Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, X.; Jin, Y.; Zhu, N.; Jin, L.Y. Pillar[n]arene-Based Supramolecular Polymers. Encyclopedia. Available online: https://encyclopedia.pub/entry/55225 (accessed on 13 January 2026).
Li X, Jin Y, Zhu N, Jin LY. Pillar[n]arene-Based Supramolecular Polymers. Encyclopedia. Available at: https://encyclopedia.pub/entry/55225. Accessed January 13, 2026.
Li, Xu, Yan Jin, Nansong Zhu, Long Yi Jin. "Pillar[n]arene-Based Supramolecular Polymers" Encyclopedia, https://encyclopedia.pub/entry/55225 (accessed January 13, 2026).
Li, X., Jin, Y., Zhu, N., & Jin, L.Y. (2024, February 20). Pillar[n]arene-Based Supramolecular Polymers. In Encyclopedia. https://encyclopedia.pub/entry/55225
Li, Xu, et al. "Pillar[n]arene-Based Supramolecular Polymers." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Supramolecular chemistry enables the manipulation of functional components on a molecular scale, facilitating a “bottom-up” approach to govern the sizes and structures of supramolecular materials. Using dynamic non-covalent interactions, supramolecular polymers can create materials with reversible and degradable characteristics and the abilities to self-heal and respond to external stimuli. Pillar[n]arene represents a novel class of macrocyclic hosts, emerging after cyclodextrins, crown ethers, calixarenes, and cucurbiturils. Its significance lies in its distinctive structure, comparing an electron-rich cavity and two finely adjustable rims, which has sparked considerable interest.
pillar[n]arene
supramolecular polymer
1. Introduction
Supramolecular chemistry, often called “chemistry beyond the molecule”, centers on non-covalent interactions such as hydrogen bonding, host–guest recognition, metal coordination, π–π interaction, cation–π interaction, electrostatic binding, salt bridge, halogen bonding, and so on [1][2]. Self-assembly, a practical technique for designing and controlling materials at the nanoscale, has emerged as a powerful synthetic tool, offering control over attributes like shape, size, geometry, topology, and morphology. Developing advanced self-assembled systems through functional building blocks and “weak” intermolecular and intramolecular interactions has garnered increasing attention since 2005.
Generally, supramolecular polymers represent a fusion of supramolecular chemistry and polymer chemistry, wherein small molecules or polymers are joined through non-covalent interactions creating assemblies with defined structures or functions [3][4]. Diverse units and architectural elements can come together through supramolecular interactions to create supramolecular polymers endowed with unique functionalities. Expanding the range of new supramolecular interactions promises to enhance both the structures and the applications of these supramolecular polymers [5]. Moreover, the process of creating inclusions based on supramolecular interactions can be dynamically regulated by external stimuli, enabling the fabrication of controllable and responsive supramolecular materials with specifically desired characteristics [6]. To date, supramolecular polymer materials have found applications across a diverse range of fields.
In many supramolecular polymer structures, macrocyclic host molecules possess appealing structural attributes, encompassing a hydrophobic cavity, sites for ion binding, versatile yet precisely defined conformations, synthetic adaptability, and adjustable functionality [7][8]. Macrocyclic molecules are used in an extremely wide range of applications, including molecular recognition, analyte detection, drug release, self-healing materials, and artificial nanochannels. Chemical structures of macrocyclic supramolecular polymers can be classified into five types (Scheme 1).
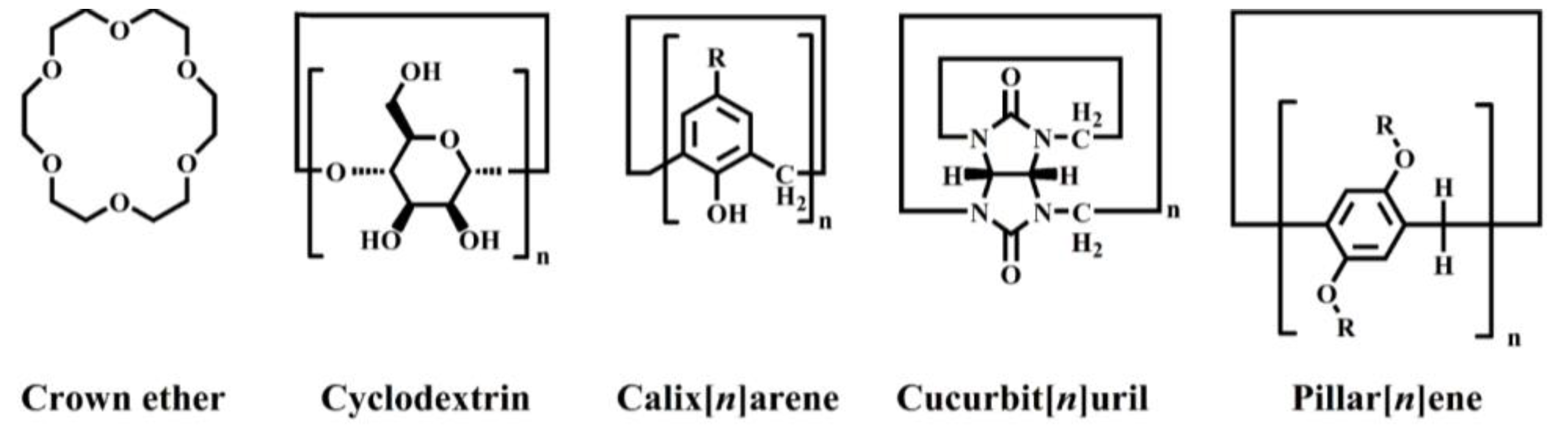
Scheme 1. Molecular structures of five different macrocyclic hosts.
Pillar[n]arene represents a next-generation macrocyclic molecule characterized by its robust complexation capabilities based on supramolecular interactions, which shows outstanding dynamic and modular performance attributes [9][10], especially in sensing [11][12] and catalytic activity [13][14]. Pillar[n]arene was first synthesized by Ogoshi in 2008. Once this unique structure had been discovered, it quickly became a research hotspot [15][16][17][18][19][20][21].
The structures and properties of pillar[n]arene are quite different from previously reported macrocyclic molecules: (i) Compared with crown ether and calixarene, pillar[n]arene has a highly symmetrical rigid structure with hydrophobic cavities at the top and bottom edges. The adjustment of cavity size also has a higher degree of freedom, which shows stronger abilities to identify guests and incorporate catalytic units. (ii) Pillar[n]arene has good solubility in organic solvents, which is unique in the main macrocyclic molecules with cavities of the same size. (iii) Compared with cucurbituril, the benzene ring of pillar[n]arene is more easily functionalized by different substituents, which can regulate the host–guest binding characteristics more effectively. As a result, a large number of derivatives with unique properties have been obtained. (iv) Pillar[n]arene can be an amphiphilic molecule with hydrophobic and hydrophilic groups. A structure with such characteristics can form self-assembly structures in both water and oil phases, which has potential applications in controlled release, gene transfer, photoelectric materials, etc. (v) Pillar[n]arene can respond to many external stimuli, including temperature, pH, redox, enzyme, etc., especially light stimulation. These stimuli are easy to operate and can be utilized in a non-invasive and lower-cost manner. This is also one of the advantages of pillar[n]arene in sensing compared with other macrocyclic structures.
At present, the mainstream work of pillar[n]arenes is focused on prominent pillar[5]arenes and pillar[6]arenes, as shown in Scheme 2. The most commonly studied structure was pillar[5]arenes with a cavity size of approximately 4.7 Å and an average bond angle of 111.3°. The structures of pillar[n]arenes of other sizes have not been systematically studied. The production yields of high-level pillar[n]arenes (where n is equal to or greater than 6) typically remain below 1%, resulting in their infrequent utilization in subsequent investigations.
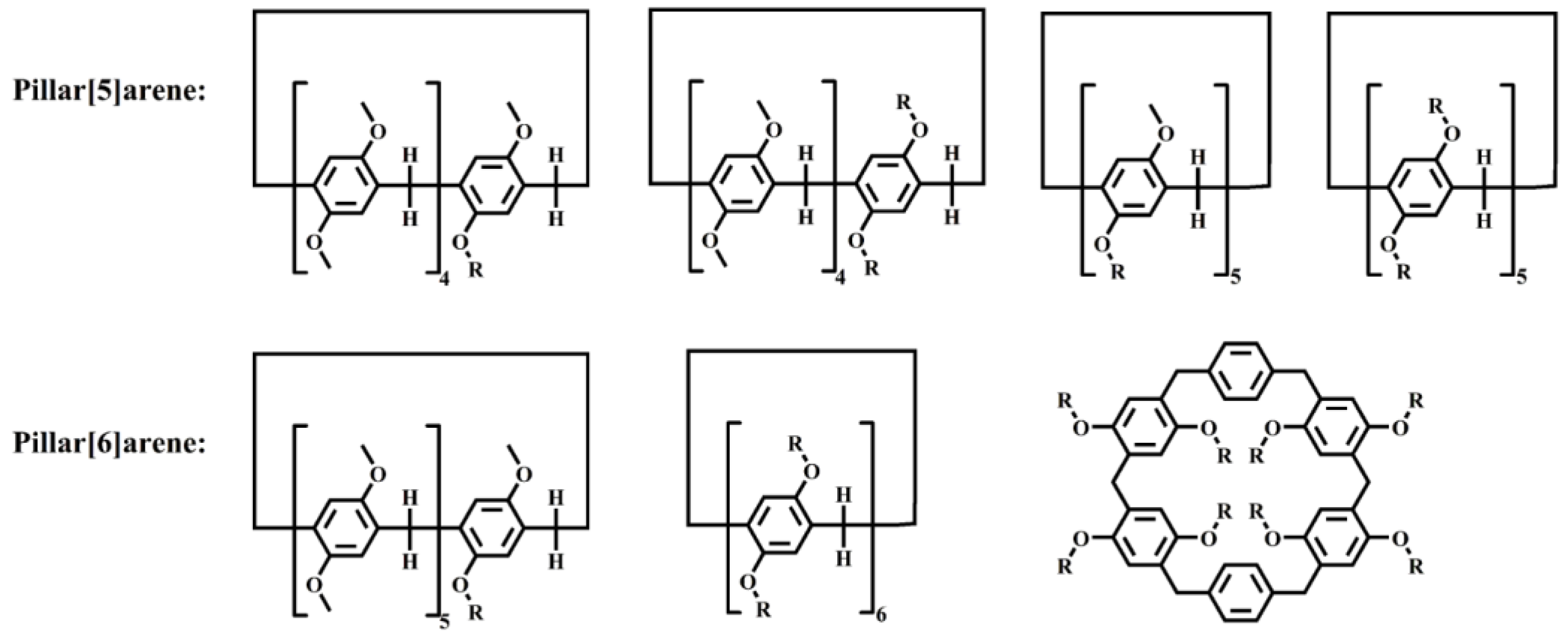
Scheme 2. Typical molecular structures of pillar[n]arenes. R represents different functional groups.
