Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Rojas | -- | 2436 | 2024-02-19 19:32:38 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 2439 | 2024-02-20 02:29:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Espinosa, M.A.; Ponce, P.; Molina, A.; Borja, V.; Torres, M.G.; Rojas, M. Home-Based Devices for Detecting Obstructive Sleep Apnea. Encyclopedia. Available online: https://encyclopedia.pub/entry/55194 (accessed on 07 February 2026).
Espinosa MA, Ponce P, Molina A, Borja V, Torres MG, Rojas M. Home-Based Devices for Detecting Obstructive Sleep Apnea. Encyclopedia. Available at: https://encyclopedia.pub/entry/55194. Accessed February 07, 2026.
Espinosa, Miguel A., Pedro Ponce, Arturo Molina, Vicente Borja, Martha G. Torres, Mario Rojas. "Home-Based Devices for Detecting Obstructive Sleep Apnea" Encyclopedia, https://encyclopedia.pub/entry/55194 (accessed February 07, 2026).
Espinosa, M.A., Ponce, P., Molina, A., Borja, V., Torres, M.G., & Rojas, M. (2024, February 19). Home-Based Devices for Detecting Obstructive Sleep Apnea. In Encyclopedia. https://encyclopedia.pub/entry/55194
Espinosa, Miguel A., et al. "Home-Based Devices for Detecting Obstructive Sleep Apnea." Encyclopedia. Web. 19 February, 2024.
Copy Citation
Obstructive Sleep Apnea (OSA) is a respiratory disorder characterized by frequent breathing pauses during sleep. The apnea–hypopnea index is a measure used to assess the severity of sleep apnea and the hourly rate of respiratory events. Despite numerous commercial devices available for apnea diagnosis and early detection, accessibility remains challenging for the general population, leading to lengthy wait times in sleep clinics. Consequently, research on monitoring and predicting OSA has surged.
sleep apnea detection
oximetry
actigraphy
respiratory effort
respiratory flow
1. Introduction
According to the American Academy of Sleep Medicine (AASM), sleep apnea is a severe disorder characterized by interrupted breathing during sleep [1]. Untreated sleep apnea in individuals involves frequent pauses in breathing, often happening numerous times throughout the night. If not addressed, this condition can result in loud snoring, daytime fatigue, and potentially more severe complications such as heart problems or high blood pressure [1]. The apnea–hypopnea index (AHI), which measures the frequency of respiratory events per hour [2], assesses the severity of sleep apnea. Despite the availability of various commercial devices for monitoring primary symptoms, it is necessary to make them more accessible to the general population. Additionally, sleep clinics often experience extended wait times. Consequently, there has been a surge in scientific research focusing on monitoring and predicting obstructive Sleep Apnea (OSA).
There are three main types of sleep apnea: OSA, central sleep apnea (CSA), and complex sleep apnea. OSA is the most common form when throat muscles relax. During these episodes, the diaphragm and chest muscles work harder than average to open the airways. The patient may start to breathe with loud gasps or jerk their body. Central sleep apnea occurs when the brain does not send a signal to breathing muscles properly. Instead, the brain fails to tell the muscles to breathe because of issues in the respiratory control center. It is related to the function of the central nervous system. Complex sleep apnea syndrome, or treatment-emergent central sleep apnea, occurs when someone has obstructive and central sleep apnea [1]. Sleep apnea is typically diagnosed through a standard test called nocturnal polysomnography (PSG) [3]. Monitoring equipment connects the patient and tracks nine specific variables during this test. The screening study can be conducted in a sleep laboratory or at home if specialized equipment is available. These variables include oximetry, respiratory flow, respiratory effort, actigraphy, electroencephalography (EEG), muscle electrical activity (EMG), eye movements, heart rate, and snoring [3]. For reference, Figure 1 illustrates the placement of each sensor on the human body in a PSG set-up [4].
