You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jacek Tabarkiewicz | -- | 2334 | 2024-02-18 14:06:48 | | | |
| 2 | Rita Xu | -3 word(s) | 2331 | 2024-02-19 02:35:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Encyclopedia. Available online: https://encyclopedia.pub/entry/55120 (accessed on 24 December 2025).
Stępień AE, Trojniak J, Tabarkiewicz J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Encyclopedia. Available at: https://encyclopedia.pub/entry/55120. Accessed December 24, 2025.
Stępień, Agnieszka Ewa, Julia Trojniak, Jacek Tabarkiewicz. "Anti-Cancer and Anti-Inflammatory Properties of Black Garlic" Encyclopedia, https://encyclopedia.pub/entry/55120 (accessed December 24, 2025).
Stępień, A.E., Trojniak, J., & Tabarkiewicz, J. (2024, February 18). Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. In Encyclopedia. https://encyclopedia.pub/entry/55120
Stępień, Agnieszka Ewa, et al. "Anti-Cancer and Anti-Inflammatory Properties of Black Garlic." Encyclopedia. Web. 18 February, 2024.
Copy Citation
Black garlic (BG) is a fermented form of garlic (Allium sativum L.), produced at precisely defined temperatures, humidities, and time periods. Although garlic has been used for thousands of years, black garlic is a relatively new discovery. There are many bioactive compounds in black garlic that give it medicinal properties, including anti-inflammatory and anti-cancer properties.
black garlic
anti-inflammatory
anti-cancer
1. Introduction
Garlic (Allium sativum L.) is a shallow-rooted vegetable plant belonging to the Alliaceae family [1]. Native to western Asia and the Mediterranean coast, this plant is widely distributed around the world [2]. Allium sativum includes two subspecies: A. sativum variety sativum (softneck garlic) and A. sativum variety ophioscorodon (hardneck garlic) [1]. There are differences between both subspecies in terms of structure. The head of hard garlic has a hard neck and six to eleven cloves surrounding a woody stem, while soft garlic has no flower top, contains up to twenty-four cloves, and has a stem that is soft and central [1]. Garlic can grow in temperate and warm climate zones and is perennial [1][3].
Garlic has been used in traditional medicine around the world since ancient times due to its valuable health-promoting properties [4]. Scientific research results indicate a number of health-promoting properties: hepatoprotective, nephroprotective, immunomodulatory, anti-allergic, antioxidant and anti-cancer properties (Figure 1) [5][6][7][8][9]. Its unfavorable taste and smell have recently significantly reduced consumption all over the world, with the exception of China and India [10]. Consuming raw garlic in quantities to achieve enormous health benefits for the patient is difficult due to its pungent taste and odor [11].