The electron-rich cavities of pillar[n]arene can form complexes with various guest molecules exhibiting neutral architectures or positive charges ascribed to supramolecular interactions. Guest molecules consisting of long carbon chains generally require cyanide, bromine, or quaternary ammonium salt as the end groups for host–guest identifications. According to the role of pillar[n]arenes in the construction of polymer materials, pillar[n]arene-based supramolecular polymers (PSPs) are mainly classified into two categories: (i) Pillar[n]arene firstly generates complexes with other guests, and such complexes are further assembled to form one-dimensional, two-dimensional, and three-dimensional spatial structures through the effects of non-covalent bonding such as hydrogen bonding, metal–ligand bonding, and π–π stacking [22][23][24][25]; (ii) Pillar[n]arene is treated as a kind of functional unit and introduced into the classical polymer substrates through covalent bonding, host–guest interactions, and other non-covalent dynamic interactions [26][27]. Based on the definition of PSPs, the PSP materials are unique in the following three points compared with the classical covalent macromolecules. Firstly, the main mechanism of supramolecular polymer formations is self-assembly. Secondly, the supramolecular polymers are able to introduce a variety of groups through host–guest recognitions or other weak interaction forces. Thirdly, pillar[n]arene-based molecules can self-assemble into chiral supramolecular polymers [28][29][30][31][32]. The constructed supramolecular polymers are a type of tunable material with features such as structural and mechanical integrity, good processability and recyclability, stimuli-responsiveness, self-healing, and shape memory, which holds outstanding advantages over classical covalent macromolecule polymers. In conclusion, PSPs not only inherit numerous advantages of conventional covalent polymers but also offer distinct characteristics [33].
2. Applications of PSPs
2.1. Fluorescence Sensor
As a novel sensing approach, supramolecular sensors have gained significant attention for their remarkable affinity towards a wide range of analytes. Pillararenes and their derivatives, with macrocyclic cavities, exhibit robust recognition capacities based on supramolecular interactions, particularly for ions and biomolecules, making them a focal point in this burgeoning field. Structure modifications can be achieved using various methods, including thermal responsiveness [34][35][36], electrochemical responsiveness [37][38][39], redox reactivity [40], and the incorporation of fluorescent [41][42][43][44][45][46][47][48][49] and other functional units [50][51][52][53][54][55][56][57][58]. The chemical structures of pillar[n]arenes and guest molecules are listed in Scheme 3.
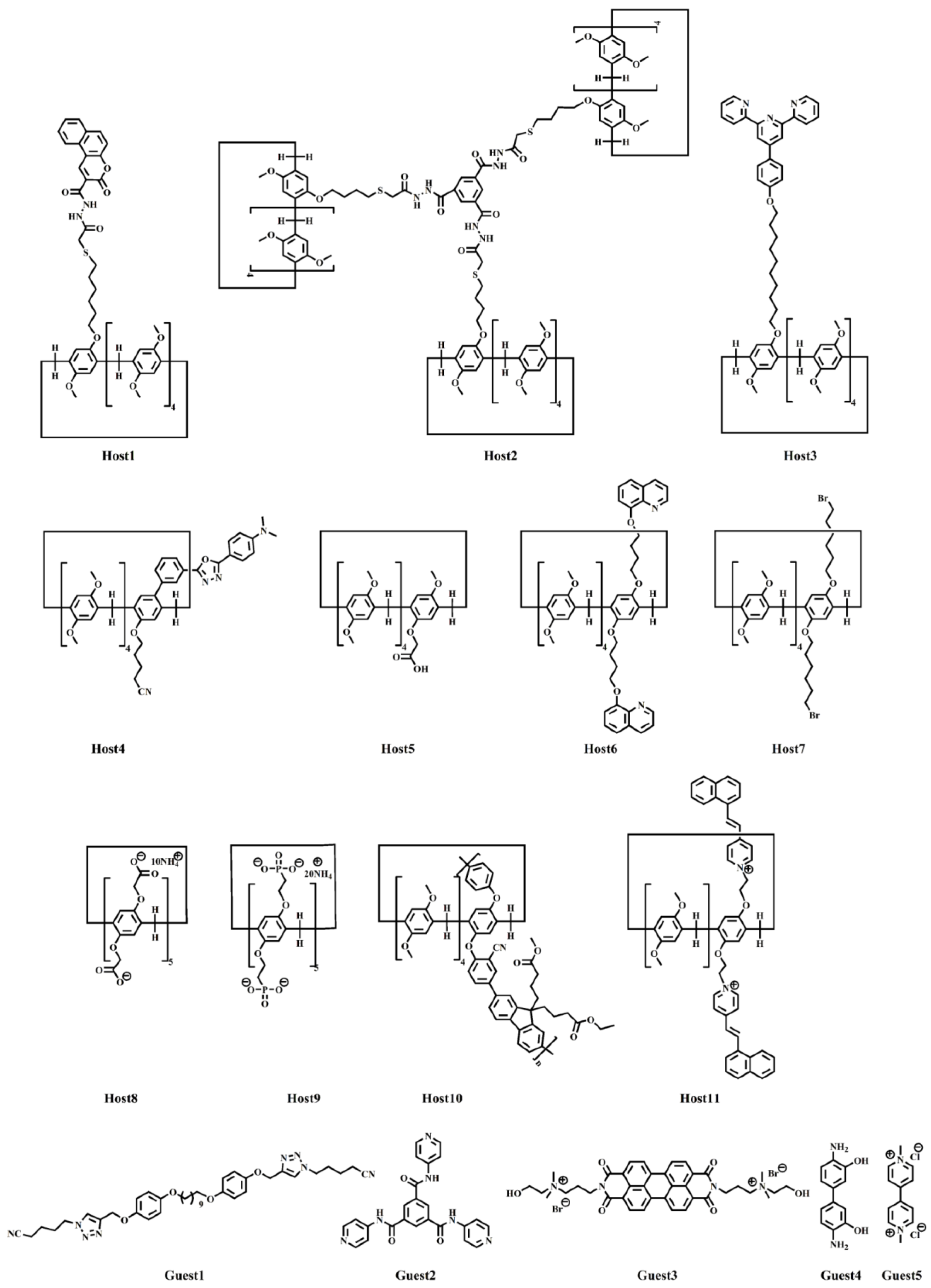
Scheme 3. Molecular structures of pillar[n]arenes and guest molecules.
Among the numerous response mechanisms available, fluorescence-based sensors have become notable due to their simple synthesis, real-time analysis, high temporal and spatial resolution, reversibility, and exceptional selectivity and limit of detection (LOD) towards analytes [59][60][61][62][63].
PSPs can capture specific analytes, leading to observable fluorescence signals that manifest as quenching, fluorescence enhancement, or shifts in response to energy transfer, charge transfer, electron transfer, and strong coordination interactions. Functionalized pillar[n]arene holds promising potential for use and advancement in fluorescence sensors. These systems respond to a broad spectrum of potential analytes, encompassing metal cations, anions, drugs, pesticides, and other biomolecules.
Metal ions and amino acids are essential components within the fields of chemistry, biology, and the environment. The selective identification and testing of specific analytes and the isolation of toxicity are critical endeavors. In addressing these challenges, numerous research teams have designed and synthesized supramolecular polymers featuring diverse morphologies, including cross-linked networks and brush architectures. Detecting and separating hazardous metal ions like Fe3+ are crucial in environmental protection and healthcare.
In 2023, Li et al. [64] introduced a stimuli-responsive supramolecular polymer network to address this challenge. The pillar[5]arene derivative Host1 engaged in supramolecular interactions with Guest1. This innovative configuration displayed impressive fluorescence characteristics attributed to the Aggregation-Induced Emission (AIE) effect and demonstrated remarkable ion-sensing capabilities in both solutions and solid states. Supramolecular polymers have the potential to undergo a transition into supramolecular gels under specific conditions. When the polymer is concentrated, it forms a polymer gel that responds to changes in temperature, mechanical force, and the competitive agent. This xerogel effectively removes Fe3+ from water. Additionally, a fluorescence test kit utilizing an ion-responsive film can be conveniently employed for detecting Fe3+.
While it is known that AIE materials based on the supramolecular organic frameworks (SOFs) possess the ability to adjust emission, it is worth noting that there are relatively few reports on stimuli-responsive soft materials with the capacity for tunable AIE. Lin et al. [65] developed an AIE supramolecular organic framework gel (SOF-TPN-G) to detect and separate various metal ions. This innovative material allows for recognizing multiple metal ions through the changes in fluorescent signals. Host2 and Guest2 join together to create supramolecular polymer networks (SPNs). TPSN serves as the π–π interactions, C−H···π interactions, and inclusion interactions. The thioacetylhydrazine moiety functions as the binding site for hydrogen bonding and coordination. The introduction of certain ions into SOF-TPN-G led to the creation of numerous metallogels exhibiting multicolor fluorescence. Leveraging these gels, an eight-unit sensor was successfully developed, with some units achieving an ultrasensitive performance. The xerogel form of SOF-TPN-G proved highly effective in adsorbing multiple metal ions. These gel materials have significant potential for applications in detection and separation processes.
Recent reports indicate that the neutral Guest1, featuring a cyano site and a triazole site, has proven to be an efficient guest molecule for Host3 in supramolecular interactions. Inspired by the stable coordination compounds formed by terpyridyl groups and zinc ions, Yao et al. [66] designed a high molecular weight and fluorescent supramolecular polymer with a cross-linked network (Figure 1). This polymer was created through the principles of molecular recognition and metal coordination involving pillararene. Three-dimensional networks were formed through the entanglement of the supramolecular bundles, and phase transitions were achieved by alternating heating and cooling. The polymer displayed concentration-dependent fluorescence and was responsive to basic stimuli. A thin film was subsequently prepared, which is a convenient test kit for detecting OH− anions.