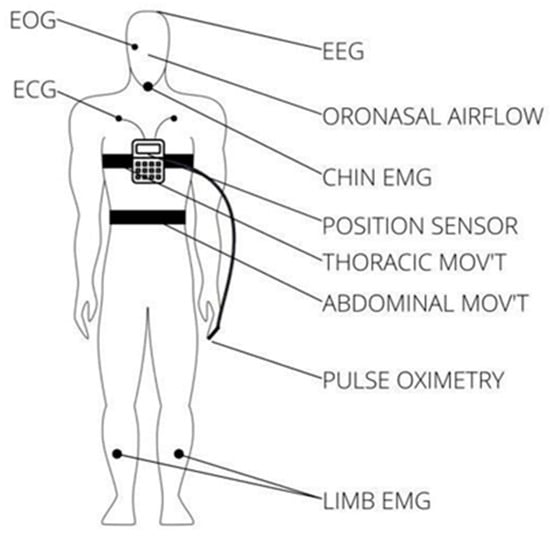
Figure 1. Position of each sensor in a PSG.
Home sleep testing is also an option for detecting OSA [5]. Despite its effectiveness as the primary method for detecting sleep apnea, polysomnography frequently leads to prolonged diagnostic wait times due to the condition manifesting during nighttime sleep. Furthermore, sleep laboratories specializing in diagnosing sleep apnea may have limited capacity to evaluate all patients.
Alternative solutions for diagnosing OSA include portable home devices, such as portable monitors or out-of-center sleep tests that measure fewer variables [5]. One notable characteristic of these mobile devices is their cost-effectiveness compared with polysomnography. However, it is essential to note that according to the AASM, portable home devices are recommended only for patients with a high likelihood of having sleep apnea. One of the main limitations of these devices is the absence of an electroencephalography (EEG) monitor.
2. Modules and Technology Available for OSA Detection
2.1. Commercial Devices for OSA Diagnosis
According to the AASM, Portable Home Sleep Monitoring (PHSM) devices are classified into Types II, III, and IV [6]. It is important to note that portable Type III and IV monitors do not directly detect sleep stages. Instead, they estimate the respiratory disturbance index (RDI) or AHI by extrapolating data from the recorder’s active period [6]. Type II monitors have at least seven channels (e.g., EEG, EOG, electromyogram, heart rate, airflow, respiratory effort, oxygen saturation). This type of device monitors sleep staging and allows the calculation of AHI. Type III monitors are devices with a restricted number of channels, typically ranging from four to seven, and they must have at least four channels. The most common channels are heart rate, oxygen saturation, and respiratory measurements. Finally, Type IV devices were typically utilized to measure only one or two parameters, such as oxygen saturation or airflow, ultimately limiting their scope. However, this changed when the Centers for Medicare and Medicaid Services (CMS) decided to include coverage for continuous positive airway pressure (CPAP) treatment based on positive tests from Type IV devices with a minimum of three channels. Also, these devices can screen pediatric patients with OSA, as mentioned by [7].
In 2003, the AASM, the American College of Chest Physicians, and the American Thoracic Society recommended the diagnostic approach for patients suspected of having OSA; thus, they advised a complete polysomnography (PSG) study for patients with a strong suspicion. In cases where a portable monitoring (PM) device of Type II with a minimum of seven channels is utilized, it must be integrated into an attended PSG. Conversely, a four-channel study is conducted using a Type III portable monitoring (PM) device in a hospital setting supervised by a technician to assess the presence of OSA. However, an unattended four-channel study is not recommended. Lastly, an IV PM device with a single or double channel is also not recommended for diagnosing OSA [8]. These recommendations are related to the number of variables for each device, and it is important to note that all devices within these categories are intended exclusively for data collection, not diagnosis. Subsequently, a specialist reviews the collected information and, with additional signals from the patient, can formulate a more precise diagnosis of their condition.