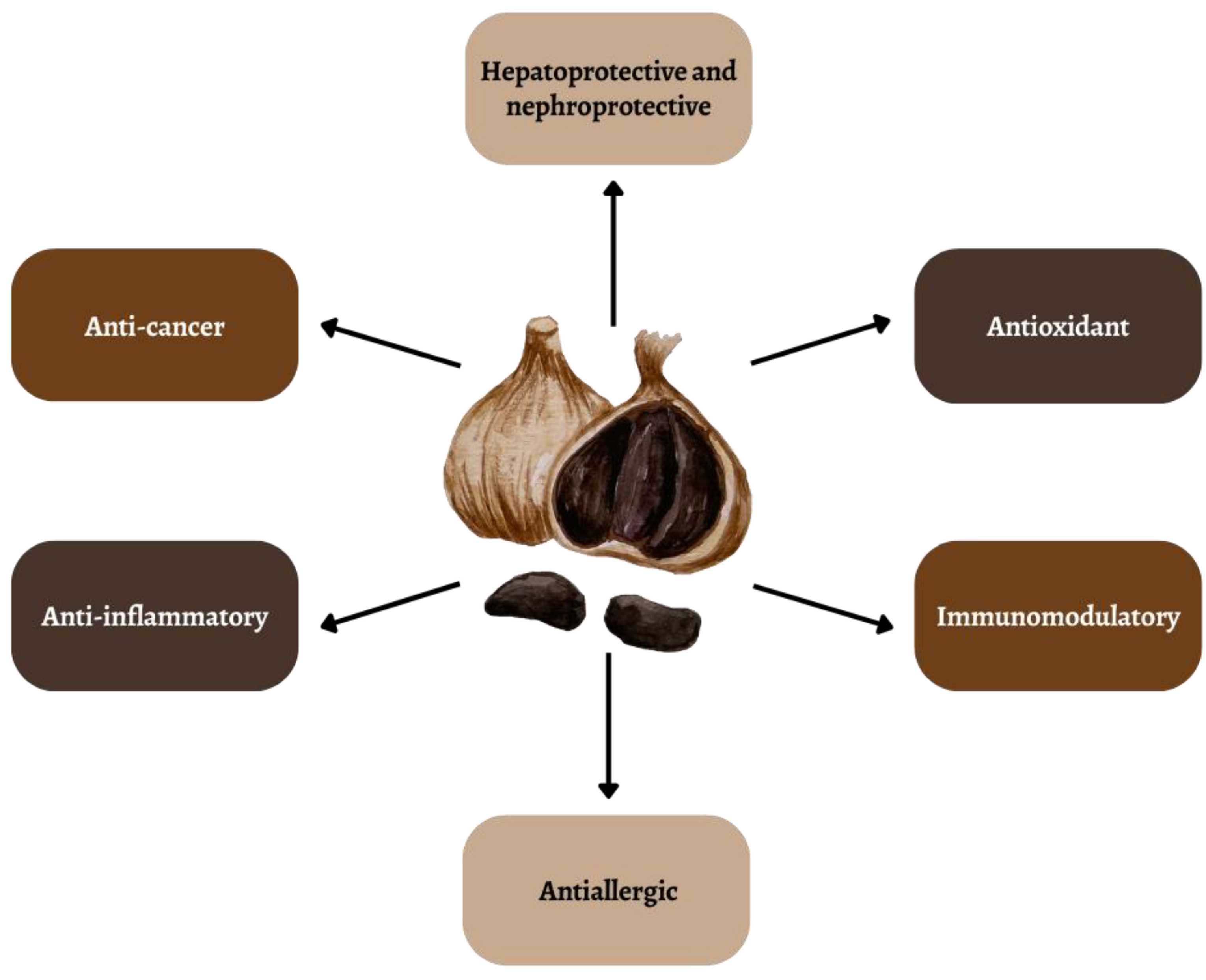
Figure 1. Selected properties of black garlic.
Garlic consumption is accompanied by an unpleasant odor from the mouth and body. There is evidence that garlic consumed in large doses has toxic effects [8]. Additionally, a significant number of consumers have stopped consuming garlic due to gastrointestinal discomfort, including damage to the stomach and intestinal walls [10][11]. When large doses of garlic are used, negative side effects are observed: anemia, calcium deficiency and contact allergies [8].
In recent years, there has been an increase in interest in the health-promoting properties of black garlic (BG) as a rich source of several bioactive compounds, mainly those with antioxidant properties [4][12]. It is obtained from raw garlic subjected to aging processes [13][14]. Black garlic is produced by fermentation and ripening under strictly defined conditions of a temperature of 60–90 °C and humidity of 60–90% for 10 to 80 days [14]. As it ages under precisely defined processing parameters, e.g., temperature or humidity, the color of garlic changes from white to dark brown/black [15]. The color change is the result of enzymatic browning in the Millard reaction—condensation between the reducing carbonyl group of sugar and the amino group [10][15][16][17]. In Figure 2, researchers can visualize the difference between fresh garlic and aged garlic. During the fermentation process, white garlic loses its sharp taste due to the alliin content in favor of a sweet or sweet–sour taste and becomes odorless and has a consistency ranging from rubbery and stringy to gelatinous [4][14][18]. Moreover, new S-allicin compounds resulting from the transformation of allicin have very strong antioxidant properties as a result of the Maillard reaction between reducing sugars and allicin [19]. During garlic aging, the chemical oxidation of phenols and thermal degradation of organic sulfur compounds also occur [4][20].

Figure 2. Fresh garlic and black garlic heads and cloves comparison: (a) fresh garlic (Allium sativum) head; (b) black garlic (Allium sativum) head; (c) fresh garlic head and gloves; (d) black garlic head and cloves; (e,f) a side-by-side comparison of a clove of fresh garlic (left) and black garlic (right) (photograph by Julia Trojniak).
