
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angel Justiz-Vaillant | -- | 5273 | 2024-02-19 15:54:22 | | | |
| 2 | Sirius Huang | -333 word(s) | 4940 | 2024-03-11 02:25:09 | | |
Video Upload Options
Systemic lupus erythematosus (SLE) is an idiopathic chronic autoimmune disease that can affect any organ in the body, including the neurological system. Multiple factors, such as environmental (infections), genetic (many HLA alleles including DR2 and DR3, and genes including C4), and immunological influences on self-antigens, such as nuclear antigens, lead to the formation of multiple autoantibodies that cause deleterious damage to bodily tissues and organs. The production of autoantibodies, such as anti-dsDNA, anti-SS(A), anti-SS(B), anti-Smith, and anti-neuronal DNA are characteristic features of this disease. Patients with SLE also present numerous neuropsychiatric manifestations. These neuropsychiatric manifestations are referred to as neuropsychiatric systemic lupus erythematosus (NPSLE). NPSLE affects both the central nervous system (CNS) and the peripheral nervous system (PNS) and can present as various symptoms, such as cognitive dysfunction, organic brain syndromes, delirium, seizures, headache, and psychosis.
1. Introduction
| Central Nervous System | Neurological syndromes (focal): Seizure disorder Aseptic meningitis Cerebrovascular disease Demyelinating syndromes Headache Myelopathy Movement disorders Neuropsychiatric syndrome (diffuse): Anxiety disorders Psychosis Mood disorders Acute confusional state Cognitive dysfunction |
| Peripheral Central Nervous System | Neurological syndromes (focal): Autonomic disorders Myasthenia gravis Polyneuropathy Guillian Barre Syndrome Plexopathy Mononeuropathy |
2. Most Systemic Lesions Are Due to Loss of Tolerance to Self-Antigens
3. SLE Immunopathogenesis
3.1. Apoptosis Cascade and Role of IFN-α in SLE
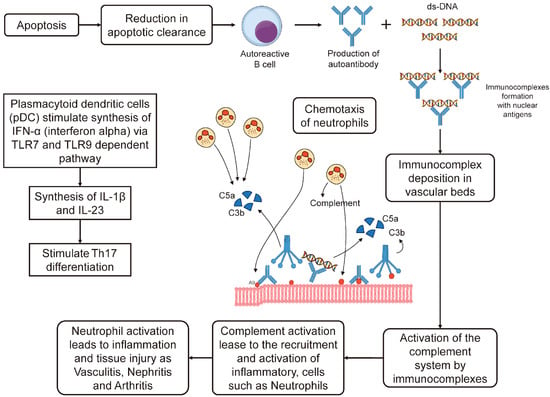
3.2. Increased Association of HLA System and SLE in a Population
3.3. Immunopathogenesis of NPSLE
3.3.1. Genetic Factors
-
Transcriptomic data analysis has revealed several pathways and immune responses that are associated with SLE, such as interferons, T cell differentiation, complement pathways, and coagulation;
-
Eight genes (SOCE, CXCL8, MMP9, IL1B, JUN, TNF, NFKBIA, and FOS) are up-regulated in SLE and have interactions with different pathways. These genes are also linked to SNPs that are identified by GWAS;
-
Several other genes with known SLE-related variations are detected by integrating GWAS and pathway analysis, such as TYK2, SH2B, C5, IL2RA, IRF5, FCGR2A, TNFAIP3, STAT4, LYN, IL7R, and HLA-DRB;
-
One of the relevant pathways that is identified by pathway-based analysis is the TSLP signaling pathway, which is connected to rs7574865, LYN, STAT4, and IL7R;
3.3.2. Comorbidities
3.3.3. Summary of NPSLE Immunopathogenesis
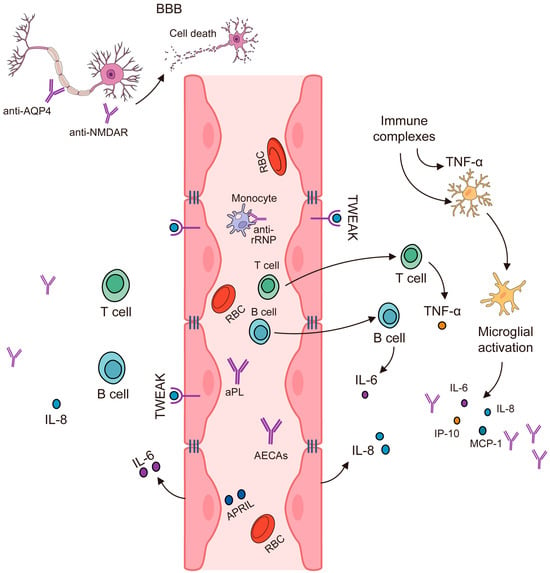
3.4. IL-2, IL-10, and IFN-γ Produced by T-Helper Cells Are Elevated in NPSLE
3.5. Noninflammatory or Thrombotic/Ischemic Vascular Injury
4. Autoantibodies Can Lead to Neuronal Damage in NPSLE
4.1. Antiphospholipid Antibodies (β2-Glycoprotein 1, Cardiolipin Anticardiolipin (Anti-CL) and Lupus Anticoagulant (LA)
4.2. Ribosomal P Protein (Anti-Ribosmal P Ab)
4.3. Anti-Human N-Methyl-D-Aspartate Receptor Abs (Anti-NMDA)
4.4. Microtubule-Associated Protein (Anti-MAP-2 Ab)
4.5. U1 Ribonucleoprotein (Anti-U1RNP Ab)
4.6. Structural Endothelial Proteins (AECA)
