- Tseng, A.A.; Chen, K.; Chen, C.D.; Ma, K.J. Electron beam lithography in nanoscale fabrication: Recent development. IEEE Trans. Electron. Packag. Manuf. 2003, 26, 141–149.
- Chen, Y.F. Nanofabrication by electron beam lithography and its applications: A review. Microelectron. Eng. 2015, 135, 57–72.
- Asadi, R.; Abdollahi, H.; Gharabaghi, M.; Boroumand, Z. Effective removal of Zn (II) ions from aqueous solution by the magnetic MnFe2O4 spinel ferrite nanoparticles with focuses on synthesis, characterization, adsortion, and desorption. Adv. Powder Technol. 2020, 31, 1480–1489.
- Niu, C.; Liu, X.; Meng, J.; Xu, L.; Yan, M.; Wang, X.; Zhang, G.; Liu, Z.; Xu, X.; Mai, L. Three dimensional V2O5/NaV6O15 Hierarchical heterostructures: Controlled synthesisand synergistic effect investigated by in situ X-ray difraction. Nanoenergia 2016, 27, 147–156.
- Zhang, X.; Yu, P.; Zhang, H.; Zhang, D.; Sun, X.; Ma, Y. Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type Mn = 2 nanosheets for supercapacitor applications. Electrochim. Acta 2013, 89, 523–529.
- Zhu, F.; Liu, Y.; Yan, M.; Shi, W. Construction of hierarchical FeCo2O4 MnO2 core-shell nanostructures on carbon fibers for High-performance asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 512, 419–427.
- Mu, C.F.; Yao, Q.Z.; Qu, X.F.; Zhou, G.T.; Li, M.L.; Fu, S.Q. Controlled synthesis of various hierarchical nanostructures of copper sulfide by a facile microwave irradation method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 14–21.
- Moshnikov, V.A.; Gracheva, I.E.; Kuznezov, V.V.; Maximov, A.I.; Karpova, S.S.; Ponomareva, A.A. Hierarchical nanostructured semiconductor porous materials for gas sensonrs. J. Non-Cryst. Solids 2010, 356, 2020–2025.
- Naqvi, T.K.; Bajpai, A.; Bharati, M.S.S.; Kulkarni, M.M.; Siddiqui, A.M.; Soma, V.R.; Dwivedi, P.K. Ultra-sensitive reusable SERS sensor for multiple hazardous materials detection on single platform. J. Hazard. Mater. 2020, 407, 124353.
- Yu, A.; Qian, J.; Pan, H.; Cui, Y.; Xu, M.; Tu, L.; Chai, Q.; Zhou, X. Micro-lotus constructured by Fe-doped ZnO hierarchically porous nanosheets: Preparation, characterization and gas sensing property. Sens. Actuators B Chem. B 2011, 158, 9–16.
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Photocatalytic performance of novel samarium-doped spherical-like ZnO hierarchical nanostructures under visible light irradiation for 2,4-dichlorophenol degradation. J. Colloid Interface Sci. 2013, 401, 40–49.
- Hu, J.; Tu, J.; Li, X.; Wang, Z.; Li, Y.; Li, Q.; Wang, F. Enhanced UV-Visible light Photocatalytic activity by constructing appropriate heterostructures between mesopore TiO2 nanospheres and Sn3O4 nanoparticles. Nanomaterials 2017, 7, 336.
- Wu, S.; Xu, Y.; Li, X.; Tong, R.; Chen, L.; Han, Y.; Wu, J.; Zhang, X. Controlled synthesis of porous hierarchical ZnFe2O4 Micro-/Nanostructures with multifunctional photovatalytic performance. Inorg. Chem. 2018, 57, 15481–15488.
- Panmand, R.P.; Sethi, Y.A.; Kadam, S.R.; Tamboli, M.S.; Nikam, L.K.; Ambekar, J.D.; Park, C.-J.; Kale, B.B. Self-assembled hierarchical nanostrcutures of Bi2WO6 for hydrogen production and dye degradation under solar light. CrystEngComm 2015, 17, 107–115.
- Tian, X.D.; Liu, B.J.; Li, J.F.; Yang, Z.L.; Ren, B.; Tian, Z.Q. Shiners and plasmonic properties of Au Core SiO2 shell nanoparticles with optimal core size and shell thickness. J. Raman Spectrosc. 2013, 44, 994–998.