2. Active Compounds of Black Garlic
The aging process of black garlic causes a change in physicochemical properties associated with an increase in the content of antioxidant compounds [21]. Compared to fresh garlic, black garlic has a significantly different chemical composition. Black garlic is characterized by a high concentration of many antioxidant compounds, including the following: phenols, flavonoids, pyruvate, S-Allyl-Cysteine (SAC), S-allyl-Mercapto-Cysteine (SAMC), and 5-hydroxymethylfurfural (5-HMF). It also contained allicin-derived organosulfur compounds (OSC): diallyl sulfides (DAS), diallyl disulfides (DADS), diallyl trisulfides (DATS), and diallyl tetrasulfide [10][22]—as shown in Figure 3a–h. Scientific research results have shown that the content of phenols differs significantly between black and fresh garlic [23]. An increase in temperature and a decrease in humidity during aging significantly increases the level of polyphenols, thiosulfonates, and allicin [24].
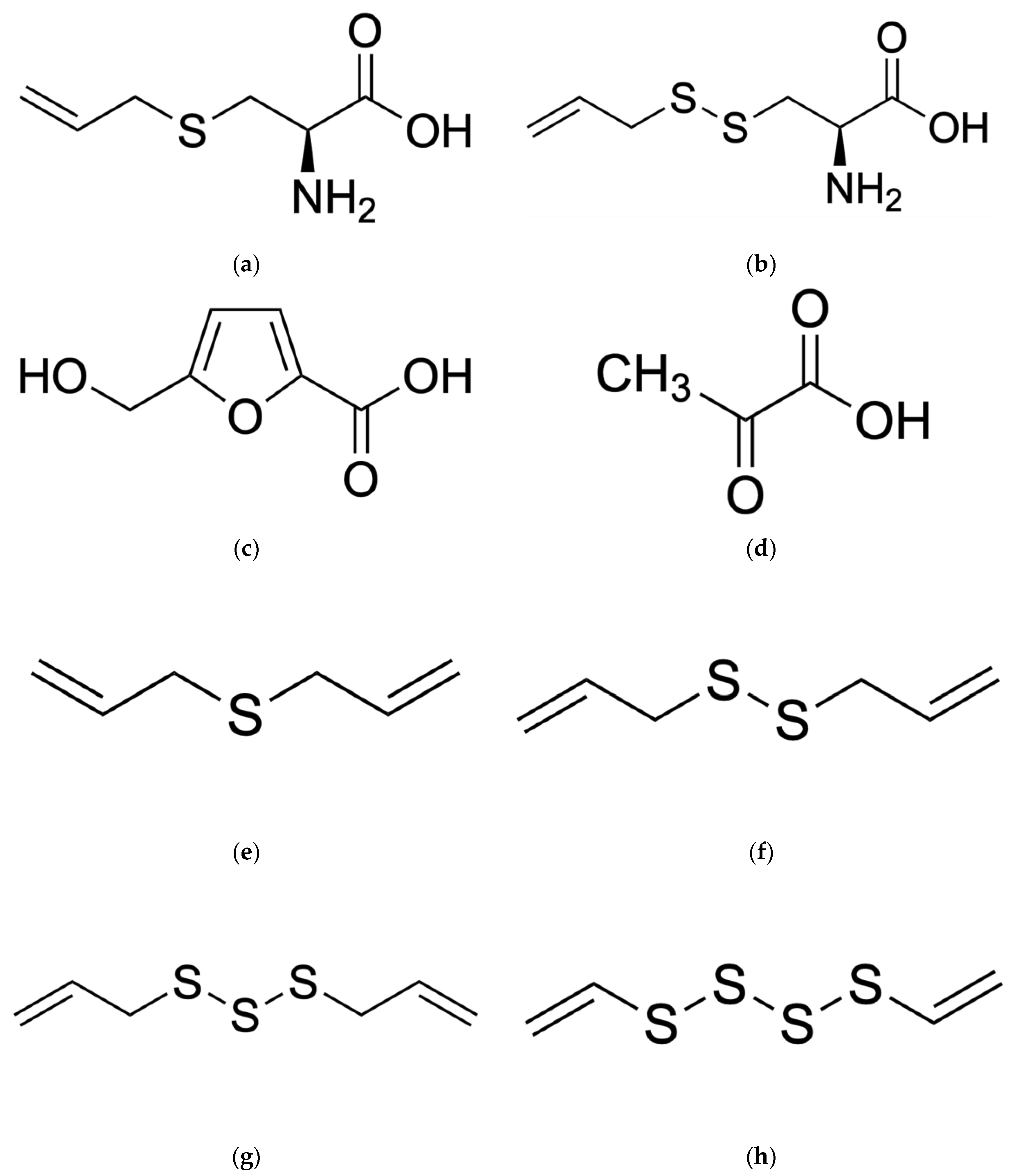
Figure 3. Chemical structures of several of the most important compounds found in black garlic (BG): (a) S-Allyl-Cysteine (SAC); (b) S-Allyl-Mercapto-Cysteine (SAMC); (c) 5-Hydroxymethylfurfural (5-HMF); (d) pyruvate/pyruvic acid; (e) dialyl sulfides (DAS); (f) dialyl disulfides (DADS); (g) dialyl trisulfides (DATS); (h) dialyl tetrasulfide.