4.7. Triosephophate Isomerase (Anti-TPI Ab)
4.8. Glyceraldehyde-3-Phosphate Dehydrogenase Antibodies (Anti-GAPDH)
4.9. Anti-Aquaporin Four Antibodies (NMO-IgG/AQP4-Ab)
4.10. Anti-Endothelial Cell Antibodies (AECAb)
4.11. Anti-Ubiquitin Carboxyl Hydrolase L 1 Antibodies (Anti-UCH-L1 Ab)
| Autoantibody | Location Isolated | Associated NPSLE Symptoms | References |
|---|---|---|---|
| Phospholipid: β2-glycoprotein 1 and cardiolipin (aCL-Ab) |
Serum, CSF | CVD, seizures, chorea cognitive dysfunction, psychosis, depression, headache | [54][55][56][57][58] |
| Ribosomal P protein (anti-ribosmal P Ab) |
Serum, CSF | psychosis, depression, cognitive impairment | [66][67][68] |
| NMDA receptor (anti-NMDA) |
Serum, CSF | depression cognitive dysfunction | [84] |
| MAP-2 (anti-MAP-2 Ab) | Serum, CSF | seizures, chorea, sensory neuropathy, psychosis, headache) | [90][91][92] |
| U1 ribonucleoprotein (Anti-U1RNP Ab) |
Serum, CSF | Diffuse NPSLE symptoms | [98] |
| Structural endothelial proteins (AECA) | Serum | Psychosis, depression | [102][103] |
| Triosephosphate isomerase(anti-TPI Ab) | Serum, CSF | aseptic meningitis | [105] |
| GAPDH (anti-GAPDH Ab) | Serum | Involved in various in neurodegenerative disorders, increased intracranial pressure, cognitive dysfunction | [108][110][111] |
References
- Schwartz, N.; Stock, A.D.; Putterman, C. Neuropsychiatric Lupus: New Mechanistic Insights and Future Treatment Directions. Nat. Rev. Rheumatol. 2019, 15, 137–152.
- Stock, A.D.; Wen, J.; Putterman, C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front. Immunol. 2013, 4, 484.
- Shin, J.I.; Lee, K.H.; Park, S.; Yang, J.W.; Kim, H.J.; Song, K.; Lee, S.; Na, H.; Jang, Y.J.; Nam, J.Y.; et al. Systemic Lupus Erythematosus and Lung Involvement: A Comprehensive Review. J. Clin. Med. Res. 2022, 11, 6714.
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the Diagnosis and Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25.
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus Nephritis: Clinical Presentations and Outcomes in the 21st Century. Rheumatology 2020, 59, v39–v51.
- Durcan, L.; O’Dwyer, T.; Petri, M. Management Strategies and Future Directions for Systemic Lupus Erythematosus in Adults. Lancet 2019, 393, 2332–2343.
- Islam, M.A.; Khandker, S.S.; Kotyla, P.J.; Hassan, R. Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review. Front. Immunol. 2020, 11, 1477.
- Santacruz, J.C.; Mantilla, M.J.; Rueda, I.; Pulido, S.; Rodriguez-Salas, G.; Londono, J. A Practical Perspective of the Hematologic Manifestations of Systemic Lupus Erythematosus. Cureus 2022, 14, e22938.
- Low, J.T.; Hughes, P.; Lin, A.; Siebenlist, U.; Jain, R.; Yaprianto, K.; Gray, D.H.D.; Gerondakis, S.; Strasser, A.; O’Reilly, L.A. Impact of Loss of NF-κB1, NF-κB2 or c-REL on SLE-like Autoimmune Disease and Lymphadenopathy in Fas(lpr/lpr) Mutant Mice. Immunol. Cell Biol. 2016, 94, 66–78.
- Garcia, D.; Young, L. Lymphocytic Interstitial Pneumonia as a Manifestation of SLE and Secondary Sjogren’s Syndrome. BMJ Case Rep. 2013, 2013, bcr2013009598.
- Hariri, L.P.; Unizony, S.; Stone, J.; Mino-Kenudson, M.; Sharma, A.; Matsubara, O.; Mark, E.J. Acute Fibrinous and Organizing Pneumonia in Systemic Lupus Erythematosus: A Case Report and Review of the Literature. Pathol. Int. 2010, 60, 755–759.
- Kudsi, M.; Nahas, L.D.; Alsawah, R.; Hamsho, A.; Omar, A. The Prevalence of Oral Mucosal Lesions and Related Factors in Systemic Lupus Erythematosus Patients. Arthritis Res. Ther. 2021, 23, 229.