- Cao, S.W.; Zhu, Y.J.; Ma, M.Y.; Li, L.; Zhang, L. Hierarchically nanostructured magnetic hollow spheres of Fe3O4 and Fe2O3: Preparation and potetialapplication in drug delivery. J. Phys. Chem. 2008, 112, 1851–1856.
- Tang, X.; Cai, W.; Yang, L.; Liu, J. Monitoring plasmon-driven surface catalyzed reactions in situ using time-dependent surface-enhanced Raman spectrsocopy on single particles of hierarchical peonylike silver microflowers. Nanoscale 2014, 6, 8612–8616.
- Kang, L.; Xu, P.; Chen, D.; Zhang, B.; Du, Y.; Han, X.; Li, Q.; Wang, H.-L. Amino Acid-Assisted Synthesis of hierarchical silver microspheres for single particle surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 10007–10012.
- Ou, Y.; Wang, L.-Y.; Zhu, L.-W.; Wan, L.-S.; Xu, Z.-K. In-Situ Immobilization of Silver Nanoparticles on Self-Assembled Honeycomb-Patterned Films Enables Surface-Enhanced Raman Scattering (SERS) Substrates. J. Phys. Chem. C 2014, 118, 11478–11484.
- Butakova, M.A.; Chernov, A.V.; Kartashov, O.O.; Soldatov, A.V. Data-Centric Architecture for Self-Driving laboratories with autonomous discovery of new Nanomaterials. Nanomaterials 2021, 12, 12.
- Escarpa, A.; Prodromidis, M.I. Microchimica Acta: A top analytical chemistry journal for disseminating research involving micro and nanomaterials. Microchim. Acta 2021, 188, 136.
- Khomutov, G.; Gubin, S. Interfacial synthesis of noble metal nanoparticles. Mater. Sci. Eng. C 2002, 22, 141–146.
- Zhang, A.Q.; Cai, L.J.; Sui, L.; Qian, D.J.; Chen, M. Reducing properties of polymers in the Synthesis of noble metal nanoparticles. Polym. Rev. 2013, 53, 240–276.
- Nunez, F.A.; Castro, A.C.; de Oliveira, V.L.; Lima, A.C.; Oliveira, J.R.; de Medeiros, G.X.; Sasahara, G.L.; Santos, K.S.; Lanfredi, A.J.C.; Alves, W.A. Electrochemical Immunosensors Based on Zinc Oxide Nanorods for Detection of Antibodies Against SARS-CoV-2 Spike Protein in Convalescent and Vaccinated Individuals. ACS Biomater. Sci. Eng. 2023, 9, 458–473.
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 037523.
- Jeon, M.J.; Kim, S.K.; Hwang, S.H.; Lee, J.U.; Sim, S.J. Lateral flow immunoassay based on surface-enhanced Raman scattering using pH-induced phage-templated hierarchical plasmonic assembly for point-of-care diagnosis of infectious disease. Biosens. Bioelectron. 2024, 250, 116061.
- Yin, Z.Z.; Liu, Z.; Zhou, M.; Yang, X.; Zheng, G.; Zhang, H.; Kong, Y. A surface molecularly imprinted electrochemical biosensor for the detection of SARS-CoV-2 spike protein by using Cu7S4-Au as built-in probe. Bioelectrochemistry 2023, 152, 108462.
- Ji, D.; Guo, M.; Wu, Y.; Liu, W.; Luo, S.; Wang, X.; Kang, H.; Chen, Y.; Dai, C.; Kong, D.; et al. Electrochemical Detection of a Few Copies of Unamplified SARS-CoV-2 Nucleic Acids by a Self-Actuated Molecular System. J. Am. Chem. Soc. 2022, 144, 13526–13537.
- El-Sherif, D.M.; Abouzid, M.; Gaballah, M.S.; Ahmed, A.A.; Adeel, M.; Sheta, S.M. New approach in SARS-CoV-2 surveillance using biosensor technology: A review. Environ. Sci. Pollut. Res. 2022, 29, 1677–1695.
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334.
- Dan, N. Synthesis of hierarchical materials. Focus 2000, 18, 370–374.
- O’Brien, M.N.; Jones, M.R.; Mirkin, C.A. The nature and implications of uniformity in the hierarchical organization of nanomaterials. Proc. Natl. Acad. Sci. USA 2016, 113, 11717–11725.