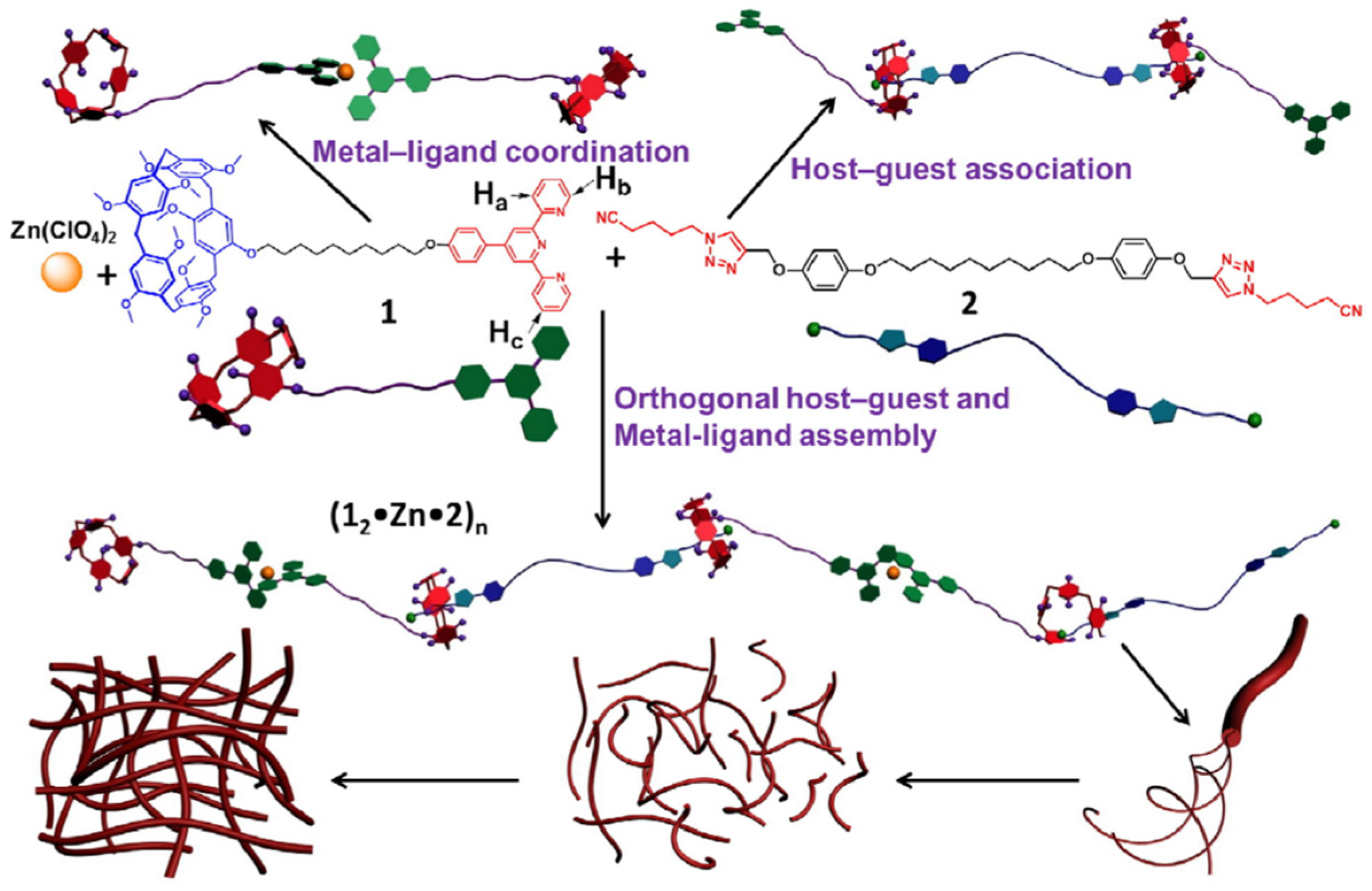
Figure 1. Representation for the formation of a fluorescent supramolecular polymer Co-P[5]Flu constructed by pillar[5]arene-based molecular recognition and zinc ion coordination.
In recent years, 1,3,4-oxadiazole (OXD) derivatives have found widespread utility in various domains owing to their exceptional photoluminescent quantum yield and chemical stability.
In collaboration with Zhang and his research team, Han [67] developed a supramolecular brush polymer named Poly(P5-OXD) through the self-assembly of Host4, featuring a 1,3,4-oxadiazole unit and a cyanobutoxy group. This polymer exhibited responsiveness to Cu2+ ions, resulting in an ON-OFF fluorescent switch. Upon adding Cu2+, the supramolecular brush polymers displayed altered structural characteristics, as evidenced by fluorescence quenching, suggesting the potential transformation into cross-linked supramolecular networks.
Over the past decade, AIE has grown significantly across numerous domains. The collaboration between supramolecular materials and coordination chemistry holds immense potential to enhance the rapid advancement of multifunctional materials further. Creating fluorescent coordination polymers incorporating supramolecular macrocyclic rings is a novel and complex research area. In this context, pillarenes are regarded as organic linkers due to their inherent porous properties. In 2018, Wu and Yang [68] developed a fluorescent coordination polymer named CP5-PCP. This polymer featured dicarboxylic acid-containing copillar[5]arene (Host5) as the organic linker and Cr3+ as the nodes. This sophisticated fluorescent material found applications in detecting Fe3+, acetone, and nitrophenols. The creation of this synthetic material has significantly contributed to the advancement of both supramolecular materials and coordination chemistry.
Recently, there has been a growing focus on ultrasensitive response materials because of their remarkable sensitivity to target substances. Furthermore, the ability to detect exceedingly low concentrations of targeting analytes in environmental or biological systems holds significant importance. Fe3+ is the most prevalent and extensively used metal ion in living organisms, ranking among the most critical transition metal ions. Nonetheless, a deficiency and an excess of Fe3+ can lead to severe biological disorders and disruptions in normal bodily functions, resulting in diseases. Fluoride ions (F−) naturally occur in the environment and are present in the human body. However, excessive fluoride levels lead to environmental contamination and pose health risks, including kidney problems, acute stomach issues, and conditions like dental and skeletal fluorosis. Hence, there is a pressing need to devise novel and straightforward ultrasensitive methods for detecting Fe3+ and F− ions. Zhang et al. [69] significantly addressed this challenge by successfully developing a supramolecular organic framework gel PQPA. They achieved this by utilizing bilateral 8-hydroxyquinoline-modified pillar[5]arene (Host6) and bilateral bromohexyl-functionalized pillar[5]arene (Host7) through supramolecular interaction binding. The LOD achieved by PQPA for Fe3+ was an impressive 0.102 nM, highlighting this gel’s selective and ultrasensitive properties. Notably, PQPA proved effective for the detection of Fe3+ in real-life samples. Simultaneously, by introducing Fe3+ into PQPA gel, a non-fluorescent Fe3+ coordination metal gel, PQPA-Fe, can be created. This gel exhibited a “turn-on” fluorescent response to F− due to competitive coordination. The LOD for F− achieved by PQPA-Fe was 9.79 nM. Furthermore, the PQPA xerogel demonstrated the ability to efficiently remove Fe3+ at lower concentrations with a high adsorption rate.
Organic solvents are undesirable for ion detection in biological and environmental systems. Consequently, there is significant importance in developing water-soluble derivatives that exhibit exceptional detection capabilities in aqueous solutions.
The sensitivity of perylene diimide derivatives was constrained due to their inherent aggregation-induced quenching (ACQ) properties in aqueous environments. In 2017, Yin et al. [70] introduced a supramolecular interaction system that relied on Host8 and a quaternized perylene diimide Guest3 designed for use in aqueous media. In this process, the hydrophobic effects and electrostatic interactions played pivotal roles. In an aqueous solution, Guest3 formed strong fluorescent aggregates. However, when Host8 was introduced into the Guest3 aqueous solution, a change in morphology occurred, leading to a fluorescence “turn-off” phenomenon due to the photoinduced electron transfer (PET) process. The addition of Fe3+ reversed this supramolecular PET process, resulting in a “turn-on” fluorescent response. The detection of Fe3+ exhibited specific, ratiometric, and reversible behavior, with an LOD of 2.13 × 10−7 M.
Inclusion compound probes that are built using macrocyclic compounds have garnered significant research interest and can detect various metal cations sensitively. Yang et al. [71] introduced a highly sensitive and selective fluorescence sensor capable of quantitatively analyzing Au3+ ions in aqueous systems. This sensor achieved an LOD as low as 6.9 × 10−8 M. The sensor relies on the supramolecular interactions between Guest4 and Host9, forming an inclusion complex prepared using the saturated solution method. Including Guest4 by Host9 significantly increased the water solubility of Guest4, boosting it by 177-fold. The inclusion complex IC exhibited a larger Stokes shift in aqueous conditions alone. The stoichiometric ratio between Host9 and Guest4 was determined to be 1:1, and the binding constant was calculated to be (4.62 ± 0.15) × 104 M−1. Notably, the IC displayed robust resistance to interference and demonstrated excellent adaptability over a wide range of pH values.
The PSPs find extensive applications in drug detection. Investigations were also focused on studying another typical pesticide, namely paraquat (PQ). PQ is a non-selective and fast-acting herbicide that, if accidentally ingested, can severely damage human lungs, liver, kidneys, and other vital organs. Given the significant clinical and environmental implications of PQ, there is a pressing need to design new methods for its high-sensitivity and accurate detection. PQ can interact not only with electron-rich molecules but also form supramolecular interaction complexes and columnar structures with macrocyclic molecules like calixarenes, cucurbiturils, and others.
Fluorene possesses a planar and rigid biphenyl structure with a high fluorescence quantum yield. To enhance the solubility of the material, chemical modifications were performed on fluorene derivatives by introducing functional side chains at the C9 position of the fluorene molecule. Covalent-bonded conjugated polymers exhibited a molecular wire effect that enhances the response signal in fluorescence sensing applications.
Building on the principles mentioned earlier, Li, along with Xiao and their team [72], synthesized a novel conjugated polymer named (Co-P[5])Flu. This polymer was obtained through the copolymerization of a difunctionalized pillar[5]arene (Host10) and a fluorene derivative monomer. The intramolecular rotations of the pillar[5]arene unit were constrained in Co-P[5]Flu, resulting in an AIE enhancement (AIEE) effect. Pesticide PQ (paraquat, Guest5) could enter the pillar[5]arene cavity within Co-P[5]Flu. In EtOH/H2O, Co-P[5]Flu exhibited fluorescence quenching in response to PQ, attributed to the synergistic effects of polypseudorotaxane formation and PET. Co-P[5]Flu demonstrated exceptional selectivity and sensitivity in detecting PQ, with a low LOD of 1.69 × 10−8 M and a determined Stern–Volmer constant (Ksv) of 2.11 × 104 M−1. This polymer chemosensor proved to be valuable for the practical detection of PQ.
Researchers are actively designing and synthesizing materials with improved performance for PQ detection to enhance the sensitivity of detection. The combination of two different macrocyclic compounds in such efforts holds significant promise and is an intriguing avenue of exploration. Indeed, there has been limited research on the potential applications of such materials thus far.
However, a new category of supramolecular polymers involving two distinct macrocyclic host molecules, namely pillar[n]arene NTP5 (Host11) and Q[10], was successfully synthesized by Xiao et al. [73]. Representation for the formation of a fluorescent supramolecular polymer was shown in Figure 2. The inclusion of the macrocyclic Q[10] significantly positively impacted the fluorescence properties of NTP5 by constraining its intramolecular rotation. Specifically, the 4-[2-(1-naphthalenyl)ethenyl]pyridine moiety of NTP5 was encapsulated by Q[10], leading to the formation of a supramolecular interaction complex. The introduction of Q[10] caused a transformation in the morphology of NTP5, changing it from a spherical structure to a porous one, resulting in enhanced fluorescence. Interestingly, the fluorescence of Q[10]-NTP5 could be quenched by adding PQ, making it suitable for molecular recognition and bioimaging applications.