- Brewer, B.N.; Kamen, D.L. Gastrointestinal and Hepatic Disease in Systemic Lupus Erythematosus. Rheum. Dis. Clin. N. Am. 2018, 44, 165–175.
- Kivity, S.; Agmon-Levin, N.; Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Neuropsychiatric Lupus: A Mosaic of Clinical Presentations. BMC Med. 2015, 13, 43.
- Uramoto, K.M.; Michet, C.J., Jr.; Thumboo, J.; Sunku, J.; O’Fallon, W.M.; Gabriel, S.E. Trends in the Incidence and Mortality of Systemic Lupus Erythematosus, 1950–1992. Arthritis Rheum. 1999, 42, 46–50.
- Liang, M.H.; Corzillius, M.; Bae, S.C.; Lew, R.A.; Fortin, P.R.; Gordon, C.; Isenberg, D.; Alarcón, G.S.; Straaton, K.V.; Denburg, J.; et al. The American College of Rheumatology Nomenclature and Case Definitions for Neuropsychiatric Lupus Syndromes. Arthritis Rheum. 1999, 42, 599–608.
- Nived, O.; Sturfelt, G.; Liang, M.H.; De Pablo, P. The ACR Nomenclature for CNS Lupus Revisited. Lupus 2003, 12, 872–876.
- Sarwar, S.; Mohamed, A.S.; Rogers, S.; Sarmast, S.T.; Kataria, S.; Mohamed, K.H.; Khalid, M.Z.; Saeeduddin, M.O.; Shiza, S.T.; Ahmad, S.; et al. Neuropsychiatric Systemic Lupus Erythematosus: A 2021 Update on Diagnosis, Management, and Current Challenges. Cureus 2021, 13, e17969.
- Chung, S.A.; Taylor, K.E.; Graham, R.R.; Nititham, J.; Lee, A.T.; Ortmann, W.A.; Jacob, C.O.; Alarcón-Riquelme, M.E.; Tsao, B.P.; Harley, J.B.; et al. Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti-dsDNA Autoantibody Production. PLoS Genet. 2011, 7, e1001323.
- Pisetsky, D.S. Pathogenesis of Autoimmune Disease. Nat. Rev. Nephrol. 2023, 19, 509–524.
- Panagopoulos, D.; Themistocleous, M. Central Nervous System Manifestation of Lupus Erythematosus Resembling Brain Abscess. Int. J. Pediatr. Adolesc. Med. 2019, 6, 29–37.
- Govoni, M.; Hanly, J.G. The Management of Neuropsychiatric Lupus in the 21st Century: Still so Many Unmet Needs? Rheumatology 2020, 59, v52–v62.
- Cohen, D.; Rijnink, E.C.; Nabuurs, R.J.A.; Steup-Beekman, G.M.; Versluis, M.J.; Emmer, B.J.; Zandbergen, M.; van Buchem, M.A.; Allaart, C.F.; Wolterbeek, R.; et al. Brain Histopathology in Patients with Systemic Lupus Erythematosus: Identification of Lesions Associated with Clinical Neuropsychiatric Lupus Syndromes and the Role of Complement. Rheumatology 2017, 56, 77–86.
- Sutanto, H.; Yuliasih, Y. Disentangling the Pathogenesis of Systemic Lupus Erythematosus: Close Ties between Immunological, Genetic and Environmental Factors. Medicina 2023, 59, 1033.
- Al-Motwee, S.; Jawdat, D.; Jehani, G.S.; Anazi, H.; Shubaili, A.; Sutton, P.; Uyar, A.F.; Hajeer, A.H. Association of HLA-DRB1*15 and HLADQB1*06 with SLE in Saudis. Ann. Saudi Med. 2013, 33, 229–234.
- Hosseini, S.A.; Labilloy, A. Genetics, TREX1 Mutations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Lehtinen, D.A.; Harvey, S.; Mulcahy, M.J.; Hollis, T.; Perrino, F.W. The TREX1 Double-Stranded DNA Degradation Activity Is Defective in Dominant Mutations Associated with Autoimmune Disease. J. Biol. Chem. 2008, 283, 31649–31656.
- Bailey, S.L.; Harvey, S.; Perrino, F.W.; Hollis, T. Defects in DNA Degradation Revealed in Crystal Structures of TREX1 Exonuclease Mutations Linked to Autoimmune Disease. DNA Repair 2012, 11, 65–73.
- Selvaraja, M.; Chin, V.K.; Abdullah, M.; Arip, M.; Amin-Nordin, S. HLA-DRB1*04 as a Risk Allele to Systemic Lupus Erythematosus and Lupus Nephritis in the Malay Population of Malaysia. Front. Med. 2020, 7, 598665.
- Gorji, A.E.; Roudbari, Z.; Alizadeh, A.; Sadeghi, B. Investigation of Systemic Lupus Erythematosus (SLE) with Integrating Transcriptomics and Genome Wide Association Information. Gene 2019, 706, 181–187.