3. Anti-Inflammatory Properties of Black Garlic
It has been shown that black garlic significantly reduces blood sugar levels, lipid peroxidation, and antioxidant defenses by activating downstream nuclear factor erythroid 2-related factor 2 (Nrf2) and Nrf2 targets such as quinone-oxidoreductase-1 (NQO1), heme oxygenase-1 (HO-1), and glutathione S-transferase alpha 2 (GSTA2) [25].
There are several compounds in black garlic that could have anti-inflammatory effects, including pyruvate, S-Allyl-Cysteine (SAC), 2-linoleoylglycerol, and 5-hydroxymethylfurfural (5-HMF) [10][26][27].
It was shown that 5-hydroxymethylfurfural suppressed cell adhesion by human umbilical vein endothelial cells (HUVEC) by inhibiting the expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule (ICAM-1), ROS generation, and nuclear factor kappa B activation [28]. Kong et al. investigated 5-Hydroxymethylfurfural’s (5-HMF) effects on LPS-stimulated RAW 264.7 macrophage inflammatory responses. It was found that 5-HMF suppressed the phosphorylation of proteins connected to MAPK, NF-κB, and Akt/mTOR signaling pathways. The inhibition of these pathways was mediated through inhibition of pro-inflammatory mediators (NO, PGE2, TNF-α, IL-6 and IL-1β) and reactive oxygen species (ROS) [29].
Using HaCaT keratinocytes as a model system, it was found that S-Allyl-Cysteine (SAC) from black garlic (BG) induces an anti-inflammatory response by inhibiting the production of pro-inflammatory cytokines TNF-α and IL-1β. In addition, S-Allyl-Cysteine significantly inhibited TNF-α-induced activation of P38 and JNK MAP kinases and NF-κB [30].
A mouse model of contact dermatitis showed that black garlic decreased the activation of macrophages, as well as the release of inflammatory mediators like nitric oxide II (NO), tumor necrosis factor (TNF-α), and interleukin 6 (IL-6) [31]. A reduction in inflammatory mediators was achieved by inhibiting iNOS, COX-2, and NF-κB. Additionally, the fraction of black garlic extract (BG10) showed a stronger anti-inflammatory effect against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced contact dermatitis in RAW264.7 cells compared to crude black garlic extract (ABG) [31]. In a study involving RAW 264.7 macrophages that had been stimulated with LPS, Kim et al. investigated the anti-inflammatory properties of BG. In aged black garlic, one compound (AGE-1) inhibited the production of pro-inflammatory mediators (NO, PGE2, IL-1β, IL-6 and TNF-α). In contrast, the second compound (AGE-2) did not show such an effect [26].
The anti-inflammatory and hepatoprotective effects of black garlic extracts were demonstrated in a mouse model of acute hepatitis by reducing the levels of alanine aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and maldialdehyde (MDA). Additionally, black garlic extracts improved the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and glutathione reductase (GSH-Rd), while reducing tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1β) levels in mouse liver, indicating a significant anti-inflammatory effect [32]. Increasing activity of SOD in hepatocytes during a toxic liver injury stay, in opposition to the in vitro results described above, can suggest that the influence of BG extracts on SOD activity could be dependent on the model researchers checked SOD activity in, that is, whether it was a simple chemical reaction or in complex model of whole organs or organisms.
The anti-inflammatory properties of black garlic were also demonstrated in a study by Lee et al. using mice with colistin-induced nephritis. An aged black garlic extract inhibited the expression of the TGF-β1 protein, which induced the NF-κB signaling pathway. CD68+ cells (“mouse macrophages”), infiltrated in kidneys, were also reduced, as were levels of IL-1β and TNF-α [33].
Using RT-PCR analysis, Recinella et al. examined the effect of aged black garlic extract on pro-inflammatory and pro-oxidant mediators (COX-2, TNF-α, IL-6, NF-kB) and iNOS mRNA levels on isolated LPS-stimulated heart samples. ABGE inhibited all the inflammatory and prooxidative mediators mentioned, which that suggests black garlic has anti-inflammatory properties [23].
Moreover, black garlic combined with vitamins D, C, and B12 had greater protective effects by inhibiting more inflammatory and oxidative pathways related to stress in LPS-exposed mice’s hearts than each vitamin alone [23].