Further, Embletta MPR [9] is a fourth-generation ambulatory recorder that offers the capacity to record data from seven channels, encompassing variables such as abdominal strain, chest strain, nasal pressure, nasal flow, snore, SpO2, heart rate, position, and audio. Crucially, this device permits the incorporation of additional sensors for a more comprehensive study, offering increased versatility in monitoring. Embletta underwent validation against PSG in [10], demonstrating a remarkable correlation in the AHI compared with PSG, boasting a sensitivity of 0.924 and a specificity of 0.857.
MediByte is another commercial device [11], and it is described as the world’s smallest recorder, measuring a mere 2.5 × 2.25 × 0.75 inches (66 × 60 × 19 mm) and weighing 3.3 ounces (93 g). It offers compatibility with CPAP devices through the Luer connector. This device measures ECG, oximetry, effort, and nasal pressure. In a thorough validation against PSG, as detailed in [12], MediByte achieved a sensitivity of 80% and a specificity of 97%, which is especially noteworthy when considering an AHI threshold exceeding 15 events per hour. Moreover, with a higher threshold (AHI exceeding 30 events per hour), the device demonstrated a positive predictive value of 100% and a negative predictive value of 88%. An essential application of this device in research involving pediatric patients against PSG is described in [13].
2.2. Hardware and Software Systems for OSA Diagnosis Available in Scientific Research
Unlike commercial devices, scientific research proposals have been focused on developing software for predicting OSA based on a few input sensors and machine learning algorithms. Also, many solutions have been proposed through alternative hardware, such as smartwatches and rings [14]. Moreover, according to [15], between 2016 and 2019, numerous Internet of Things (IoT)-based solutions emerged; thus, collaborative efforts in this domain, leveraging technologies like smart devices, fog computing, cloud, big data, and machine learning, have facilitated the development of innovative solutions. According to [16], the proliferation of wearable watches with photoplethysmography (PPG) sensors allows the monitoring of continuous pulse wave data during daily activities. This study investigated the use of PPG data from a smartwatch for diagnosing OSA, showing that smartwatch information can be a viable alternative with a final accuracy of 85%.
Other recent trends include the development of smartphone apps, as highlighted in [17]. While these apps may have a promising future, they are less accurate than traditional methods. An example is introduced by [18], where a wireless pulse oximeter is used together with an app to diagnose OSA. In this solution, the smartphone did not record information from any internal sensor but instead received data from the oximeter. Further, [19] demonstrates that app-based solutions are unreliable, requiring an expert review for an accurate diagnosis. The primary benefits of these solutions include their noninvasiveness and the avoidance of patients needing to visit a sleep center for a comprehensive diagnosis. Nevertheless, since preventive studies do not encompass the monitoring of all variables measured in a PSG study, the diagnosis should be reassessed by a sleep expert. As reported in [20], recent years have witnessed the emergence of novel detection methods based on noncontact sensors, such as radio frequency, audio, and video.
In [21], a study is presented with the primary objective of evaluating the potential of a microbend fiber optic sensor (MFOS) to detect vital signs and sleep apnea in the controlled environment of an in-lab sleep study. Ten participants underwent full polysomnography (PSG) with discreet placement of the microbend fiber optic sensor (MFOS) beneath the patient’s mattress to capture bed-embedded ballistocardiogram (BCG) data. In addition, the vital signs were assessed within a 30 s time frame with a 15 s overlap. Electrocardiograms and thoracic effort signals were critical reference points in the assessment process. The research outcomes revealed commendable results for sleep apnea detection, with an accuracy rate of 49.96%, a sensitivity rate of 57.07%, and a specificity rate of 45.26%. These findings suggest promising advancements in sleep-related disorder diagnosis in clinical settings using a nonintrusive and practical approach.
Another study with an innovative monitoring system is proposed in [22], using a tracheal sound (TS) sensor during sleep to identify apnea. Polysomnographic recordings from 32 patients served as the dataset, enabling an efficacy comparison of four airflow signal methods: the oronasal thermal airflow sensor (thermistor), a nasal pressure transducer (NP), respiratory inductance plethysmography (RIPsum), and the TS. Notably, the thermistor signal served as the reference for scoring, and it showed that with this method, there were 4167 apneas detected: 5416 with the NP, 2959 with the RIPsim, and 5019 with the TS caught. The findings suggest that placing TS sensors has the potential to identify apneas that might be overlooked by RIPsum and detect apneas that NP sensors may miss. However, it is essential to note that TS sensors may tend to overscore apneas due to mouth breathing.