- Wang, C.; Sandling, J.K.; Hagberg, N.; Berggren, O.; Sigurdsson, S.; Karlberg, O.; Rönnblom, L.; Eloranta, M.-L.; Syvänen, A.-C. Genome-Wide Profiling of Target Genes for the Systemic Lupus Erythematosus-Associated Transcription Factors IRF5 and STAT4. Ann. Rheum. Dis. 2013, 72, 96–103.
- Gupta, V.; Kumar, S.; Pratap, A.; Singh, R.; Kumari, R.; Kumar, S.; Aggarwal, A.; Misra, R. Association of ITGAM, TNFSF4, TNFAIP3 and STAT4 Gene Polymorphisms with Risk of Systemic Lupus Erythematosus in a North Indian Population. Lupus 2018, 27, 1973–1979.
- Cheikh, M.M.; Bahakim, A.K.; Aljabri, M.K.; Alharthi, S.M.; Alharthi, S.M.; Alsaeedi, A.K.; Alqahtani, S.F. Neuropsychiatric Lupus and Lupus Nephritis Successfully Treated with Combined IVIG and Rituximab: An Alternative to Standard of Care. Case Rep. Rheumatol. 2022, 2022, 5899188.
- UpToDate. Available online: https://www.uptodate.com/contents/neurologic-and-neuropsychiatric-manifestations-of-systemic-lupus-erythematosus (accessed on 15 December 2023).
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596.
- Liu, Y.; Tu, Z.; Zhang, X.; Du, K.; Xie, Z.; Lin, Z. Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus: A Review. Front. Cell Dev. Biol. 2022, 10, 998328.
- Jeltsch-David, H.; Muller, S. Neuropsychiatric Systemic Lupus Erythematosus: Pathogenesis and Biomarkers. Nat. Rev. Neurol. 2014, 10, 579–596.
- Moore, E.; Huang, M.W.; Putterman, C. Advances in the Diagnosis, Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 2020, 32, 152–158.
- Wang, M.; Wang, Z.; Zhang, S.; Wu, Y.; Zhang, L.; Zhao, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Progress in the Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus. J. Clin. Med. Res. 2022, 11, 4955.
- Gono, T.; Kawaguchi, Y.; Yamanaka, H. Discoveries in the Pathophysiology of Neuropsychiatric Lupus Erythematosus: Consequences for Therapy. BMC Med. 2013, 11, 91.
- Study Discovers Antibodies Leading to Development of Neuropsychiatric Lupus. Available online: https://www.lupus.org/news/study-discovers-antibodies-leading-to-development-of-neuropsychiatric-lupus (accessed on 15 December 2023).
- Sato, S.; Temmoku, J.; Fujita, Y.; Yashiro-Furuya, M.; Matsuoka, N.; Asano, T.; Kobayashi, H.; Watanabe, H.; Migita, K. Autoantibodies Associated with Neuropsychiatric Systemic Lupus Erythematosus: The Quest for Symptom-Specific Biomarkers. Fukushima J. Med. Sci. 2020, 66, 1–9.
- Yoshio, T.; Okamoto, H. Pathogenesis of Neuropsychiatric Syndromes of Systemic Lupus Erythematosus. Open J. Rheumatol. Autoimmune Dis. 2015, 5, 46–56.
- Okamoto, H.; Kobayashi, A.; Yamanaka, H. Cytokines and Chemokines in Neuropsychiatric Syndromes of Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 268436.
- Zhang, E.; Jorgensen, T.N. Neuropsychiatric SLE: From Immune Mechanisms to Clinical Management. In Lupus—New Advances and Challenges; IntechOpen: London, UK, 2020; ISBN 9781838801694.
- Barile-Fabris, L.; Hernández-Cabrera, M.F.; Barragan-Garfias, J.A. Vasculitis in Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2014, 16, 440.
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential Diagnosis of Multiple Sclerosis and Other Inflammatory CNS Diseases. Mult. Scler. Relat. Disord. 2020, 37, 101452.
- Sciascia, S.; Bertolaccini, M.L.; Roccatello, D.; Khamashta, M.A.; Sanna, G. Autoantibodies Involved in Neuropsychiatric Manifestations Associated with Systemic Lupus Erythematosus: A Systematic Review. J. Neurol. 2014, 261, 1706–1714.
- Saleki, K.; Shirzad, M.; Banazadeh, M.; Hosein Mohamadi, M.; Alijanizadeh, P.; Javanmehr, N.; Pourahmad, R.; Shakeri, M.; Nikkhoo Amiri, R.; Payandeh, P.; et al. Lupus and the Nervous System: A Neuroimmunoloigcal Update on Pathogenesis and Management of Systemic Lupus Erythematosus with Focus on Neuropsychiatric SLE. In Systemic Lupus Erythematosus—Pathogenesis and Management; IntechOpen: London, UK, 2022.
- Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Autoantibodies Involved in Neuropsychiatric SLE and Antiphospholipid Syndrome. Semin. Arthritis Rheum. 2007, 36, 297–315.