- Shi, S.; Li, Y.; Ngo-Dinh, B.-N.; Markmann, J.; Weissmüller, J. Scaling behavior of stiffness and strength of hierarchical network nanomaterials. Science 2021, 371, 1026–1033.
- Xu, Y. Chapter 19—Hierarchical Materials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–574.
- Kamada, A.; Herneke, A.; Lopez-Sanchez, P.; Harder, C.; Ornithopoulou, E.; Wu, Q.; Wei, X.; Schwartzkopf, M.; Müller-Buschbaum, P.; Roth, S.V.; et al. Hierarchical propagation of structural features in protein nanomaterials. Nanoscale 2022, 14, 2502–2510.
- Baranov, O.; Belmonte, T.; Levchenko, I.; Bazaka, K.; Košiček, M.; Cvelbar, U. Hierarchical Nanomaterials by Selective Deposition of Noble Metal Nanoparticles: Insight into Control and Growth Processes. Adv. Theory Simul. 2023, 6, 2300288.
- Tseng, P.; Napier, B.; Zhao, S.; Mitropoulos, A.N.; Applegate, M.B.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Directed assembly of bio-inspired hierarchical materials with controlled nanofibrillar architectures. Nat. Nanotechnol. 2017, 12, 474–480.
- Hamon, C.; Novikov, S.; Scarabelli, L.; Basabe-Desmonts, L.; Liz-Marzán, L.M. Hierarchical Self-Assembly of Gold Nanoparticles into Patterned Plasmonic Nanostructures. ACS Nano 2014, 8, 10694–10703.
- Bonacchi, S.; Antonello, S.; Dainese, T.; Maran, F. Atomically Precise Metal Nanoclusters: Novel Building Blocks for Hierarchical Structures. Chem. A Eur. J. 2021, 27, 30–38.
- Teo, W.E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817.
- Zelada-Guillén, G.A.; Escárcega-Bobadilla, M.V.; Wegrzyn, M.; Giménez, E.; Maier, G.; Kleij, A.W. Enhanced Conductivity for Carbon Nanotube Based Materials through Supramolecular Hierarchical Self-Assembly. Adv. Mater. Interfaces 2018, 5, 1701585.
- Osada, M.; Sasaki, T. Nanosheet architectonics: A hierarchically structured assembly for tailored fusion materials. Polym. J. 2015, 47, 89–98.
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Haidaraly, K.M.; Newton, G.N.; Izzet, G. Supramolecular assemblies of organo-functionalised hybrid polyoxometalates: From functional building blocks to hierarchical nanomaterials. R. Soc. Chem. 2022, 51, 293–328.
- Mitzi, D.B. Thin-Film Deposition of Organic−Inorganic Hybrid Materials. Chem. Mater. 2001, 13, 3283–3298.
- Schubert, U. Cluster-based inorganic–organic hybrid materials. Chem. Soc. Rev. 2011, 40, 575–582.
- Fahmi, A.; Pietsch, T.; Mendoza, C.; Cheval, N. Functional hybrid materials. Mater. Today 2009, 12, 44–50.
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51.
- Sun, G.; Chen, D.; Zhu, G.; Li, Q. Lightweight hybrid materials and structures for energy absorption: A state-of-the-art review and outlook. Thin-Walled Struct. 2022, 172, 108760.
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829.
- Ashby, M. Hybrid Materials to Expand the Boundaries of Material-Property Space. J. Am. Ceram. Soc. 2011, 94, s3–s14.
- Nicole, L.; Laberty-Robert, C.; Rozes, L.; Sanchez, C. Hybrid materials science: A promised land for the integrative design of multifunctional materials. Nanoscale 2014, 6, 6267–6292.
- Houbertz, R.; Domann, G.; Cronauer, C.; Schmitt, A.; Martin, H.; Park, J.U.; Fröhlich, L.; Buestrich, R.; Popall, M.; Streppel, U.; et al. Inorganic–organic hybrid materials for application in optical devices. Thin Solid Films 2003, 442, 194–200.
- Semenova, D.; Silina, Y.E. The Role of Nanoanalytics in the Development of Organic-Inorganic Nanohybrids—Seeing Nanomaterials as They Are. Nanomaterials 2019, 9, 1673.
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985.