Also, there are scientific research papers based on software development for predicting OSA. An example of a noncontact method for estimating OSA is provided in [23]. The main objective of this study is to pioneer a noncontact method aimed at assessing the severity of sleep apnea while discerning between positional and nonpositional sleep apnea cases. This cutting-edge approach leverages the power of a deep learning algorithm, which diligently scrutinizes infrared sleep videos to gauge and quantify the AHI. A noteworthy facet of this algorithm is its capacity to pinpoint patients affected by positional sleep apnea, a condition predominantly associated with individuals who favor sleeping on their backs [24].
Further, a novel approach is introduced in [25], presenting an automatic feature extraction method that combines convolutional neural networks (CNNs) and long short-term memory (LSTM) recurrent networks to accurately differentiate individuals with apnea from those without, employing the apnea–hypopnea index (AHI) as a crucial diagnostic measure. The method demonstrates advancements, featuring a sensitivity of 94.41%, a specificity of 98.94%, and an overall accuracy of 97.21%. Furthermore, extensive testing on the St. Vincent’s University Hospital/University College Dublin Sleep Apnea Database (UCDDB) dataset underscores its robustness, achieving a high accuracy rate of 93.70%, sensitivity of 90.69%, and specificity of 95.82%. A deep learning approach establishes the credibility of deep learning methodologies in diagnosing OSA, utilizing electrocardiogram (ECG) signals as the primary diagnostic modality [26]. The ECG signal undergoes meticulous preprocessing, normalization, and segmentation into 10 s intervals for efficient analysis. With data from 86 patients, the study allocates 69 patients’ data for training. It reserves the remaining 17 patients’ data for testing. The best-performing model achieves an exceptional accuracy rate of 99%, emphasizing the potential of deep learning to enhance the accuracy and effectiveness of OSA diagnosis.
2.3. Applicable Regulations for Signal Monitoring Modules
Oximetry: Pulse oximeters employ the principle of differential light absorption to determine SpO2. These devices utilize a sensor placed on a body region, such as a finger, toe, or earlobe, to transmit light of different wavelengths through the skin. The primary standard applicable is ISO 80601-2-61 [27]. The Pan American Health Organization and the World Health Organization developed the Technical and Regulatory Aspects of the Use of Pulse Oximeters [28], where some of the following specifications are mentioned: SpO2 detection to include the range 70–99%, SpO2 resolution of 1% or less, SpO2 Accuracy (in the range at least 70–99%) within ± 3%, pulse rate detection range to include 30–240 bpm, and others.
Respiratory flow: The primary standard that applies, in this case, is ISO 23747:2015, titled “Aesthetic and respiratory equipment—Peak expiratory flow meters for assessing pulmonary function in spontaneously breathing humans” [29].
Respiratory effort: The standard applicable is ISO 4135:2022 Anaesthetic and respiratory equipment [30]. This ISO standard ensures the uniformity of terminology used in all relevant anesthesiology and respiratory care equipment standards. It establishes a common language that manufacturers, test laboratories, and regulatory agencies can use to effectively communicate and regulate such equipment.
Actigraphy: Although no principal applicable standard exists for an actigraph, the ISO 13485:2016 Medical Devices—Quality Management Systems—Requirements for regulatory purposes can be used [31]. According to the company Actigraph, which develops Medical-Grade Actigraphy monitors, their devices are certified by ISO 13485:2016, the European Union Medical Device Directive (EU MDD) 93/42/EEC, the Health Canada Medical Devices Regulations (CMDR), and the US FDA’s Quality System Regulations (QSRs) [32].