- Salmon, J.E.; de Groot, P.G. Pathogenic Role of Antiphospholipid Antibodies. Lupus 2008, 17, 405–411.
- Mackworth-Young, C.G. Antiphospholipid Syndrome: Multiple Mechanisms. Clin. Exp. Immunol. 2004, 136, 393–401.
- Harper, B.E.; Wills, R.; Pierangeli, S.S. Pathophysiological Mechanisms in Antiphospholipid Syndrome. Int. J. Clin. Rheumtol. 2011, 6, 157–171.
- Katzav, A.; Ben-Ziv, T.; Blank, M.; Pick, C.G.; Shoenfeld, Y.; Chapman, J. Antibody-Specific Behavioral Effects: Intracerebroventricular Injection of Antiphospholipid Antibodies Induces Hyperactive Behavior While Anti-Ribosomal-P Antibodies Induces Depression and Smell Deficits in Mice. J. Neuroimmunol. 2014, 272, 10–15.
- de Carvalho, J.F.; Pasoto, S.G.; Appenzeller, S. Seizures in Primary Antiphospholipid Syndrome: The Relevance of Smoking to Stroke. Clin. Dev. Immunol. 2012, 2012, 981519.
- Kitagawa, Y. Antiphospholipid syndrome and stroke. Rinsho Shinkeigaku 2005, 45, 852–855.
- Harris, E.N.; Pierangeli, S. Antiphospholipid Antibodies and Cerebral Lupus. Ann. N. Y. Acad. Sci. 1997, 823, 270–278.
- Peluso, S.; Antenora, A.; De Rosa, A.; Roca, A.; Maddaluno, G.; Brescia Morra, V.; De Michele, G. Antiphospholipid-Related Chorea. Front. Neurol. 2012, 3, 150.
- Lerjefors, L.; Andretta, S.; Bonato, G.; Mainardi, M.; Carecchio, M.; Antonini, A. Antiphospholipid-Related Chorea: Two Case Reports and Role of Metabolic Imaging. Mov. Disord. Clin. Pract. 2022, 9, 516–521.
- Sanna, G.; Bertolaccini, M.L.; Cuadrado, M.J.; Laing, H.; Khamashta, M.A.; Mathieu, A.; Hughes, G.R.V. Neuropsychiatric Manifestations in Systemic Lupus Erythematosus: Prevalence and Association with Antiphospholipid Antibodies. J. Rheumatol. 2003, 30, 985–992.
- Shi, Z.-R.; Han, Y.-F.; Yin, J.; Zhang, Y.-P.; Jiang, Z.-X.; Zheng, L.; Tan, G.-Z.; Wang, L. The Diagnostic Benefit of Antibodies against Ribosomal Proteins in Systemic Lupus Erythematosus. Adv. Rheumatol. 2020, 60, 45.
- Caponi, L.; Bombardieri, S.; Migliorini, P. Anti-Ribosomal Antibodies Bind the Sm Proteins D and B/B’. Clin. Exp. Immunol. 1998, 112, 139–143.
- Mader, S.; Brimberg, L.; Diamond, B. The Role of Brain-Reactive Autoantibodies in Brain Pathology and Cognitive Impairment. Front. Immunol. 2017, 8, 1101.
- Brimberg, L.; Mader, S.; Fujieda, Y.; Arinuma, Y.; Kowal, C.; Volpe, B.T.; Diamond, B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015, 36, 709–724.
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Singla, N.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Blood-Brain Barrier: Emerging Trends on Transport Models and New-Age Strategies for Therapeutics Intervention against Neurological Disorders. Mol. Brain 2022, 15, 49.
- Abdel-Nasser, A.M.; Ghaleb, R.M.; Mahmoud, J.A.; Khairy, W.; Mahmoud, R.M. Association of Anti-Ribosomal P Protein Antibodies with Neuropsychiatric and Other Manifestations of Systemic Lupus Erythematosus. Clin. Rheumatol. 2008, 27, 1377–1385.
- Hirohata, S.; Arinuma, Y.; Takayama, M.; Yoshio, T. Association of Cerebrospinal Fluid Anti-Ribosomal P Protein Antibodies with Diffuse Psychiatric/neuropsychological Syndromes in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2007, 9, R44.
- Leng, Q.; Su, J.; Wang, X.; Zhuang, B.; Liu, L.; Deng, X.; Li, Y. Anti-Ribosomal P Protein Antibodies and Insomnia Correlate with Depression and Anxiety in Patients Suffering from Systemic Lupus Erythematosus. Heliyon 2023, 9, e15463.
- Zhang, S.; Li, M.; Zhang, L.; Wang, Z.; Wang, Q.; You, H.; Wang, Y.; Li, M.; Zeng, X. Clinical Features and Outcomes of Neuropsychiatric Systemic Lupus Erythematosus in China. J. Immunol. Res. 2021, 2021, 1349042.