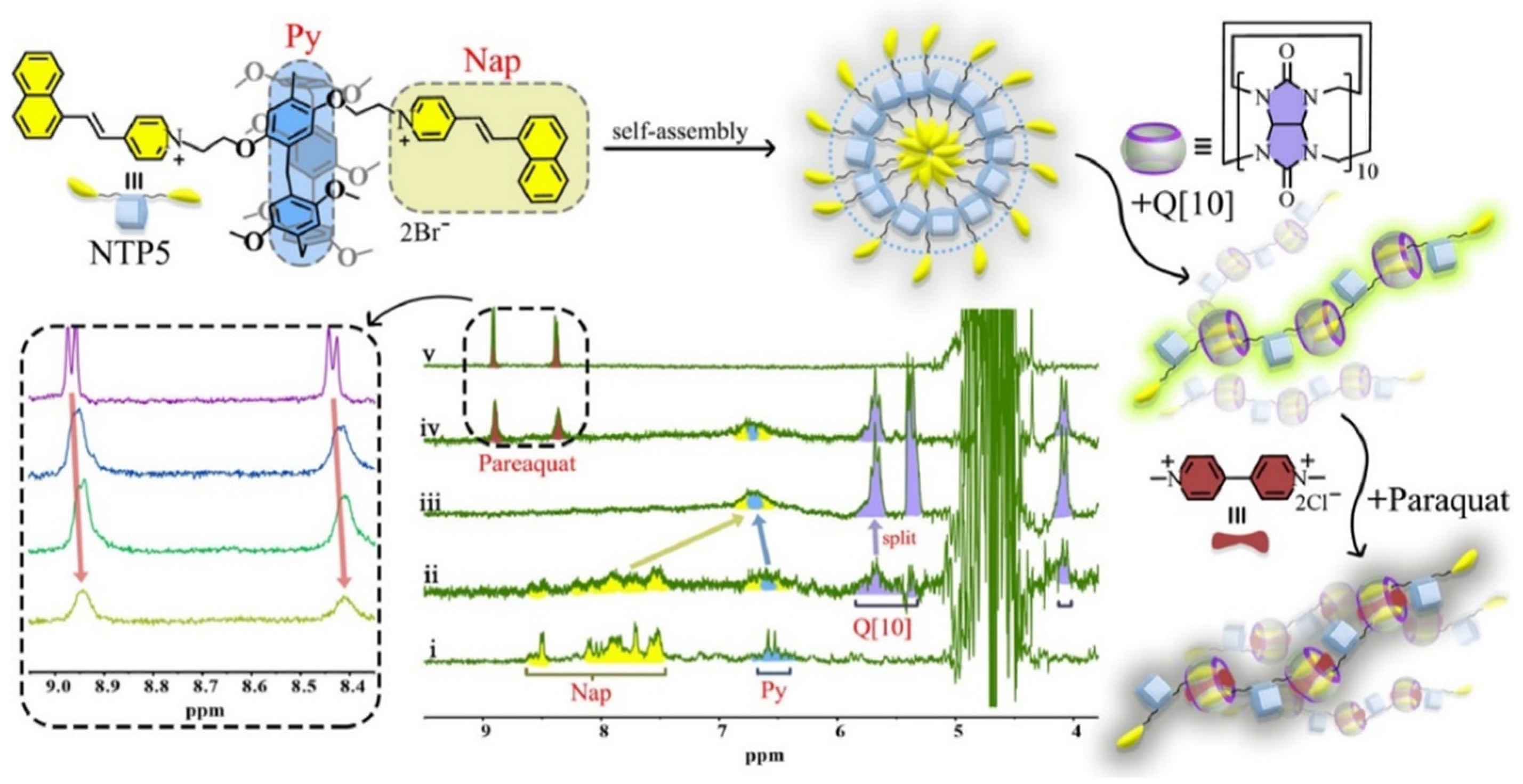
Figure 2. Schematic representation of a novel pillar[5]arene-cucurbit[10]uril−based complex and its application for the detection of paraquat. Illustration (top) of the interactions of NTP5, Q[10]-NTP5, Q[10]-NTP5-PQ, and the 1H NMR spectrum (bottom) (inset: Q[10]-NTP5 at different concentrations of PQ).
2.2. Substance Adsorption and Separation
Pillar[n]arene offers a diverse range of supramolecular interactions, making it a highly sought-after building block for SPs (supramolecular polymers) with multifunctionality [74][75]. Additionally, the structure of the polymers can create novel voids within the networks and spaces [76], complementing inherent pillar[n]arene cavities, which functions as new binding sites for guest molecules [77][78]. Achieving the environmentally friendly and efficient removal of various pollutants is a crucial challenge in environmental science and industrial applications. This issue directly impacts human health and the health of aquatic ecosystems [79][80][81][82][83][84][85]. Pillararene materials serve as protective containers, accurately capturing and safely storing harmful molecules [86][87][88]. The properties and applications of polymers are influenced by factors such as the size of the macrocyclic pore, connectivity, and the chemical environment within the hosts. Self-assembly aggregates contribute to the removal effect. This concept provides a novel approach to adsorbing and separating ions and organic substances. Meanwhile, the chemical structures of pillar[n]arenes and guest molecules are listed in Scheme 4.
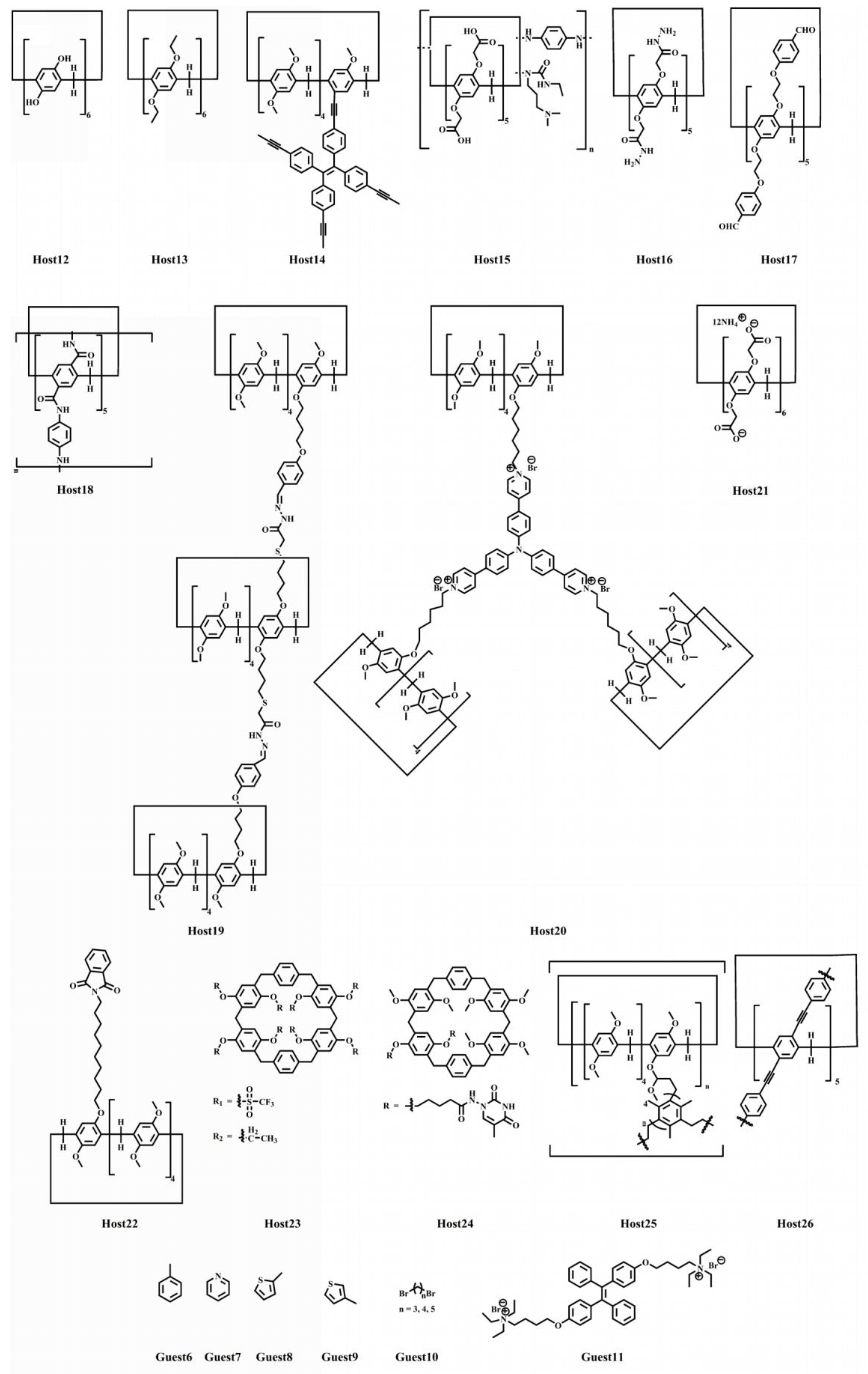
Scheme 4. Molecular structures of pillar[n]arenes and guest molecules.
Materials featuring micropores are highly suitable for gas storage and separation applications. A growing focus has recently been on discrete organic molecules instead of 1D-3D networked materials. This shift is driven by the advantages of solubility, flexibility, and the feasibility of obtaining these materials through organic synthesis. Ogoshi et al. [89] found that the aromatic molecule within the pillar[6]arene (Host12) forms a highly ordered 1D channel with a diameter of 6.7 Å. This supramolecular assembly channel can capture various gases, including water vapor-saturated hydrocarbons. The organic gas and vapor adsorption capacity of pillar[6]arene in a solid state presents a promising avenue for further exploration.
In industrial processes, toluene is primarily derived from coal and petroleum sources through catalytic reforming, pyrolysis of hydrocarbons, and coking. However, these processes often leave trace amounts of heterocyclic compounds behind, which can significantly impact the properties of toluene. Therefore, efficiently removing these heterocyclic compounds is crucial for obtaining high-purity toluene.
Zhu et al. [90] introduced a separation strategy for mixtures of toluene and heterocyclic compounds using non-porous adaptive guest-loaded crystals of perethylated Host13. The approach successfully enhanced the purity of toluene, increasing it from 96.78% to 99%. The stability difference strongly influenced the selectivity of this material in the EtP6 crystal structure after incorporating various guest molecules (Guest6, 7, 8, and 9). Upon removing the guest molecules, the structure of EtP6 reverted to its original state.
Tetraphenylethylene (TPE), which stands for AIE properties, was linked to P[5] using a Sonogashira–Hagihara cross-coupling reaction, resulting in the formation of P[5]-TPE (Host14). In their study, Yang et al. [91] introduced a macrocyclic cross-linked reticular polymer, P[5]-TPE-CMP, which demonstrated remarkable properties, including resistance to photobleaching, insolubility, recyclability, and selective sensing capabilities for Fe3+ ions and 4-amino azobenzene (Figure 3). P[5]-TPE-CMP also functionalized as a two-photon fluorescence sensor, detecting wavelengths in the 300–400 nm and 650–800 nm ranges. In solution, it exhibited a range of microstructures, including various sizes of spherical and/or rodlike structures. Both P[5]-TPE-CMP materials displayed remarkable thermal stability, remaining stable up to 300 °C in air. The material demonstrated sensitivity and selectivity in the detection process, making it a promising candidate for removing harmful safety-related substances.