2.4. Applicable Regulations for Medical Materials
ISO 10993-1 identifies the standard endpoints for evaluating the biological effects of skin contact devices, which include cytotoxicity, sensitization, and irritation [33]. The FDA considers these factors when guiding the biocompatibility of specific devices in contact with intact skin. Further, this guidance only applies to medical devices composed of certain materials, including synthetic polymers, polycarbonate, polyoxymethylene, and some fabrics, including Lycra.
3. Conclusions
OSA has garnered substantial attention, resulting in increased efforts for independent and commercial developments to tackle this issue. Commercially available devices mainly belong to Type III, emphasizing ECG and oximetry variables. On the scientific front, single-variable approaches, enhanced by postprocessing techniques, offer reasonably accurate predictions. Notably, artificial neural networks and deep learning methods have emerged as prominent strategies for postprocessing, achieving accuracy rates of up to 99% when combined with the ECG variable.
A future direction involves the development of a device specifically designed to predict sleep apnea in Mexican patients. This device should leverage the best-detected characteristics while prioritizing cost-effectiveness. It is essential to weigh the advantages and disadvantages of commercial and independently developed devices, emphasizing the need to define the primary objective and approach of the proposed device. A novel and effective sleep apnea detection system could be achieved by carefully considering these factors.
References
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 479–504.
- Apnea Hypopnea Index (AHI). Available online: https://www.webmd.com/sleep-disorders/sleep-apnea/sleep-apnea-ahi-numbers (accessed on 7 September 2022).
- Rundo, J.V.; Downey, R. Polysomnography. Handb. Clin. Neurol. 2019, 160, 381–392.
- Learn about Polysomnography. Available online: https://www.chegg.com/learn/medicine-and-health/medical-terminology/polysomnography (accessed on 8 September 2022).
- Kapoor, M.; Greenough, G. Home sleep tests for obstructive sleep apnea (OSA). J. Am. Board Fam. Med. 2015, 28, 504–509.
- Obstructive Sleep Apnea and Home Sleep Monitoring: Overview of Obstructive Sleep Apnea, Efficacy of Home Sleep Tests, Advantages of HSTsPublication: Medscape–eMedicine. Available online: https://emedicine.medscape.com/article/1518830-overviewa7 (accessed on 12 September 2022).
- Gao, X.; Li, Y.; Xu, W.; Han, D. Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 2021, 87, 127–137.
- Chesson, A.L., Jr.; Berry, R.B.; Pack, A.; American Academy of Sleep Medicine; American Thoracic Society; American College of Chest Physicians. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep 2003, 26, 907–913.
- Embletta® MPR Sleep System. Available online: https://natus.com/neuro/embletta-mpr-sleep-system/ (accessed on 27 September 2022).
- Ng, S.S.S.; Chan, T.-O.; To, K.-W.; Ngai, J.; Tung, A.; Ko, F.W.S.; Hui, D.S.C. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnea syndrome (OSAS). Respirology 2010, 15, 336–342.
- Braebon-Medibyte Features. Available online: https://www2.braebon.com/products/medibyte (accessed on 30 September 2022).
- Driver, H.S.; Pereira, E.J.; Bjerring, K.; Toop, F.; Stewart, S.C.; Munt, P.W.; Fitzpatrick, M.F. Validation of the MediByte® type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can. Respir. J. J. Can. Thorac. Soc. 2011, 18, 137–143.
- Masoud, A.I.; Patwari, P.P.; Adavadkar, P.A.; Arantes, H.; Park, C.; Carley, D.W. Validation of the MediByte Portable Monitor for the Diagnosis of Sleep Apnea in Pediatric Patients. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2019, 15, 733–742.
- Penzel, T.; Schöbel, C.; Fietze, I. New technology to assess sleep apnea: Wearables, smartphones, and accessories. F1000Research 2018, 7, 413.
- Abdel-Basset, M.; Ding, W.; Abdel-Fatah, L. The fusion of Internet of Intelligent Things (IoIT) in remote diagnosis of obstructive Sleep Apnea: A survey and a new model. Inf. Fusion 2020, 61, 84–100.