- Kerry, R.G.; Ukhurebor, K.E.; Kumari, S.; Maurya, G.K.; Patra, S.; Panigrahi, B.; Majhi, S.; Rout, J.R.; Rodriguez-Torres, M.D.P.; Das, G.; et al. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Sensor 2015, 5, 14539–14568.
- Al-Ahmady, Z.S.; Ali-Boucetta, H. Nanomedicine & Nanotoxicology Future Could Be Reshaped Post-COVID-19 Pandemic. Front. Nanotechnol. 2020, 2, 610465.
- Singh, C.K.; Sodhi, K.K. The emerging significance of nanomedicine-based approaches to fighting COVID-19 variants of concern: A perspective on the nanotechnology’s role in COVID-19 diagnosis and treatment. Front. Nanotechnol. 2023, 4, 1084033.
- Singh, P.; Yadava, R. Chapter 26—Nanosensors for health care. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–450.
- Hulanicki, A.; Glab, S.; Ingman, F. Analytical Chemistry division commission on general aspects of analytical chemistry. Pure Appl. Chem. 1991, 63, 1247–1250.
- Riu, J.; Maroto, A.; Rius, F.X. Nanosensors in environmental analysis. Talanta 2006, 69, 288–301.
- Tonyushkina, K.; Nichols, J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009, 3, 971–980.
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239.
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250.
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80.
- Qi, H.; Yue, S.; Bi, S.; Ding, C.; Song, W. Isothermal exponential amplification techniques: From basic principles to applications in electrochemical biosensors. Biosens. Bioelectron. 2018, 110, 207–217.
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126.
- Jia, Y.; Yi, X.; Li, Z.; Zhang, L.; Yu, B.; Zhang, J.; Wang, X.; Jia, X. Recent advance in biosensing applications based on two-dimensional transition metal oxide nanomaterials. Talanta 2019, 219, 121308.
- Fang, L.; Liu, B.; Liu, L.; Li, Y.; Huang, K.; Zhang, Q. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sens. Actuators B Chem. 2016, 222, 1096–1102.
- Tripathy, N.; Kim, D.-H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27.
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183.
- Xia, Y.; Halas, N.J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005, 30, 338–348.
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392.
- Ni, J.; Liu, D.; Wang, W.; Wang, A.; Jia, J.; Tian, J.; Xing, Z. Hierarchical defect-rich flower-like BiOBr/Ag nanoparticles/ultrathin g-C3N4 with transfer channels plasmonic Z-scheme heterojunction photocatalyst for accelerated visible-light-driven photothermal-photocatalytic oxytetracycline degradation. Chem. Eng. J. 2021, 419, 129969.
- Xiang, Z.; Xiong, J.; Deng, B.; Cui, E.; Yu, L.; Zeng, Q.; Pei, K.; Chen, R.; Lu, W. Rational design of 2D hierarchically laminated Fe3O4@ nanoporous carbon@rGO nanocomposites with strong magnetic coupling for excellent electromagnetic absorption applications. J. Mater. Chem. C 2020, 8, 2123–2134.
- Pietá, I.S.; Rathib, A.; Piedad, P.; Nowakowski, R.; Holdynski, M.; Pisarek, M.; Kaminska, A.; Gawandeb, M.; Zborilb, R. Electrocatalytic methanol oxidation over Cu, Ni and bimetallic Cu-Ni nanoparticles supported on graphitic carbon nitride. Appl. Catal. B Environ. 2019, 244, 272–283.
- Wen, F.; Zhang, W.; Wei, G.; Wang, Y.; Zhang, J.; Zhang, M.; Shi, L. Synthesis of noble metal nanoparticles embedded in the shell layer of core-shell poly(styrene-co-4-vinylpyridine) microspheres and their application in catalysis. Chem. Mater. 2008, 20, 2144–2150.
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs@Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106.
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes ‘Meet’ Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930.
- Holler, R.P.M.; Dulle, M.; Thoma, S.; Mayer, M.; Steiner, A.M.; Förster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-Assisted Assembly of modular 3D Plasmonic Raspberry-like Core/Satellite Nanoclusters: Correlation of Structure and Optical Properties. ACS Nano 2016, 6, 5740–5750.
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H. Challenges and Opportunities in the Bottom-up Mechanochemical Synthesis of Noble Metal Nanoparticles. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 16114–16141.
- Yan, N.; Liu, H.; Zhu, Y.; Jiang, W.; Dong, Z. Entropy-driven hierarchical nanostructures from cooperative self-assembly of gold nanoparticles/block copolymers under three-dimensional confinement. Macromolecules 2015, 48, 5980–5987.