The chloroform extract of aged black garlic (CEABG) inhibited TNF-α-stimulated VCAM-1 expression by decreasing reactive oxygen species (ROS) production and inhibiting the activation of the redox-sensitive transcription factor NF-κB in human umbilical vein endothelial cells (HUVEC) [34].
4. Anti-Cancer Properties of Black Garlic (BG)
Throughout the world, cancer is one of the leading causes of death. Despite scientific progress, cancer therapy remains a challenge for many types of cancer. Oncological therapies often cause unfavorable side effects for patients, leading to reductions in their quality of life. Often, it also affects the deterioration of health.
In recent years, scientists have been focusing on researching the role of phytochemicals, mainly antioxidants, present in plants to determine new and effective methods for supporting anticancer therapy, especially in terms of limiting side effects. Intensive research is being conducted to find new substances with anti-cancer properties and therapeutic potential.
Carcinogenesis is influenced by internal and environmental factors. The consequences of the action of free radicals, e.g., reactive oxygen species generated in the human body, are one of the main internal etiological factors of cancer [35]. Oxidative stress is frequently associated with chronic inflammation, which can be followed by neighboring cell mutation and increased proliferation, often creating an environment that is conducive to the development of cancer. The antioxidative and anti-inflammatory properties of black garlic described above are also indirect anti-cancer mechanisms. In the text below, researchers focused on the direct anti-cancer features of aged garlic.
It has been shown that mature black garlic extract inhibits the proliferation, migration, invasion, and metastasis of ER+ breast cancer cells in the MCF-7 and MDA-MB-361 cell lines [16]. Moreover, it stimulates apoptosis in ER+ breast cancer cells via the inhibition of the expression of anti-apoptotic proteins MCL-1 and BCL-2, while stimulating the expression of pro-apoptotic proteins BIM and BAK [16]. The reduction in MCL-1 expression was mediated by JNK activation caused by an increase in the amount of reactive oxygen species in cancer cells [16].
It has been shown that hexane extract from ripening black garlic induces apoptosis of the human leukemic cells (U937). The process of caspase-dependent apoptosis was initiated by both intrinsic and extrinsic pathways [36].
The use of ABGE in the treatment of colon cancer also has potential therapeutic value. In the study by Dong et al., it was observed that ABGE inhibited proliferation and stimulated the apoptosis of HT29 colon cancer cells. The possible mechanism of the anticancer effect is the modulation of the PI3K/Akt signaling pathway, increasing the expression of PTEN and reducing the expression of Akt and p-Akt [37].
The effects of mature black garlic on 1,2-dimethylhydrazine (DMH)-induced colon cancer models in rats and the anti-proliferative mechanisms of action were determined. Its inhibitory effect on the proliferative activity in cancerous lesions was observed without affecting the normal colonic mucosa. AGE (aged garlic extract) gradually inhibited the progression of DLD-1 by delaying cell proliferation by reducing the expression level of cyclin B1 and cdk1, which in turn was caused by the weakening of NF-κB activity [38].
The anticancer effects of aqueous extract of aged garlic on diethylnitrosamine (DEN)-induced liver cancer in rats were observed. The administration of this extract for 7 weeks resulted in a reduction in liver mass, with a significant reduction in the levels of alanine aminotransferase (ALT), aminotransferase (AST), and total bilirubin (TBIL), and an increase in antioxidant activity (TEAC test). The authors emphasize its extraordinary hepatoprotective and antioxidant effects in rats with DEN-induced liver cancer [39].
Next to S-Allyl-Cysteine (SAC), S-Allyl-Mercapto-Cysteine (SAMC) is another compound in black garlic with health-promoting properties. According to Zhang et al.’s study, SAMC induced apoptosis in human colon cancer cell line SW620 in vitro, which may explain garlic’s antiproliferative properties. Also, these results suggested that S-Allyl-Mercapto-Cysteine induces apoptosis through the JNK and p38 pathways, which activate Bax and p53 [40].
Hep-G2 cells, prostate cancer (PC-3), MCF-7 breast cancer, and mouse macrophage line (TIB-71) were inhibited by 80%–90% after 72 h by inhibiting cell proliferation and the cell cycle and causing apoptosis [41][42]. Black garlic showed dose-dependent cytotoxic effects on HL-60 leukemia cells. Unlike fresh garlic, black garlic did not induce pro-apoptotic internucleosomal DNA fragmentation; therefore, cytotoxic activity was induced in a pro-apoptotic manner only in white garlic [43]. It is worth noting that black garlic was not found to be genotoxic when tested on fruit flies (Drosophila melanogaster) and is even antigenotoxic [43].