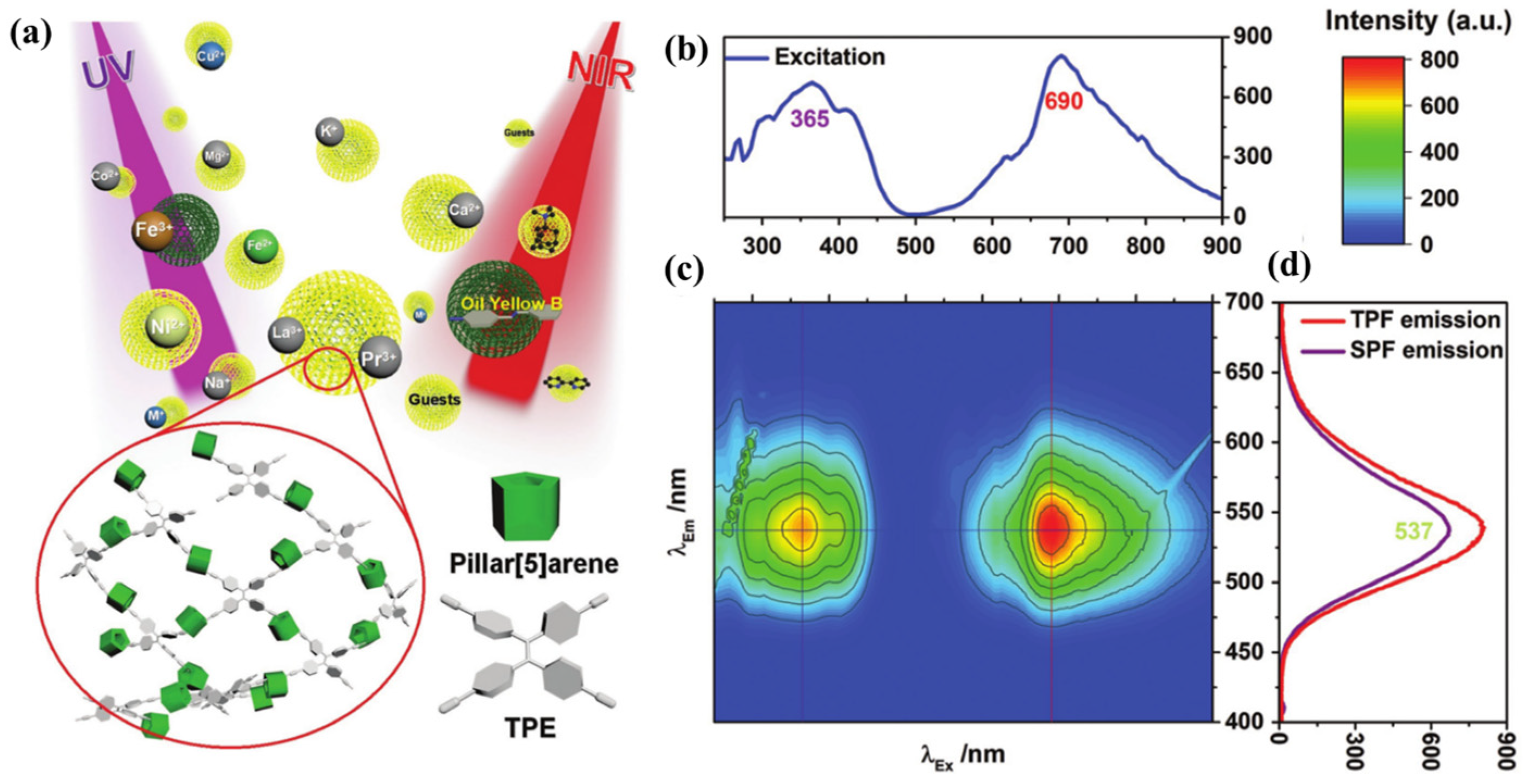
Figure 3. (a) Schematic representation of a conjugated macrocycle polymer (P[5]-TPE-CMP) based on pillar[5]arene and TPE and its two-photon sensing for metal ions and organic molecules. (b) Excitation spectrum (blue). (c) The 3D fluorescence contour spectroscopy of P[5]-TPE-CMP. (d) Single-photon fluorescent emission spectrum (purple) and two-photon fluorescent (TPF) emission spectrum (red).
Organic micropollutants, including drugs, hormones, pesticides, and industrial chemicals in water systems, threaten human health and aquatic ecosystems. Consequently, creating rapid, efficient, and cost-effective chemical and physical water treatment methods is a pressing challenge for material chemists. The design and synthesis of sustainable adsorbent materials that offer fast adsorption and high absorption capacity remain significant challenges in addressing the removal of organic micropollutants from water.
In 2017, Shi et al. [92] developed a 3D network polymer known as P5-P (Host15), which was cross-linked using carboxyl-derived pillar[5]arene and p-phenylenediamine. This polymer exhibited excellent capabilities for adsorbing and removing organic micropollutants from water. Furthermore, this polymer demonstrated recyclable adsorption performance, particularly for fluorescein sodium and methyl orange. The 3D network polymer exhibited the potential for rapid waste-water treatment. Two years later, they applied hydrazide-functionalized pillar[5]arene (Host16) and 4-aldehydephenyl-functionalized pillar[5]arene (Host17) to obtain another intelligent hydrogel [93]. The hydrogel exhibited a remarkable removal efficiency of 91.5% for organic micropollutants in water. The primary driving force behind its action was the supramolecular interactions between the macrocycle and pollutant molecules. The hydrogel material could be regenerated at least five times by disrupting the supramolecular interaction complex.
The most prevalent sources of water pollution stem from industrial waste discharges and municipal sewage, which release a combination of chemicals and microorganisms. Recognizing the severity of this issue, Wen et al. [94] developed a conjugated polymer based on pillar[5]arene (Host18), which is an acid-derivatized poly-p-phenylene pillar[4]arene[1]terephthamide (P[5]-PPTA). In P[5]-PPTA, the pillar[5]arene rings engage in supramolecular interactions with short-chain alkyl derivatives. The polymer exhibited a unique absorption capability for low-molecular-weight alkylamines, alcohols, and carboxylic acids in water, and it maintained its performance across five successive cycles of reuse without degradation. Furthermore, P[5]-PPTA could be thermally reactivated, enhancing its versatility for water treatment applications.
In the studies of separation materials, Wang et al. [95] designed an adsorbent material for PQ by harnessing the combined effect of linear tri-pillar[5]arene-based compounds (Host19). This design linked three pillar[5]arene groups within a single acceptor molecule. This material demonstrated several advantages, including a faster adsorption rate for PQ compared to activated carbon and single-pillar[5]arene-based materials. Additionally, its removal efficiency met expectations, with a remarkable 98% adsorption rate for commercial pesticide PQ in water. A year later, Zhang et al. [96] introduced a highly effective tripodal molecule based on tri-pillar[5]arene (Host20), which exhibited a “synergistic effect” for the efficient detection and removal of the pesticide PQ through supramolecular interactions. Each molecule featured two adjacent pillar[5]arene groups capable of binding one PQ molecule through multi-non-covalent interactions. The LOD was impressively low, reaching 2.23 × 10−7 M. The testing paper utilizing Host20 could be a detection kit for PQ in water across a wide concentration range (10−6–10−1 M). Moreover, it demonstrated exceptional adsorption capabilities in water, with an impressive adsorption efficiency of 98.40% for commercial PQ and a removal capacity of 108.24 mg/g. Yang et al. [97] designed and successfully prepared a controllable supramolecular coordination polymer Zn2+@WP5 (Host21) in a related development. The polymer displayed outstanding adsorption and removal capabilities for PQ, owing to its negative charge, high surface area, and recognition capacity based on supramolecular interactions. The maximum adsorption capacity for PQ reached approximately 203.60 mg/g. The polymer was successfully employed in real sample applications and could be regenerated by exchanging the guest structure.
Anions are crucial in maintaining human health, biological systems, and environmental stability. The precise detection and separation of these ions are of paramount importance. Consequently, the ultrasensitive detection and separation of various environmental ions are gaining significance. Lin et al. [98] developed a smart blue-white AIE gel material (PMDP-G), which was constructed using functionalized Host17 and Host22, utilizing “exo-wall” π–π and supramolecular interactions. PMDP-G demonstrated remarkable sensitivity in detecting and separating multiple analytes, achieved through the interplay between “exo-wall” π–π and cation–π interactions. The low LOD underlined its high sensitivity. PMDP-G exhibited the capacity to adsorb and separate multiple metal ions from aqueous solutions, including Fe3+, Hg2+, and Ag+, with adsorption rates ranging from 90.12 to 99.95%.
Water contamination from heavy metal ions poses a grave threat to humans and animals globally. The development of a comprehensive approach for real-time sensing and the swift removal of mercury(II) ions holds immense significance in materials chemistry, chemical engineering, and environmental science. In recent years, there has been a growing emphasis on designing and synthesizing cost-effective and efficient materials for the adsorption and removal of Hg2+ ions. Developing novel technologies for detecting Hg2+ in the atmosphere and industrial waste water is essential to enhance environmental protection and safeguard life on our planet.
As reported by Yang in 2018, leaning tower[6]arenes (LT6, Host23) exhibit a distinctive leaning tower conformation with minimal substituents, which sets them apart from the traditional pillar[6]arenes structures. These molecules have shown excellent performance in supramolecular interaction systems and macrocyclic chemistry. Yang’s group developed a set of four novel CMPs (CMP-n, n = 1–4) using per-triflate-functionalized and [2]biphenyl-extended pillar[6]arene (Figure 4) [99]. CMP-4 exhibited an exceptional affinity for I2, with a vapor uptake capacity of 208 wt % and a remarkable removal efficiency of 94 %. These properties were attributed to the well-suited cavity size of the tower[6]arene and numerous aromatic rings within the framework. CMP-2 displayed no capacity to adsorb N2, making it highly selective for CO2 capture.