- Hanly, J.G.; Urowitz, M.B.; Siannis, F.; Farewell, V.; Gordon, C.; Bae, S.C.; Isenberg, D.; Dooley, M.A.; Clarke, A.; Bernatsky, S.; et al. Autoantibodies and Neuropsychiatric Events at the Time of Systemic Lupus Erythematosus Diagnosis: Results from an International Inception Cohort Study. Arthritis Rheum. 2008, 58, 843–853.
- Yoshio, T.; Hirata, D.; Onda, K.; Nara, H.; Minota, S. Antiribosomal P Protein Antibodies in Cerebrospinal Fluid Are Associated with Neuropsychiatric Systemic Lupus Erythematosus. J. Rheumatol. 2005, 32, 34–39.
- Katzav, A.; Solodeev, I.; Brodsky, O.; Chapman, J.; Pick, C.G.; Blank, M.; Zhang, W.; Reichlin, M.; Shoenfeld, Y. Induction of Autoimmune Depression in Mice by Anti-Ribosomal P Antibodies via the Limbic System. Arthritis Rheum. 2007, 56, 938–948.
- Matus, S.; Burgos, P.V.; Bravo-Zehnder, M.; Kraft, R.; Porras, O.H.; Farías, P.; Barros, L.F.; Torrealba, F.; Massardo, L.; Jacobelli, S.; et al. Antiribosomal-P Autoantibodies from Psychiatric Lupus Target a Novel Neuronal Surface Protein Causing Calcium Influx and Apoptosis. J. Exp. Med. 2007, 204, 3221–3234.
- Bravo-Zehnder, M.; Toledo, E.M.; Segovia-Miranda, F.; Serrano, F.G.; Benito, M.J.; Metz, C.; Retamal, C.; Álvarez, A.; Massardo, L.; Inestrosa, N.C.; et al. Anti-Ribosomal P Protein Autoantibodies from Patients with Neuropsychiatric Lupus Impair Memory in Mice. Arthritis Rheumatol. 2015, 67, 204–214.
- González, A.; Massardo, L. Antibodies and the Brain: Antiribosomal P Protein Antibody and the Clinical Effects in Patients with Systemic Lupus Erythematosus. Curr. Opin. Neurol. 2018, 31, 300–305.
- Marín, J.-D.; Vargas, S.; Ruiz-Ordoñez, I.; Posso-Osorio, I.; Nieto-Aristizábal, I.; Barrera, M.C.; Ríos-Serna, L.J.; Tobón, G.J. Association of Antiribosomal P Antibody with Neurological and Systemic Manifestations in Patients with Systemic Lupus Erythematosus in Southwestern Colombia. J. Appl. Lab. Med. 2022, 7, 3–11.
- Li, F.; Tsien, J.Z. Memory and the NMDA Receptors. N. Engl. J. Med. 2009, 361, 302–303.
- Levite, M. Glutamate Receptor Antibodies in Neurological Diseases: Anti-AMPA-GluR3 Antibodies, Anti-NMDA-NR1 Antibodies, Anti-NMDA-NR2A/B Antibodies, Anti-mGluR1 Antibodies or Anti-mGluR5 Antibodies Are Present in Subpopulations of Patients with Either: Epilepsy, Encephalitis, Cerebellar Ataxia, Systemic Lupus Erythematosus (SLE) and Neuropsychiatric SLE, Sjogren’s Syndrome, Schizophrenia, Mania or Stroke. These Autoimmune Anti-Glutamate Receptor Antibodies Can Bind Neurons in Few Brain Regions, Activate Glutamate Receptors, Decrease Glutamate Receptor’s Expression, Impair Glutamate-Induced Signaling and Function, Activate Blood Brain Barrier Endothelial Cells, Kill Neurons, Damage the Brain, Induce Behavioral/psychiatric/cognitive Abnormalities and Ataxia in Animal Models, and Can Be Removed or Silenced in Some Patients by Immunotherapy. J. Neural Transm. 2014, 121, 1029–1075.
- Schüler, T.; Mesic, I.; Madry, C.; Bartholomäus, I.; Laube, B. Formation of NR1/NR2 and NR1/NR3 Heterodimers Constitutes the Initial Step in N-Methyl-D-Aspartate Receptor Assembly. J. Biol. Chem. 2008, 283, 37–46.
- Zhang, S.; Yang, Y.; Long, T.; Li, Z. Systemic Lupus Erythematosus Associated with Recurrent Anti-NMDA Receptor Encephalitis during Pregnancy. Arch. Women’s Ment. Health 2021, 24, 525–528.
- Samanta, D.; Lui, F. Anti-NMDA Receptor Encephalitis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Lauvsnes, M.B.; Omdal, R. Systemic Lupus Erythematosus, the Brain, and Anti-NR2 Antibodies. J. Neurol. 2012, 259, 622–629.
- Hanly, J.G.; Legge, A.; Kamintsky, L.; Friedman, A.; Hashmi, J.A.; Beyea, S.D.; Fisk, J.; Omisade, A.; Calkin, C.; Bardouille, T.; et al. Role of Autoantibodies and Blood-Brain Barrier Leakage in Cognitive Impairment in Systemic Lupus Erythematosus. Lupus Sci. Med. 2022, 9, e000668.