- Bianchi, M.T. Sleep devices: Wearables and nearables, informational and interventional, consumer and clinical. Metab. Clin. Exp. 2018, 84, 99–108.
- Baptista, P.M.; Martin, F.; Ross, H.; Reina, C.O.; Plaza, G.; Casale, M. A systematic review of smartphone applications and devices for obstructive sleep apnea. Braz. J. Otorhinolaryngol. 2022, 88, S188–S197.
- Pinheiro, G.D.L.; Cruz, A.F.; Domingues, D.M.; Genta, P.R.; Drager, L.F.; Strollo, P.J.; Lorenzi-Filho, G. Validation of an Overnight Wireless High-Resolution Oximeter plus Cloud-Based Algorithm for the Diagnosis of Obstructive Sleep Apnea. Clinics 2020, 75, e2414.
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Diagnostic value of smartphone in obstructive sleep apnea syndrome: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268585.
- Shelgikar, A.V.; Anderson, P.F.; Stephens, M.R. Sleep Tracking, Wearable Technology, and Opportunities for Research and Clinical Care. Chest 2016, 150, 732–743.
- Sadek, I.; Heng, T.T.S.; Seet, E.; Abdulrazak, B. A New Approach for Detecting Sleep Apnea Using a Contactless Bed Sensor: Comparison Study. J. Med. Internet Res. 2020, 22, e18297.
- Sabil, A.; Glos, M.; Günther, A.; Schöbel, C.; Veauthier, C.; Fietze, I.; Penzel, T. Comparison of Apnea Detection Using Oronasal Thermal Airflow Sensor, Nasal Pressure Transducer, Respiratory Inductance Plethysmography and Tracheal Sound Sensor. J. Clin. Sleep Med. 2019, 15, 285–292.
- Akbarian, S.; Ghahjaverestan, N.M.; Yadollahi, A.; Taati, B. Non-contact Sleep Monitoring With Infrared Video Data to Estimate Sleep Apnea Severity and Distinguish Between Positional and Non-positional Sleep Apnea: Model Development and Experimental Validation. J. Med. Internet Res. 2021, 23, e26524.
- Oksenberg, A.; Arons, E.; Radwan, H.; Silverberg, D.S. Positional vs non-positional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest 1997, 112, 629–639.
- Zarei, A.; Beheshti, H.; Asl, B.M. Detection of sleep apnea using deep neural networks and single-lead ECG signals. Biomed. Signal Process. Control 2022, 71, 103125.
- Erdenebayar, U.; Kim, Y.J.; Park, J.-U.; Joo, E.Y.; Lee, K.-J. Deep learning approaches for automatic detection of sleep apnea events from an electrocardiogram. Comput. Methods Programs Biomed. 2019, 180, 105001.
- ISO 80601-2-61:2017(en); Medical Electrical Equipment—Part 2-61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/67963.html (accessed on 23 December 2022).
- Panamerican Health Organization. Technical and Regulatory Aspects of the Use of Pulse Oximeters in Monitoring COVID-19 Patients. 7 August 2020. Available online: https://iris.paho.org/handle/10665.2/52589 (accessed on 24 December 2022).
- ISO 23747:2015; Anaesthetic and Respiratory Equipment—Peak Expiratory Flow Meters for the Assessment of Pulmonary Function in Spontaneously Breathing Humans. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/64926.html (accessed on 10 January 2023).
- ISO 4135:2022(en); Anaesthetic and Respiratory Equipment-Vocabulary. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/obp/ui#iso:std:iso:4135:ed-4:v1:en (accessed on 12 January 2023).
- ISO 13485:2016; Anaesthetic and Respiratory Equipment. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59752.html (accessed on 13 January 2023).
- Compliance Center|ActiGraph. Available online: https://actigraphcorp.com/compliance/ (accessed on 14 January 2023).
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 14 February 2023).
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
767
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