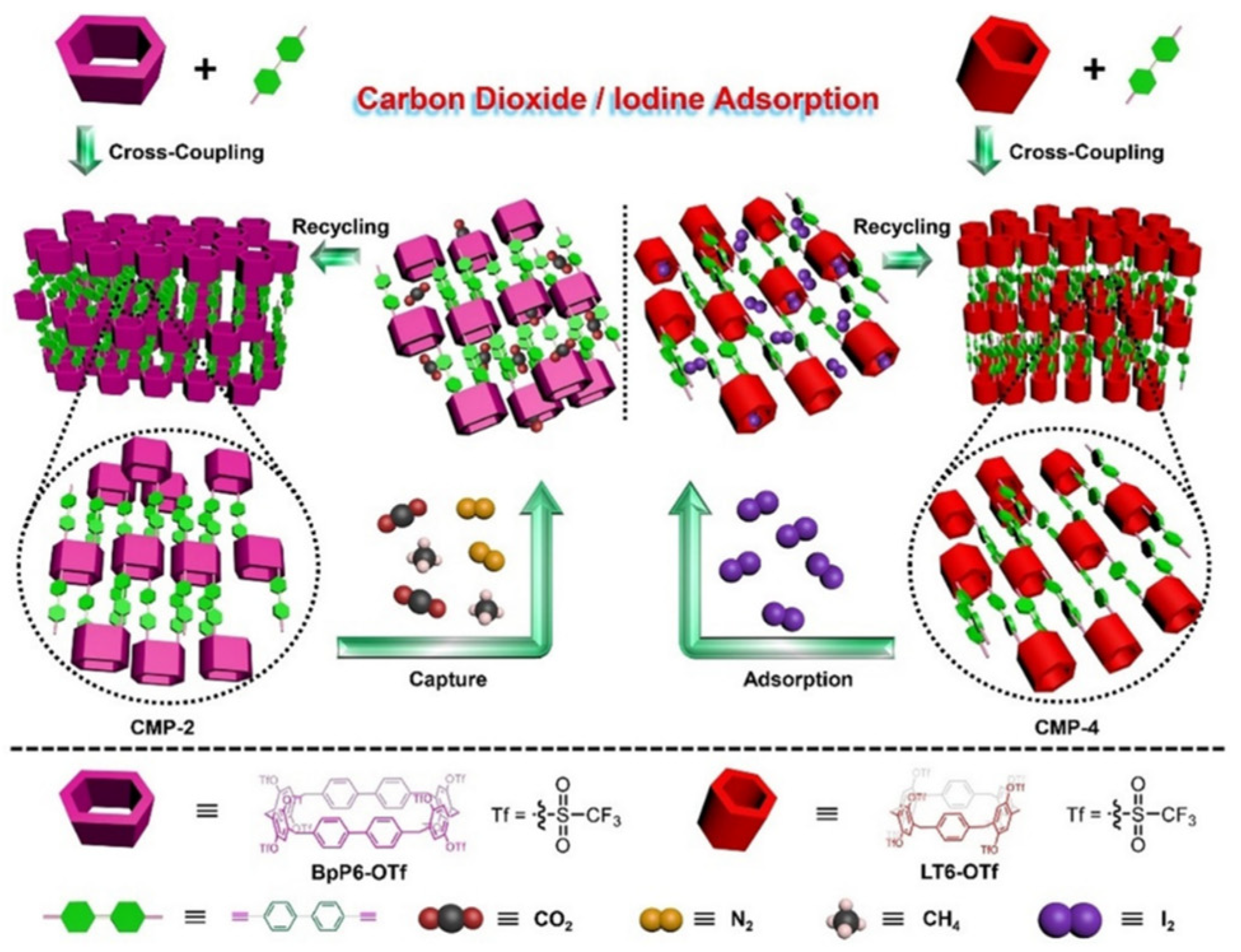
Figure 4. Illustration of the construction of four CMPs (CMP-n, n = 1–4), selective capture of CO2, and efficient adsorption of I2.
In 2019, Wu et al. [100] introduced a straightforward separation method utilizing a novel type of non-porous adaptive crystals built upon inclined pillar[6]arene (Host23). These deoxidized perethyl-inclined pillar[6]arene crystals (EtLP6) displayed non-porous characteristics, exhibiting a preference for 1-bromoalkane isomers over 2-bromoalkane isomers in the separation process. EtLP6 demonstrated the ability to separate 1-bromopropane, 1-bromobutane, and 1-bromopentane from their 1/2 isomer mixtures at a 1:1 (v/v) ratio in a single adsorption cycle. The obtained purities ranged from 89.6% to 96.3%. This selectivity is due to the distinct supramolecular interaction binding modes and varying stability of EtLP6 crystals loaded with one-site and two-site isomers.
In 2019, Dai et al. [101] introduced a fluorescent supramolecular polymer. This polymer was created using a [2]biphenyl-extended Host24 with two thymine sites as arms, along with a tetraphenylethylene-bridged bis(quaternary ammonium) Guest11. Spherical nanoparticles were generated upon adding Hg2+, increasing the emission intensity. This approach was suitable for the real-time detection and removal of Hg2+. It demonstrated a rapid response, high selectivity, and fast adsorption rates. Additionally, the material could be recycled by treatment with Na2S.
Arunachalam et al. [102] made significant contributions to the removal of iodine vapor by successfully synthesizing a recyclable 3D cross-linked microporous polymer based on pillar[5]arene Host25, which demonstrated an iodine vapor capture capability of 3.84 g/g at 80 °C. The polymer adsorbent efficiently removed over 83% of iodine within 15 min of shaking in an aqueous solution. Pillar[n]arene holds significant potential for applications in environmental remediation. Additionally, their versatility allows for various possibilities for further functionalization, enhancing their utility in environmental improvement.
To address the issue of water stability in separation systems, Talapaneni et al. [103] introduced an efficient conjugated microporous polymer based on pillar[5]arenes (P5-CMPs, Host26). These P5-CMPs were created by attaching triflate-functionalized P5 to 1,4-diethynylbenzene and 4,4′-diethynyl-1,1′-biphenyl linkers. The P5-CMPs exhibited an impressive surface area of up to 400 m2∙g−1. In earlier studies, porous materials displayed isosteric heats of adsorption (Qst) for propane at zero coverage in the range of 32.9–36.9 kJ/mol. The Qst value increased as the propane loading increased, indicating stronger intermolecular interactions between propane molecules. The Qst value reached a maximum of 53 kJ/mol at zero coverage and then gradually decreased to approximately 35 kJ/mol as the loading of propone increased. This phenomenon was attributed to the formation of a robust supramolecular complex between propane and pillar[5]arenes, facilitated by multiple C–H···π interactions. This method introduced a thermodynamic selective separation of saturated hydrocarbons with low polarizability. The strong affinity of P5-CMPs for propane allowed for efficient breakthrough encapsulation and separation from natural gas mixtures under simulated conditions at 298 K.
References
- Mujahid, A.; Afzal, A.; Dickert, F.L. Transitioning from supramolecular chemistry to molecularly imprinted polymers in chemical sensing. Sensors 2023, 23, 7457.
- Lu, J.; Deng, Y.; Liu, P.; Han, Q.; Jin, L.Y. Self-assembly of β-cyclodextrin-pillararene molecules into supramolecular nanoassemblies: Morphology control by stimuli responsiveness and Host-Guest interactions. Nanoscale 2023, 15, 4282–4290.
- Zheng, F.; Du, W.; Yang, M.; Liu, K.; Zhang, S.; Xu, L.; Wen, Y. Constructing ROS-responsive supramolecular gel with innate antibacterial properties. Pharmaceutics 2023, 15, 2161.
- Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable construction of temperature-sensitive supramolecular hydrogel based on cellulose and cyclodextrin. Polymers 2022, 14, 3801.
- Ohtani, S.; Kato, K.; Fa, S.; Ogoshi, T. Host-Guest chemistry based on solid-state pillararenes. Coord. Chem. Rev. 2022, 462, 214503.
- Hou, C.; Liu, L.; Meng, S.; Wu, Y.; Xie, M.; Shan, Y.; He, P.; Sun, P.; Liao, X. Hybrid vesicles of pillararene/silica: Host-Guest complexation and application in pH-triggered release. Chin. Chem. Lett. 2021, 32, 214–217.
- Luo, Y.; Gan, S.; Zhang, W.; Jia, M.; Chen, L.; Redshaw, C.; Tao, Z.; Xiao, X. A new cucurbituril-based AIE fluorescent supramolecular polymer for cellular imaging. Mater. Chem. Front. 2022, 6, 1021–1025.
- Yin, H.; Bardelang, D.; Wang, R. Macrocycles and related hosts as supramolecular antidotes. Trends Chem. 2021, 3, 1–4.
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Structural diversification of pillararene macrocycles. Angew. Chem. Int. Ed. 2020, 59, 6314–6316.
- Kaizerman-Kane, D.; Hadar, M.; Tal, N.; Dobrovetsky, R.; Zafrani, Y.; Cohen, Y. pH-responsive pillararene-based water-soluble supramolecular hexagonal boxes. Angew. Chem. Int. Ed. 2019, 58, 5302–5306.
- Cao, X.; Gao, A.; Hou, J.T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coordin. Chem. Rev. 2021, 434, 213792.
- Cao, S.; Zhou, L.; Liu, C.; Zhang, H.; Zhao, Y.; Zhao, Y. Pillararene-based self-assemblies for electrochemical biosensors. Biosens. Bioelectron. 2021, 181, 113164.
- Wang, X.; Liu, Z.J.; Hill, E.H.; Zheng, Y.; Guo, G.; Wang, Y.; Weiss, P.S.; Yu, J.; Yang, Y.W. Organic-inorganic hybrid pillarene-based nanomaterial for label-free sensing and catalysis. Matter 2019, 1, 848–861.
- Qiang, H.; Chen, T.; Wang, Z.; Li, W.; Guo, Y.; Yang, J.; Jia, X.; Yang, H.; Hu, W.; Wen, K. Pillararene based conjugated macrocycle polymers with unique photocatalytic selectivity. Chin. Chem. Lett. 2020, 31, 3225–3229.
- Han, C.; Zhang, Z.; Yu, G.; Huang, F. Syntheses of a pillararenequinone and a difunctionalized pillararene by partial oxidation. Chem. Commun. 2012, 48, 9876–9878.
- Shao, L.; Hua, B.; Yang, J.; Yu, G. Pillararene-based Host-Guest complex in water: Dual-responsiveness and application in controllable self-assembly. RSC Adv. 2016, 6, 60029–60033.
- Duan, Q.; Cao, Y.; Li, Y.; Hu, X.; Xiao, T.; Lin, C.; Pan, Y.; Wang, L. pH-Responsive Supramolecular Vesicles Based on Water-Soluble Pillararene and Ferrocene Derivative for Drug Delivery. J. Am. Chem. Soc. 2013, 135, 10542–10549.
- Zhang, D.; Tang, H.; Zhang, G.; Wang, L.; Cao, D. A cucurbituril–pillararene ring-on-ring complex. Chem. Commun. 2021, 57, 6562–6565.
- Lee, E.; Park, I.; Ju, H.; Kim, S.; Jung, J.; Habata, Y.; Lee, S. Formation of a Pillararene-Based Two-Dimensional Poly-Pseudo-Rotaxane: Threading and Crosslinking by the Same Guest Molecules. Angew. Chem. Int. Ed. 2019, 58, 11296–11300.
- Lou, X.; Yang, Y. Pyridine-Conjugated Pillararene: From Molecular Crystals of Blue Luminescence to Red-Emissive Coordination Nanocrystals. J. Am. Chem. Soc. 2021, 143, 11976–11981.
- Chen, J.; Tian, G.; Liu, K.; Zhang, N.; Wang, N.; Yin, X.; Chen, P. Pillararene-based Neutral Radicals with Doublet Red Emissions and Stable Chiroptical Properties. Org. Lett. 2022, 24, 1935–1940.
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host-guest interactions. Chem. Rev. 2020, 120, 6070–6123.
- Ji, X.; Ahmed, M.; Long, L.; Khashab, N.M.; Huang, F.; Sessler, J.L. Adhesive supramolecular polymeric materials constructed from macrocycle-based Host-Guest interactions. Chem. Soc. Rev. 2019, 48, 2682–2697.
- Qin, B.; Yin, Z.; Tang, X.; Zhang, S.; Wu, Y.; Xu, J.; Zhang, X. Supramolecular polymer chemistry: From structural control to functional assembly. Prog. Polym. Sci. 2020, 100, 101167.
- Aida, T.; Meijer, E.W. Supramolecular polymers–we’ve come full circle. Isr. J. Chem. 2020, 60, 33–47.
- Zhang, F.; Ma, J.; Sun, Y.; Boussouar, I.; Tian, D.; Li, H.; Jiang, L. Fabrication of a mercaptoacetic acid pillararene assembled nanochannel: A biomimetic gate for mercury poisoning. Chem. Sci. 2016, 7, 3227–3233.
- Wei, X.F.; Sun, Z.H.; Mihail, B. Pillararenes for construction of artificial transmembrane channels. Isr. J. Chem. 2018, 58, 1–11.