- Hirohata, S.; Arinuma, Y.; Yanagida, T.; Yoshio, T. Blood-Brain Barrier Damages and Intrathecal Synthesis of Anti-N-Methyl-D-Aspartate Receptor NR2 Antibodies in Diffuse Psychiatric/neuropsychological Syndromes in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2014, 16, R77.
- Tomalla, V.; Schmeisser, M.J.; Weinmann-Menke, J. Mouse Models, Antibodies, and Neuroimaging: Current Knowledge and Future Perspectives in Neuropsychiatric Systemic Lupus Erythematosus (NPSLE). Front. Psychiatry 2023, 14, 1078607.
- DeGiorgio, L.A.; Konstantinov, K.N.; Lee, S.C.; Hardin, J.A.; Volpe, B.T.; Diamond, B. A Subset of Lupus Anti-DNA Antibodies Cross-Reacts with the NR2 Glutamate Receptor in Systemic Lupus Erythematosus. Nat. Med. 2001, 7, 1189–1193.
- Sánchez, C.; Díaz-Nido, J.; Avila, J. Phosphorylation of Microtubule-Associated Protein 2 (MAP2) and Its Relevance for the Regulation of the Neuronal Cytoskeleton Function. Prog. Neurobiol. 2000, 61, 133–168.
- Izant, J.G.; McIntosh, J.R. Microtubule-Associated Proteins: A Monoclonal Antibody to MAP2 Binds to Differentiated Neurons. Proc. Natl. Acad. Sci. USA 1980, 77, 4741–4745.
- Williams, R.C., Jr.; Sugiura, K.; Tan, E.M. Antibodies to Microtubule-Associated Protein 2 in Patients with Neuropsychiatric Systemic Lupus Erythematosus. Arthritis Rheum. 2004, 50, 1239–1247.
- Jones, L.B.; Johnson, N.; Byne, W. Alterations in MAP2 Immunocytochemistry in Areas 9 and 32 of Schizophrenic Prefrontal Cortex. Psychiatry Res. 2002, 114, 137–148.
- Rosoklija, G.; Keilp, J.G.; Toomayan, G.; Mancevski, B.; Haroutunian, V.; Liu, D.; Malespina, D.; Hays, A.P.; Sadiq, S.; Latov, N.; et al. Altered Subicular MAP2 Immunoreactivity in Schizophrenia. Prilozi 2005, 26, 13–34.
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased Expression of Synapse-Related Genes and Loss of Synapses in Major Depressive Disorder. Nat. Med. 2012, 18, 1413–1417.
- Vlachoyiannopoulos, P.G.; Guialis, A.; Tzioufas, G.; Moutsopoulos, H.M. Predominance of IgM Anti-U1RNP Antibodies in Patients with Systemic Lupus Erythematosus. Br. J. Rheumatol. 1996, 35, 534–541.
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies 2016, 5, 2.
- van Venrooij, W.J.; Hoet, R.; Castrop, J.; Hageman, B.; Mattaj, I.W.; van de Putte, L.B. Anti-(U1) Small Nuclear RNA Antibodies in Anti-Small Nuclear Ribonucleoprotein Sera from Patients with Connective Tissue Diseases. J. Clin. Investig. 1990, 86, 2154–2160.
- Kattah, N.H.; Kattah, M.G.; Utz, P.J. The U1-snRNP Complex: Structural Properties Relating to Autoimmune Pathogenesis in Rheumatic Diseases. Immunol. Rev. 2010, 233, 126–145.
- Benito-Garcia, E.; Schur, P.H.; Lahita, R.; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for Immunologic Laboratory Testing in the Rheumatic Diseases: Anti-Sm and Anti-RNP Antibody Tests. Arthritis Rheum. 2004, 51, 1030–1044.
- Sato, T.; Fujii, T.; Yokoyama, T.; Fujita, Y.; Imura, Y.; Yukawa, N.; Kawabata, D.; Nojima, T.; Ohmura, K.; Usui, T.; et al. Anti-U1 RNP Antibodies in Cerebrospinal Fluid Are Associated with Central Neuropsychiatric Manifestations in Systemic Lupus Erythematosus and Mixed Connective Tissue Disease. Arthritis Rheum. 2010, 62, 3730–3740.
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91.
- Bergkamp, S.C.; Wahadat, M.J.; Salah, A.; Kuijpers, T.W.; Smith, V.; Tas, S.W.; van den Berg, J.M.; Kamphuis, S.; Schonenberg-Meinema, D. Dysregulated Endothelial Cell Markers in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. J. Inflamm. 2023, 20, 18.
- Alessandri, C.; Bombardieri, M.; Valesini, G. Pathogenic Mechanisms of Anti-Endothelial Cell Antibodies (AECA): Their Prevalence and Clinical Relevance. Adv. Clin. Chem. 2006, 42, 297–326.