- Prominski, A.; Tomczyk, E.; Pawlak, M.; Jedrych, A.; Mieczkowki, J.; Lewandowski, W.; Wójcik, M. Size-dependent thermal- and photoresponsive plasmonic properties of liquid crystalline gold nanoparticles. Materials 2020, 13, 875.
- Adesuji, E.T.; Torres-Guerrero, V.O.; Arizpe-Zapata, J.A.; Videa, M.; Sánchez-Domínguez, M.; Fuentes, K.M. Bicontinuous microemulsion as confined reaction media for the synthesis of plasmonic silver self-ass. Nanotechnology 2020, 31, 425601.
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724.
- Henry, A.-I.; Bingham, J.M.; Ringe, E.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Correlated Structure and Optical property Studies of plasmonic nanoparticles. J. Phys. Chem. 2011, 115, 9291–9305.
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostrutures: Engineering their plasmonic properties for biomedical application. Chem. Soc. Rev. 2006, 35, 1084–1094.
- Sayan, J.S.; Kuruvilla, J.; Appukuttan, S. Development of Hierarchical Nanostructures for Energy Storage. In Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 663–695.
- Knebel, A.; Wulfert-Holzman, P.; Friebe, S.; Pavel, J.; Srauß, I.; Mundstock, A.; Steibach, F.; Caro, J. Hierarchical nanostructures of metal-Organic Frameworks applied in gas separating ZIF-8on-ZIF-67 membranes. Chemistry 2017, 24, 5728–5733.
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Organ. Nanostruct. 2003, 42, 2350–2365.
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363.
- Kurnia Sari, A. The optimization of an electrochemical aptasensor to detect RBD protein S SARS-CoV-2 as a biomarker of COVID-19 using screen-printed carbon electrode/AuNP. J. Electrochem. Sci. Eng. 2022, 12, 219–235.
- Gao, Y.; Han, Y.; Wang, C.; Qiang, L.; Gao, J.; Wang, Y.; Liu, H.; Han, L.; Zhang, Y. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Anal. Chim. Acta 2021, 1154, 338330.
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666.
- Wang, X.; Kong, D.; Guo, M.; Wang, L.; Gu, C.; Dai, C.; Wang, Y.; Jiang, Q.; Ai, Z.; Zhang, C.; et al. Rapid SARS-CoV-2 Nucleic Acid Testing and Pooled Assay by Tetrahedral DNA Nanostructure Transistor. Nanoletters 2021, 21, 9450–9457.
- Dighe, K.; Moitra, P.; Alafeef, M.; Gunaseelan, N.; Pan, D. A rapid RNA extraction-free lateral flow assay for molecular point-of-care detection of SARS-CoV-2 augmented by chemical probes. Biosens. Bioelectron. 2022, 200, 113900.
- White, A.M.; Lin, W.; Cheng, X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV-2 Nsp13 Helicase. J. Phys. Chem. Lett. 2020, 11, 9144–9151.
- Salcedo, N.; Reddy, A.; Gomez, A.R.; Bosch, I.; Herrera, B.B. Monoclonal antibody pairs against SARS-CoV-2 for rapid antigen test development. PLoS Negl. Trop. Dis. 2022, 16, e0010311.
- Younes, N.; Al-Sadeq, D.W.; Al-Jinghefee, H.; Younes, S.; AL-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582.
- Wang, J.; Drelich, A.J.; Hopkins, C.M.; Mecozzi, S.; Li, L.; Know, G.; Hong, S. Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1754.
- Özmen, E.N.; Kartal, E.; Turan, M.B.; Yaziciglu, A.; Niazi, J.H.; Qureshi, A. Graphene and carbon nanotubes interfaced electrochemical nanobiosensors for the detection of SARS-CoV-2 (COVID-19) and other respiratory viral infections: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112356.
- Mattioli, I.A.; Hassan, A.; Oliveira, O.N.; Crespilho, F.N. On the Challenges for the Diagnosis of SARS-CoV-2 Based on a Review of Current Methodologies. ACS Sens. 2020, 5, 3655–3677.
- Yadav, S.; Sadique, M.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995.
More
Information
Subjects:
Physics, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
639
Revisions:
2 times
(View History)
Update Date:
21 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