Both in vitro and in vivo, black garlic extract also demonstrated anticancer and immunomodulatory properties against SGC-7901 human gastric cancer cells as well as in the mouse model. In a dose-dependent manner, ABGE significantly increased SOD and GSH-Px activity compared to the negative control. By inducing apoptosis in vitro, ABGE inhibited the growth of cancer cells [44].
On the other hand, no anti-proliferative and pro-apoptotic effects were demonstrated in the MCF-10A breast cancer cell line [16]. In a study on three lung cancer cell lines (H1975, H520, A549), water and alcohol extracts also showed negligible anti-proliferative properties [45]. A study by Kim et al. found that black garlic extract had no cytotoxic effects when applied to RAW264.7 and RBL-2H3 cells [8].
Angiogenesis is an important process in tumor development and the inhibition of new blood vessel formation could be effective in cancer treatment. Arianingrum R et al. performed in vivo experiments using the Chorio-Allantoic Membrane Assay (CAM) for the evaluation of blood vessel formation as well as in silico study via docking analysis of BG compounds on the VEGF receptor [46]. The authors showed that ethanol extract, ethyl acetate, and n-hexane fractions of BG inhibit angiogenesis, with n-hexane fraction having the highest efficacy. The molecular docking assay indicated some possible inhibitory interaction between the black garlic bioactive compounds and VEGFR. On the other hand, the induction of angiogenesis was described in vivo in a zebrafish model or male Wistar rat model and in vitro with the use of HUVEC cells [47][48].
References
- Alam, K.; Hoq, O.; Uddin, S. Medicinal plant Allium sativum. A review. J. Med. Plant Stud. 2016, 4, 72–79.
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and Antimicrobial Activities of Fresh Garlic and Aged Garlic By-Products Extracted with Different Solvents. Food Sci. Biotechnol. 2018, 27, 219–225.
- Ammarellou, A.; Yousefi, A.R.; Heydari, M.; Uberti, D.; Mastinu, A. Biochemical and Botanical Aspects of Allium sativum L. Sowing. BioTech 2022, 11, 16.
- Kilic-Buyukkurt, O.; Kelebek, H.; Bordiga, M.; Keskin, M.; Selli, S. Changes in the Aroma and Key Odorants from White Garlic to Black Garlic Using Approaches of Molecular Sensory Science: A Review. Heliyone 2023, 9, e19056.
- Saryono; Sarmoko; Nani, D.; Proverawati, A.; Taufik, A. Black Solo Garlic Protects Hepatic and Renal Cell Function in Streptozotocin-Induced Rats. Front. Nutr. 2022, 9, 962993.
- Saikat, A.S.M.; Hossain, R.; Mina, F.B.; Das, S.; Khan, I.N.; Mubarak, M.S.; Islam, M.T. Antidiabetic Effect of Garlic. Rev. Bras. Farmacogn. 2022, 32, 1–11.
- Song, X.; Xue, L.; Geng, X.; Wu, J.; Wu, T.; Zhang, M. Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic. Foods 2023, 12, 2004.
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A Comparative Study on the Antioxidative and Anti-Allergic Activities of Fresh and Aged Black Garlic Extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182.
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of Garlic and Their Anticancer and Antioxidant Activity: A Review of the Epidemiologic and Experimental Evidence. Eur. J. Nutr. 2021, 60, 3585–3609.
- Ryu, J.H.; Kang, D.; Slusarenko, A.J.; Gruhlke, M.C.H.; Mcphee, D.J. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919.
- Chae, J.; Lee, E.; Oh, S.M.; Ryu, H.W.; Kim, S.; Nam, J.O. Aged Black Garlic (Allium sativum L.) and Aged Black Elephant Garlic (Allium ampeloprasum L.) Alleviate Obesity and Attenuate Obesity-Induced Muscle Atrophy in Diet-Induced Obese C57BL/6 Mice. Biomed. Pharmacother. 2023, 163, 114810.
- Wang, B.; Zhong, Y.; Wang, D.; Meng, F.; Li, Y.; Deng, Y.; Wang, B.; Zhong, Y.; Wang, D.; Meng, F.; et al. Formation, Evolution, and Antioxidant Activity of Melanoidins in Black Garlic under Different Formation, Evolution, and Antioxidant Activity of Melanoidins in Black Garlic under Different Storage Conditions. Foods 2023, 12, 3727.