- Li, H.; Quan, K.; Yang, X.; Li, Z.; Zhao, L.; Qiu, H. Recent developments for the investigation of chiral properties and applications of pillararenes in analytical chemistry. TrAC Trend. Anal. Chem. 2020, 131, 116026.
- Fa, S.X.; Shi, T.H.; Akama, S.; Adachi, K.; Wada, K.; Tanaka, S.; Oyama, N.; Kato, K.; Ohtani, S.; Nagata, Y.; et al. Real-time chirality transfer monitoring from statistically random to discrete homochiral nanotubes. Nat. Commun. 2022, 13, 7378.
- Fa, S.X.; Adachi, K.; Nagata, Y.; Egami, K.; Kato, K.; Ogoshi, T. Pre-regulation of the planar chirality of pillararenes for preparing discrete chiral nanotubes. Chem. Sci. 2021, 12, 3483.
- Nagata, Y.; Suzuki, M.; Shimada, Y.; Sengoku, H.; Nishida, S.; Kakuta, T.; Yamagishi, T.; Suginome, M.; Ogoshi, T. Holding of planar chirality of pillararene by kinetic trapping using Host-Guest interactions with achiral guest solvents. Chem. Commun. 2020, 56, 8424–8427.
- Yao, J.; Wu, W.; Liang, W.; Feng, Y.; Zhou, D.; Chruma, J.J.; Fukuhara, G.; Mori, T.; Inoue, Y.; Yang, C. Temperature-driven planar chirality switching of a pillararene-based molecular universal joint. Angew. Chem. Int. Ed. 2017, 56, 6869–6873.
- Lu, J.; Deng, Y.; Zhong, K.; Huang, Z.; Jin, L.Y. Construction of nanoaggregates from amphiphilic supramolecules containing barbiturate and Hamilton wedge units. Polym. Int. 2022, 71, 478–486.
- Bi, J.; Zeng, X.; Tian, D.; Li, H. Temperature-Responsive Switch Constructed from an Anthracene-Functionalized Pillararene-Based Host-Guest System. Org. Lett. 2016, 18, 1092–1095.
- Zheng, H.; Fu, L.; Wang, R.; Jiao, J.; Song, Y.; Shi, C.; Chen, Y.; Jiang, J.; Lin, C.; Ma, J.; et al. Cation controlled rotation in anionic pillararenes and its application for fluorescence switch. Nat. Commun. 2023, 14, 590.
- Chen, J.; Yin, X.; Wang, B.; Zhang, K.; Meng, G.; Zhang, S.; Shi, Y.; Wang, N.; Wang, S.; Chen, P. Planar Chiral Organoboranes with Thermoresponsive Emission and Circularly Polarized Luminescence: Integration of Pillararenes with Boron Chemistry. Angew. Chem. Int. Ed. 2020, 59, 11267–11272.
- Luo, D.; Liu, Z.; Su, A.; Zhang, Y.; Wang, H.; Yang, L.; Yang, W.; Pang, P. An electrochemical biosensor for detection of T4 polynucleotide kinase activity based on host-guest recognition between phosphate pillararene and methylene blue. Talanta 2024, 266, 124956.
- Wu, Y.; Guo, K.; Zhao, J.; Duan, Q.; Wang, F.; Lu, K. Highly sensitive and selective electrochemical detection of clothianidin using reduced graphene oxide-anionic pillararene composite film. Microchem J. 2022, 179, 107465.
- Qian, X.; Yang, H.; Liu, S.; Yang, L.; Li, J.; Gao, W.; Du, G.; Qu, Q.; Ran, X. Supramolecular DNA sensor based on the integration of host-guest immobilization strategy and WP5-Ag/PEHA supramolecular aggregates. Anal. Chim. Acta 2022, 1220, 340077.
- Chen, Y.; Rui, L.; Liu, L.; Zhang, W. Redox-responsive supramolecular amphiphiles based on a pillararene for enhanced photodynamic therapy. Polym. Chem. 2016, 7, 3268–3276.
- Hu, Z.; Yan, B. Facile fabrication of luminescent Tb@HOF-based films as a highly sensitive platform for detecting nicotine and its metabolite cotinine via fluorescence sensing and a smartphone. J. Mater. Chem. A 2023, 11, 4739–4750.
- Zhao, G.; Yang, L.; Wu, S.; Zhao, H.; Tang, E.; Li, C. The synthesis of amphiphilic pillararene functionalized reduced graphene oxide and its application as novel fluorescence sensing platform for the determination of acetaminophen. Biosens. Bioelectron. 2017, 91, 863–869.
- Zhu, W.; Wei, T.; Fan, Y.; Qu, W.; Zhu, W.; Ma, X.; Yao, H.; Zhang, Y.; Lin, Q. A pillararene-based and OH− dependent dual-channel supramolecular chemosensor for recyclable CO2 gas detection: High sensitive and selective off-on-off response. Dyes Pigment. 2020, 174, 108073.
- Jiang, X.; Huang, X.; Song, S.; Ma, X.; Zhang, Y.; Yao, H.; Wei, T.; Lin, Q. Tri-pillararene-based multi-stimuli-responsive supramolecular polymers for fluorescence detection and separation of Hg2+. Polym. Chem. 2018, 9, 4625–4630.
- Zhou, Y.; Yang, L.; Ma, L.; Han, Y.; Yan, C.; Yao, Y. Nano-Theranostics Constructed from Terpyridine-Modified Pillar arene-Based Supramolecular Amphiphile and Its Application in Both Cell Imaging and Cancer Therapy. Molecules 2022, 27, 6428.
- Lu, J.; Gou, X.; Deng, Y.; Pei, Y.; Huang, Z.; Jin, L. Nanoassemblies formed from amphiphilic pillararene–rod–coil macromolecules in water for the detection of aliphatic diamines. Dye. Pigment. 2022, 199, 110052.
- Ahumada, G.; Borkowska, M. Fluorescent Polymers Conspectus. Polymers 2022, 14, 1118.
- Lu, J.; Liu, P.; Deng, Y.; Zhu, N.; Jin, L. Supramolecular nanoassemblies of rim-differentiated pillararene-rod-coil macromolecules via host-guest interactions for the sensing of cis-trans isomers of 1,4-diol-2-butene. J. Mol. Struct. 2023, 1291, 136054.
- Santonocito, R.; Tuccitto, N.; Pappalardo, A.; Trusso Sfrazzetto, G. Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor. Molecules 2022, 27, 7503.
- Wang, X.; Wu, J.; Liang, F.; Yang, Y. In Situ Gold Nanoparticle Synthesis Mediated by a Water-Soluble Leaning Pillararene for Self-Assembly, Detection, and Catalysis. Org. Lett. 2019, 21, 5215–5218.
- Kursunlu, A.N.; Acikbas, Y.; Ozmen, M.; Erdogan, M.; Capan, R. Haloalkanes and aromatic hydrocarbons sensing using Langmuir–Blodgett thin film of pillararene-biphenylcarboxylic acid. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 565, 108–117.
- Wu, Q.; Zhang, T.; Li, X.; Tu, X.; Zhang, H.; Han, J. Construction of pillararene-based photochromic supramolecular polymeric system with tunable thermal bleaching rate. Polymer 2021, 231, 124112.
- Li, Z.; Zhang, Y.; Zhang, C.; Chen, L.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H. Cross-Linked Supramolecular Polymer Gels Constructed from Discrete Multi-pillararene Metallacycles and Their Multiple Stimuli-Responsive Behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589.
- Xia, L.; Tian, J.; Yue, T.; Cao, H.; Chu, J.; Cai, H.; Zhang, W. Pillararene-Based Acid-Triggered Supramolecular Porphyrin Photosensitizer for Combating Bacterial Infections and Biofilm Dispersion. Adv. Healthc. Mater. 2022, 11, 2102015.
- Ding, Y.; Wang, C.; Ma, Y.; Zhu, L.; Lu, B.; Wang, Y.; Wang, J.; Chen, T.; Dong, C.; Yao, Y. pH/ROS dual-responsive supramolecular polypeptide prodrug nanomedicine based on host-guest recognition for cancer therapy. Acta Biomater. 2022, 143, 381–391.
- Chen, J.; Meng, G.; Zhu, Q.; Zhang, S.; Chen, P. Pillararenes: A new class of AIEgen macrocycles used for luminescence sensing of Fe3+ ions. J. Mater. Chem. C 2019, 7, 11747–11751.
- Zhang, Y.; Li, Y.; Fang, H.; He, J.; Yong, B.; Yao, H.; Wei, T.; Lin, Q. Multi-stimuli-responsive supramolecular gel constructed by pillararene-based pseudorotaxanes for efficient detection and separation of multi-analytes in aqueous solution. Soft Matter 2018, 14, 8529–8536.
- Liu, Y.; Shi, K.; Ma, D. Water-Soluble Pillararene Mediated Supramolecular Self-Assembly: Multi-Dimensional Morphology Controlled by Host Size. Chem.–Asian J. 2019, 14, 307–312.
- Wang, J.; Cen, M.; Wang, J.; Wang, D.; Ding, Y.; Zhu, G.; Lu, B.; Yuan, X.; Wang, Y.; Yao, Y. Water-soluble pillararenequinone: Synthesis, host-guest property and application in the fluorescence turn-on sensing of ethylenediamine in aqueous solution, organic solvent and air. Chin. Chem. Lett. 2022, 33, 1475–1478.
- Chen, J.; Chen, P. Pillararene-Based Resilient Supramolecular Gel with Dual-Stimuli Responses and Self-Healing Properties. ACS Appl. Polym. Mater. 2019, 1, 2224–2229.
- Zhou, W.L.; Dai, X.Y.; Lin, W.J.; Chen, Y.; Liu, Y. A pillararene noncovalent assembly boosts a full-color lanthanide supramolecular light switch. Chem. Sci. 2023, 14, 6457–6466.
- Shi, B.; Zhang, Y.; Zhang, Z.; Yao, H.; Qu, W.; Zhang, Y.; Wei, T.; Lin, Q. Formation of a lead chalcogenide quantum dot-based supramolecular polymer network via pillararene-based Host-Guest complexation. Mater. Chem. Front. 2021, 5, 5833–5840.
- Muhammed, M.A.H.; Cruz, L.K.; Emwas, A.H.; El-Zohry, A.M.; Moosa, B.; Mohammed, O.F.; Khashab, N.M. Pillararene-Stabilized Silver Nanoclusters: Extraordinary Stability and Luminescence Enhancement Induced by Host-Guest Interactions. Angew. Chem. Int. Ed. 2019, 58, 15665–15670.
- Li, Y.; Lou, X.; Wang, C.; Wang, Y.; Jia, Y.; Lin, Q.; Yang, Y. Synthesis of stimuli-responsive pillararene-based supramolecular polymer materials for the detection and separation of metal ions. Chin. Chem. Lett. 2023, 34, 107877.
- Liu, J.; Fan, Y.; Song, S.; Gong, G.; Wang, J.; Guan, X.; Yao, H.; Zhang, Y.; Wei, T.; Lin, Q. Aggregation-Induced Emission Supramolecular Organic Framework (AIE SOF) Gels Constructed from Supramolecular Polymer Networks Based on Tripodal Pillararene for Fluorescence Detection and Efficient Removal of Various Analytes. ACS Sustain. Chem. Eng. 2019, 7, 11999–12007.