- Del Papa, N.; Raschi, E.; Moroni, G.; Panzeri, P.; Borghi, M.O.; Ponticelli, C.; Tincani, A.; Balestrieri, G.; Meroni, P.L. Anti-Endothelial Cell IgG Fractions from Systemic Lupus Erythematosus Patients Bind to Human Endothelial Cells and Induce a pro-Adhesive and a pro-Inflammatory Phenotype in Vitro. Lupus 1999, 8, 423–429.
- Atehortúa, L.; Rojas, M.; Vásquez, G.M.; Castaño, D. Endothelial Alterations in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Potential Effect of Monocyte Interaction. Mediat. Inflamm. 2017, 2017, 9680729.
- Myers, T.D.; Palladino, M.J. Newly Discovered Roles of Triosephosphate Isomerase Including Functions within the Nucleus. Mol. Med. 2023, 29, 18.
- Sato, S.; Yashiro, M.; Asano, T.; Kobayashi, H.; Watanabe, H.; Migita, K. Association of Anti-Triosephosphate Isomerase Antibodies with Aseptic Meningitis in Patients with Neuropsychiatric Systemic Lupus Erythematosus. Clin. Rheumatol. 2017, 36, 1655–1659.
- Sasajima, T.; Watanabe, H.; Sato, S.; Sato, Y.; Ohira, H. Anti-Triosephosphate Isomerase Antibodies in Cerebrospinal Fluid Are Associated with Neuropsychiatric Lupus. J. Neuroimmunol. 2006, 181, 150–156.
- Sirover, M.A. Role of the Glycolytic Protein, Glyceraldehyde-3-Phosphate Dehydrogenase, in Normal Cell Function and in Cell Pathology. J. Cell. Biochem. 1997, 66, 133–140.
- Sun, J.; Li, X.; Zhou, H.; Liu, X.; Jia, J.; Xie, Q.; Peng, S.; Sun, X.; Wang, Q.; Yi, L. Anti-GAPDH Autoantibody Is Associated with Increased Disease Activity and Intracranial Pressure in Systemic Lupus Erythematosus. J. Immunol. Res. 2019, 2019, 7430780.
- Delunardo, F.; Soldati, D.; Bellisario, V.; Berry, A.; Camerini, S.; Crescenzi, M.; Alessandri, C.; Conti, F.; Ceccarelli, F.; Francia, A.; et al. Anti-GAPDH Autoantibodies as a Pathogenic Determinant and Potential Biomarker of Neuropsychiatric Diseases. Arthritis Rheumatol. 2016, 68, 2708–2716.
- Kudo, H.; Miyanaga, K.; Yamamoto, N. Immunomodulatory Effects of Extracellular Glyceraldehyde 3-Phosphate Dehydrogenase of Exopolysaccharide-Producing Lactiplantibacillus Plantarum JCM 1149. Food Funct. 2023, 14, 489–499.
- Gil, M.L.; Dagan, S.; Eren, R.; Gozalbo, D. Evaluation of the Usefulness of Anti-Glyceraldehyde-3-Phosphate Dehydrogenase Antibodies as a Treatment for Invasive Candidiasis in a Murine Model. Antonie Van Leeuwenhoek 2006, 89, 345–350.
- Závada, J.; Nytrová, P.; Wandinger, K.P.; Jarius, S.; Svobodová, R.; Probst, C.; Peterová, V.; Tegzová, D.; Pavelka, K.; Vencovský, J. Seroprevalence and Specificity of NMO-IgG (anti-Aquaporin 4 Antibodies) in Patients with Neuropsychiatric Systemic Lupus Erythematosus. Rheumatol. Int. 2013, 33, 259–263.
- Graham, A.; Ford, I.; Morrison, R.; Barker, R.N.; Greaves, M.; Erwig, L.-P. Anti-Endothelial Antibodies Interfere in Apoptotic Cell Clearance and Promote Thrombosis in Patients with Antiphospholipid Syndrome. J. Immunol. 2009, 182, 1756–1762.
- Guo, Y.; Li, X.; Li, R.; Li, Y.; Wang, Z.; Liu, H.; Cao, S.; Li, R.; Zhao, Y.; Wang, Q.; et al. Utility of Autoantibody against an UCH-L1 Epitope as a Serum Diagnostic Marker for Neuropsychiatric Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2022, 40, 2078–2087.
- Li, X.; Sun, J.; Mu, R.; Gan, Y.; Wang, G.; He, J.; Yi, L.; Wang, Q.; Sun, X.; Li, Z. The Clinical Significance of Ubiquitin Carboxyl Hydrolase L1 and Its Autoantibody in Neuropsychiatric Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2019, 37, 474–480.
- Hu, C.; Huang, W.; Chen, H.; Song, G.; Li, P.; Shan, Q.; Zhang, X.; Zhang, F.; Zhu, H.; Wu, L.; et al. Autoantibody Profiling on Human Proteome Microarray for Biomarker Discovery in Cerebrospinal Fluid and Sera of Neuropsychiatric Lupus. PLoS ONE 2015, 10, e0126643.