- Bedrníček, J.; Laknerová, I.; Lorenc, F.; Probio de Moraes, P.; Jarošová, M.; Samková, E.; Tříska, J.; Vrchotová, N.; Kadlec, J.; Smetana, P.; et al. The Use of a Thermal Process to Produce Black Garlic: Differences in the Physicochemical and Sensory Characteristics Using Seven Varieties of Fresh Garlic. Foods 2021, 10, 2703.
- Villaño, D.; Marhuenda, J.; Arcusa, R.; Moreno-Rojas, J.M.; Cerdá, B.; Pereira-Caro, G.; Zafrilla, P. Effect of Black Garlic Consumption on Endothelial Function and Lipid Profile: A Before-and-After Study in Hypercholesterolemic and Non-Hypercholesterolemic Subjects. Nutrients 2023, 15, 3138.
- Zhao, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Effect of High Hydrostatic Pressure Conditions on the Composition, Morphology, Rheology, Thermal Behavior, Color, and Stability of Black Garlic Melanoidins. Food Chem. 2021, 337, 127790.
- Yang, Q.; Li, F.; Jia, G.; Liu, R. Aged Black Garlic Extract Inhibits the Growth of Estrogen Receptor-Positive Breast Cancer Cells by Downregulating MCL-1 Expression through the ROS-JNK Pathway. PLoS ONE 2023, 18, e0286454.
- Venir, E.; Pittia, P.; Giavon, S.; Maltini, E. Structure and Water Relations of Melanoidins Investigated by Thermal, Rheological, and Microscopic Analysis. Int. J. Food Prop. 2009, 12, 819–833.
- Pakakaew, P.; Phimolsiripol, Y.; Taesuwan, S.; Kumphune, S.; Klangpetch, W.; Utama-ang, N. The Shortest Innovative Process for Enhancing the S-Allylcysteine Content and Antioxidant Activity of Black and Golden Garlic. Sci. Rep. 2022, 12, 11493.
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812.
- Ríos-Ríos, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical Changes and Sensorial Properties during Black Garlic Elaboration: A Review. Trends Food Sci. Technol. 2019, 88, 459–467.
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black Garlic: A Critical Review of Its Production, Bioactivity, and Application. J. Food Drug Anal. 2017, 25, 62–70.
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.-H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts. Molecules 2016, 21, 430.
- Recinella, L.; Libero, M.L.; Citi, V.; Chiavaroli, A.; Martelli, A.; Foligni, R.; Mannozzi, C.; Acquaviva, A.; Di Simone, S.; Calderone, V.; et al. Anti-Inflammatory and Vasorelaxant Effects Induced by an Aqueous Aged Black Garlic Extract Supplemented with Vitamins D, C, and B12 on Cardiovascular System. Foods 2023, 12, 1558.
- Sun, Y.E.; Wang, W. Changes in Nutritional and Bio-Functional Compounds and Antioxidant Capacity during Black Garlic Processing. J. Food Sci. Technol. 2018, 55, 479–488.
- Lee, Y.-M.; Gweon, O.-C.; Seo, Y.-J.; Im, J.; Kang, M.-J.; Kim, M.-J.; Kim, J.-I. Antioxidant Effect of Garlic and Aged Black Garlic in Animal Model of Type 2 Diabetes Mellitus. Nutr. Res. Pract. 2009, 3, 156–161.
- Kim, D.G.; Kang, M.J.; Hong, S.S.; Choi, Y.H.; Shin, J.H. Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytother. Res. 2017, 31, 53–61.
- Ha, A.W.; Kim, W.K. Antioxidant Mechanism of Black Garlic Extract Involving Nuclear Factor Erythroid 2-like Factor 2 Pathway. Nutr. Res. Pract. 2017, 11, 206–213.
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-Hydroxymethylfurfural from Black Garlic Extract Prevents TNFα-Induced Monocytic Cell Adhesion to HUVECs by Suppression of Vascular Cell Adhesion Molecule-1 Expression, Reactive Oxygen Species Generation and NF-ΚB Activation. Phytother. Res. 2011, 25, 965–974.
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-ΚB and MTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275.
- Basu, C.; Chatterjee, A.; Bhattacharya, S.; Dutta, N.; Sur, R. S-Allyl Cysteine Inhibits TNF-α-Induced Inflammation in HaCaT Keratinocytes by Inhibition of NF- ΚB-Dependent Gene Expression via Sustained ERK Activation. Exp. Dermatol. 2019, 28, 1328–1335.