- Shi, B.; Jie, K.; Zhou, Y.; Xia, D.; Yao, Y. Formation of fluorescent supramolecular polymeric assemblies via orthogonal pillararene-based molecular recognition and metal ion coordination. Chem. Commun. 2015, 51, 4503–4506.
- Liu, S.; Wu, Q.; Zhang, T.; Zhang, H.; Han, J. Supramolecular brush polymers prepared from 1,3,4-oxadiazole and cyanobutoxy functionalised pillararene for detecting Cu2+. Org. Biomol. Chem. 2021, 19, 1287–1291.
- Wu, M.; Yang, Y. A fluorescent pillarene coordination polymer. Polym. Chem. 2019, 10, 2980–2985.
- Zhang, Y.; He, J.; Zhu, W.; Li, Y.; Fang, H.; Yao, H.; Wei, T.; Lin, Q. Novel pillararene-based supramolecular organic framework gel for ultrasensitive response Fe3+ and F− in water. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 62–69.
- Yao, Q.; Lü, B.; Ji, C.; Cai, Y.; Yin, M. Supramolecular Host-Guest System as Ratiometric Fe3+ Ion Sensor Based on Water-Soluble Pillararene. ACS Appl. Mater. Interfaces 2017, 9, 36320–36326.
- Yang, Y.; Tao, X.; Bao, Q.; Yang, J.; Su, L.; Zhang, J.; Chen, Y.; Yang, L. A highly selective supramolecular fluorescent probe for detection of Au3+ based on supramolecular complex of pillararene with 3, 3′-dihydroxybenzidine. J. Mol. Liq. 2023, 370, 121018.
- Xiao, Y.; Li, H.; Tu, M.; Sun, L.; Wang, F. Novel AIEE pillararene-fluorene fluorescent copolymer for selective recognition of paraquat by forming polypseudorotaxane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123112.
- Luo, Y.; Zhang, W.; Zhao, J.; Yang, M.; Ren, Q.; Redshaw, C.; Tao, Z.; Xiao, X. A novel pillararene-cucurbituril based host-guest complex: Synthesis, characterization and detection of paraquat. Chin. Chem. Lett. 2023, 34, 107780.
- Shi, B.; ShangGuan, L.; Wang, H.; Zhu, H.; Xing, H.; Liu, P.; Liu, Y.; Liu, J.; Huang, F. Pillararene-Based Molecular Recognition Induced Crystal-to-Crystal Transformation and Its Application in Adsorption of Adiponitrile in Water. ACS Mater. Lett. 2019, 1, 111–115.
- Song, C.; Li, Z.; Zhang, Y.; Zhang, G.; Yang, Y. Hydrazide–pillararene-mediated silver nanoparticles for highly efficient reductive degradation of organic dyes. Supramol. Mater. 2023, 2, 100035.
- Jie, K.; Zhou, Y.; Sun, Q.; Li, B.; Zhao, R.; Jiang, D.; Guo, W.; Chen, H.; Yang, Z.; Huang, F.; et al. Mechanochemical synthesis of pillarquinone derived multi-microporous organic polymers for radioactive organic iodide capture and storage. Nat. Commun. 2020, 11, 1086.
- Cao, J.; Zhu, H.; Shangguan, L.; Liu, Y.; Liu, P.; Li, Q.; Wu, Y.; Huang, F. A pillararene-based 3D polymer network for efficient iodine capture in aqueous solution. Polym. Chem. 2021, 12, 3517–3521.
- Ogoshi, T.; Takashima, S.; Yamagishi, T. Molecular Recognition with Microporous Multilayer Films Prepared by Layer-by-Layer Assembly of Pillararenes. J. Am. Chem. Soc. 2015, 137, 10962–10964.
- Yao, H.; Wang, Y.; Quan, M.; Farooq, M.U.; Yang, L.; Jiang, W. Adsorptive Separation of Benzene, Cyclohexene, and Cyclohexane by Amorphous Nonporous Amide Naphthotube Solids. Angew. Chem. Int. Ed. 2020, 59, 19945–19950.
- Alzate-Sánchez, D.M.; Ling, Y.; Li, C.; Frank, B.P.; Bleher, R.; Fairbrother, D.H.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymers on Microcrystalline Cellulose as a Granular Media for Organic Micropollutant Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 8089–8096.
- Liang, J.; Nuhnen, A.; Millan, S.; Breitzke, H.; Gvilava, V.; Buntkowsky, G.; Janiak, C. Encapsulation of a Porous Organic Cage into the Pores of a Metal–Organic Framework for Enhanced CO2 Separation. Angew. Chem. Int. Ed. 2020, 59, 6068–6073.
- Li, Q.; Jie, K.; Huang, F. Highly Selective Separation of Minimum-Boiling Azeotrope Toluene/Pyridine by Nonporous Adaptive Crystals of Cucurbituril. Angew. Chem. Int. Ed. 2020, 59, 5355–5358.
- Xu, L.; Xing, C.; Ke, D.; Chen, L.; Qiu, Z.; Zeng, S.; Li, B.; Zhang, S. Amino-Functionalized β-Cyclodextrin to Construct Green Metal–Organic Framework Materials for CO2 Capture. ACS Appl. Mater. Interfaces 2020, 12, 3032–3041.
- Yang, L.; Ke, H.; Yao, H.; Jiang, W. Effective and Rapid Removal of Polar Organic Micropollutants from Water by Amide Naphthotube-Crosslinked Polymers. Angew. Chem. Int. Ed. 2021, 60, 21404–21411.
- Davletbaeva, I.M.; Alentiev, A.Y.; Faizulina, Z.Z.; Zaripov, I.I.; Nikiforov, R.Y.; Parfenov, V.V.; Arkhipov, A.V. Organosilica-Modified Multiblock Copolymers for Membrane Gas Separation. Polymers 2021, 13, 3579.
- Yuan, B.; Xu, J.; Sun, C.; Nicolas, H.N.; Schönhoff, M.; Yang, Q.; Zhang, X. Pillararene Containing Multilayer Films: Reversible Uptake and Release of Guest Molecules with Methyl Viologen Moieties. ACS Appl. Mater. Interfaces 2016, 8, 3679–3685.
- Yan, X.; Huang, Y.; Cen, M.; Wang, J.; Shi, J.; Lu, B.; Wang, Y.; Yao, Y. Pillararene-based supramolecular polymeric materials constructed via electrostatic interactions for rapid and efficient organic dye removal from water. Nanoscale Adv. 2021, 3, 1906–1909.
- Yao, Y.; Xue, M.; Chen, J.; Zhang, M.; Huang, F. An Amphiphilic Pillararene: Synthesis, Controllable Self-Assembly in Water, and Application in Calcein Release and TNT Adsorption. J. Am. Chem. Soc. 2012, 134, 15712–15715.
- Ogoshi, T.; Sueto, R.; Yoshikoshi, K.; Yamagishi, T. One-dimensional channels constructed from per-hydroxylated pillararene molecules for gas and vapour adsorption. Chem. Commun. 2014, 50, 15209–15211.
- Zhu, W.; Li, E.; Zhou, J.; Zhou, Y.; Sheng, X.; Huang, F. Highly selective removal of heterocyclic impurities from toluene by nonporous adaptive crystals of perethylated pillararene. Mater. Chem. Front. 2020, 4, 2325–2329.
- Li, X.; Li, Z.; Yang, Y. Tetraphenylethylene-Interweaving Conjugated Macrocycle Polymer Materials as Two-Photon Fluorescence Sensors for Metal Ions and Organic Molecules. Adv. Mater. 2018, 30, 1800177.
- Shi, B.; Guan, H.; Shangguan, L.; Wang, H.; Xia, D.; Kong, X.; Huang, F. A pillararene-based 3D network polymer for rapid removal of organic micropollutants from water. J. Mater. Chem. A 2017, 5, 24217–24222.
- Ju, H.; Zhou, X.; Shi, B.; Kong, X.; Xing, H.; Huang, F. A pillararene-based hydrogel adsorbent in aqueous environments for organic micropollutants. Polym. Chem. 2019, 10, 5821–5828.
- Wang, Z.; Chen, T.; Wang, G.; Hu, W.; Liu, Y.A.; Yang, H.; Wen, K. A Pillararene Conjugated Polymer for Removal of Low-Molecular-Weight Organic Acids, Amines, and Alcohols from Water. ACS Appl. Polym. Mater. 2020, 2, 5566–5573.
- Wang, Z.; Zhang, Y.; Sun, X.; Li, Y.; Zhang, Y.; Wei, T.; Yao, H.; Lin, Q. Linear tri-pillararene-based acceptor for efficiently separate paraquat from water through collaboration effect. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111358.
- Zhang, Y.; Wang, Z.; Yao, X.; Zhang, Y.; Wei, T.; Yao, H.; Lin, Q. Novel tripodal-pillararene-based chemical sensor for efficient detection and removal paraquat by synergistic effect. Sens. Actuators B Chem. 2021, 327, 128885.
- Li, Y.; Wen, J.; Li, J.; Wu, Z.; Li, W.; Yang, K. Controllable preparation of supramolecular coordination polymers based on carboxylated pillararenes and zinc ions: An efficient tool for paraquat removal from water. Appl. Surf. Sci. 2023, 613, 156071.
- Zhang, Y.; Zhu, W.; Huang, X.; Qu, W.; He, J.; Fang, H.; Yao, H.; Wei, T.; Lin, Q. Supramolecular Aggregation-Induced Emission Gels Based on Pillararene for Ultrasensitive Detection and Separation of Multianalytes. ACS Sustain. Chem. Eng. 2018, 6, 16597–16606.
- Dai, D.; Yang, J.; Zou, Y.; Wu, J.; Tan, L.; Wang, Y.; Li, B.; Lu, T.; Wang, B.; Yang, Y. Macrocyclic Arenes-Based Conjugated Macrocycle Polymers for Highly Selective CO2 Capture and Iodine Adsorption. Angew. Chem. Int. Ed. 2021, 60, 8967–8975.
- Wu, J.; Li, B.; Yang, Y. Separation of Bromoalkanes Isomers by Nonporous Adaptive Crystals of Leaning Pillararene. Angew. Chem. Int. Ed. 2020, 59, 2251–2255.
- Dai, D.; Li, Z.; Yang, J.; Wang, C.; Wu, J.; Wang, Y.; Zhang, D.; Yang, Y. Supramolecular Assembly-Induced Emission Enhancement for Efficient Mercury(II) Detection and Removal. J. Am. Chem. Soc. 2019, 141, 4756–4763.
- Kiruthika, J.; Arunachalam, M. Pillararene-based cross-linked polymer for the rapid adsorption of iodine from water and vapor phases. Polymer 2022, 259, 125322.
- Talapaneni, S.N.; Kim, D.; Barin, G.; Buyukcakir, O.; Je, S.; Coskun, A. Pillararene Based Conjugated Microporous Polymers for Propane/Methane Separation through Host-Guest Complexation. Chem. Mater. 2016, 28, 4460–4466.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
988
Revisions:
2 times
(View History)
Update Date:
21 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