- You, B.R.; Yoo, J.M.; Baek, S.Y.; Kim, M.R. Anti-Inflammatory Effect of Aged Black Garlic on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Dermatitis in Mice. Nutr. Res. Pract. 2019, 13, 189–195.
- Tsai, J.C.; Chen, Y.A.; Wu, J.T.; Cheng, K.C.; Lai, P.S.; Liu, K.F.; Lin, Y.K.; Huang, Y.T.; Hsieh, C.W. Extracts from Fermented Black Garlic Exhibit a Hepatoprotective Effect on Acute Hepatic Injury. Molecules 2019, 24, 1112.
- Lee, T.W.; Bae, E.; Kim, J.H.; Jang, H.N.; Cho, H.S.; Chang, S.H.; Park, D.J. The Aqueous Extract of Aged Black Garlic Ameliorates Colistin-Induced Acute Kidney Injury in Rats. Ren. Fail. 2019, 41, 24–33.
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.H.; Bae, S.S.; Kim, B.S.; et al. Chloroform Extract of Aged Black Garlic Attenuates TNF-α-Induced ROS Generation, VCAM-1 Expression, NF-ΚB Activation and Adhesiveness for Monocytes in Human Umbilical Vein Endothelial Cells. Phytother. Res. 2011, 25, 92–100.
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235.
- Park, C.; Park, S.; Chung, Y.H.; Kim, G.Y.; Choi, Y.W.; Kim, B.W.; Choi, Y.H. Induction of Apoptosis by a Hexane Extract of Aged Black Garlic in the Human Leukemic U937 Cells. Nutr. Res. Pract. 2014, 8, 132–137.
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged Black Garlic Extract Inhibits HT29 Colon Cancer Cell Growth via the PI3K/Akt Signaling Pathway. Biomed. Rep. 2014, 2, 250–254.
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged Garlic Extract Inhibits 1,2-Dimethylhydrazine-Induced Colon Tumor Development by Suppressing Cell Proliferation. Oncol. Rep. 2015, 33, 1131–1140.
- Al-Shehri, S.A. Efficacy of Black Garlic Extract on Anti-Tumor and Anti-Oxidant Activity Enhancement in Rats. Clin. Nutr. Open Sci. 2021, 36, 126–139.
- Zhang, Y.; Li, H.Y.; Zhang, Z.H.; Bian, H.L.; Lin, G. Garlic-Derived Compound S-Allylmercaptocysteine Inhibits Cell Growth and Induces Apoptosis via the JNK and P38 Pathways in Human Colorectal Carcinoma Cells. Oncol. Lett. 2014, 8, 2591–2596.
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H. Bin Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246.
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude Garlic Extract Inhibits Cell Proliferation and Induces Cell Cycle Arrest and Apoptosis of Cancer Cells in Vitro. J. Med. Food 2015, 18, 731–737.
- Medina, M.Á.T.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Font, R.; Del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Alonso-Moraga, Á.; Moreno-Rojas, R. Physicochemical Characterization and Biological Activities of Black and White Garlic: In Vivo and in Vitro Assays. Foods 2019, 8, 220.
- Wang, X.; Jiao, F.; Wang, Q.W.; Wang, J.; Yang, K.; Hu, R.R.; Liu, H.C.; Wang, H.Y.; Wang, Y.S. Aged Black Garlic Extract Induces Inhibition of Gastric Cancer Cell Growth in Vitro and in Vivo. Mol. Med. Rep. 2012, 5, 66–72.
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance-Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221.
- Arianingrum, R.; Aznam, N.; Atun, S.; Senam, S.; Irwan, A.R.; Juhara, N.Q.; Anisa, N.F.; Devani, L.K. Antiangiogenesis Activity Of Indonesian Local Black Garlic (Allium sativum ‘Solo): Experiments And Bibliometric Analysis. Indones. J. Sci. Technol. 2023, 8, 487–498.
- Naderi, R.; Mohaddes, G.; Mohammadi, M.; Alihemmati, A.; Khamaneh, A.; Ghyasi, R.; Ghaznavi, R. The Effect of Garlic and Voluntary Exercise on Cardiac Angiogenesis in Diabetes: The Role of MiR-126 and MiR-210. Arq. Bras. Cardiol. 2019, 112, 154–162.
- Pan, D.; Gong, X.; Wang, X.; Li, M. Role of Active Components of Medicinal Food in the Regulation of Angiogenesis. Front. Pharmacol. 2021, 11, 594050.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
19 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




