Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jonas Jose Perez Bravo | -- | 1953 | 2024-02-17 09:00:57 | | | |
| 2 | Sirius Huang | Meta information modification | 1953 | 2024-02-18 03:07:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Perez Bravo, J.J.; Gerbehaye, C.; Raquez, J.; Mincheva, R. Solid-State Modification for Thermoplastic Polymers. Encyclopedia. Available online: https://encyclopedia.pub/entry/55110 (accessed on 07 February 2026).
Perez Bravo JJ, Gerbehaye C, Raquez J, Mincheva R. Solid-State Modification for Thermoplastic Polymers. Encyclopedia. Available at: https://encyclopedia.pub/entry/55110. Accessed February 07, 2026.
Perez Bravo, Jonas José, Carolane Gerbehaye, Jean-Marie Raquez, Rosica Mincheva. "Solid-State Modification for Thermoplastic Polymers" Encyclopedia, https://encyclopedia.pub/entry/55110 (accessed February 07, 2026).
Perez Bravo, J.J., Gerbehaye, C., Raquez, J., & Mincheva, R. (2024, February 17). Solid-State Modification for Thermoplastic Polymers. In Encyclopedia. https://encyclopedia.pub/entry/55110
Perez Bravo, Jonas José, et al. "Solid-State Modification for Thermoplastic Polymers." Encyclopedia. Web. 17 February, 2024.
Copy Citation
Solid-state modification of post-synthetic thermoplastic polymers is a rapidly evolving technique with numerous advantages and potential applications. This approach is particularly attractive because it allows adding new functionalities to existing polymer matrices, thereby extending their utility or assigning new purposes.
solid state modification
thermoplastic polymers
recycling
upcycling
1. Introduction
Have you ever considered the amazing journey of plastics from being a novel invention to a global environmental concern? In the past century, the use of plastic materials has increased significantly in various applications, from single-use items to engineering-grade polymers (e.g., food preservation, healthcare, transportation, and energy), thanks to their extensive range of properties such as permeability, waterproofing, hydrophobicity or hydrophilicity, stiffness, or flexibility. Plastic production has grown to an estimated annual production of over 390 million tons in 2021, with a growth rate of 4% per year [1].
What gives them such diverse properties as permeability, waterproofing, and flexibility? It is a blend of polymeric co-partners and other additives. But here is the catch: while these additives optimize properties, they can also lead to mechanical weaknesses, compatibility issues, and even environmental and health hazards due to toxic substances like phthalate plasticizers and brominated flame retardants [2][3][4]. These additives have been found to cause various health issues, including endocrine disruption and reproductive problems. These harmful substances can seep out of products and accumulate in the environment, putting both wildlife and humans at risk. Additionally, brominated flame retardants, which are used to reduce the flammability of materials, have been linked to neurodevelopmental issues and are highly persistent in the environment, leading to long-term ecological consequences [5][6]. Therefore, plastic materials are often associated with various additives, such as polymeric co-partners, to achieve specific properties [7][8][9]. These adjustments can improve processability, reduce costs, or extend the material’s lifespan. However, when added in excess, certain additives can significantly affect the final material’s mechanical properties and cause compatibility issues within the matrix [10]. It is crucial to maintain a good balance between the additive’s loading and its impact on the initial properties of the material, which often requires a compatibilization step. Additionally, some additives are considered to be controversial due to their toxicity during fabrication and use of plastic material. This can have harmful effects on the environment and human health, as certain compounds can migrate out of plastics [11][12][13]. Examples of such additives include phthalate plasticizers and brominated flame retardants [14][15][16]. On the other hand, the global plastic waste crisis requires urgent action from science and industry to manage plastic waste through reuse, recycling, or upcycling [17]. These methodologies involve modifying thermoplastic polymers, including polymers used during the melting stage, which requires high temperatures. However, these processes can produce undesirable changes in the properties of plastics due to various factors, including thermal and oxidative degradation, as well as microstructural changes [18][19].
Therefore, there is a growing interest in developing efficient techniques to modify thermoplastic materials by adding desired functionalities using low-content additives and avoiding detrimental reactions during their processing [20][21]. Such a technique relies on the solid-state modification (SSM) of polymeric materials, which exploits the mobility of the amorphous phase within semi-crystalline polymers [22]. This method involves using mechanical forces to drive through the incorporation of monomers or oligomers into the amorphous phase of the polymer through multiple exchange reactions [23]. The temperature plays a critical role during this process as it needs to be above the Tg of the semi-crystalline polymer and close to its Tm. The exchange reactions mainly occur in the amorphous phase, which means that the main crystalline phase and its associated properties remain unaffected. This technique can be considered a post-synthetic modification method that allows for the preservation and/or modulation of desired properties.
2. Thermo-Mechanochemistry: A Scientific Field That Can Be Focused on Solid-State Modification through Mechanical Means
SSM is a technique that has not been widely explored in the literature, despite being at the forefront of thermo-mechanochemistry. It involves modifying polymeric matrices after synthesis to extend their range of applications or improve a specific targeted property. This technique is related to solid-state polymerization, which increases the molecular weight of semi-crystalline polycondensates. SSP is commonly used in the production of glass fiber-reinforced PET grades [24]. This technique enables the formation of copolymers without requiring a high-temperature melt polycondensation reaction. This process demands the precise control of reaction parameters and significant thermal stability. SSM involves heating the starting material in an inert atmosphere or vacuum to a temperature below its melting point. This temperature is high enough to initiate and propagate the exchange reaction in the presence of a comonomer. However, the material must be above its glass transition temperature to ensure enough mobility of the end groups in the amorphous phase. It is also essential to prevent the crystalline phase from participating in the reaction. The image of Figure 1 illustrates this basic principle [22].
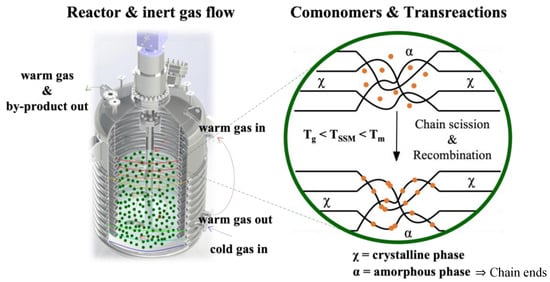
Figure 1. Schematic representation of solid-state modification.
The reaction mechanisms involved in SSM are similar to those in melt modification (Figure 2) [23]. However, a solid-state matrix is utilized as the reaction medium instead of a melt polymeric matrix. It is important to note that SSM has limitations on end-group diffusion due to restricted mobility. This is not an issue in melt or dissolution technology. These limitations become more severe over long reaction times when nearby functional groups already react. As a result, the concentration and distribution of these groups are reduced locally, making the migration of unreactive chain ends crucial for the reaction to continue. Removing condensate effectively through diffusion is crucial for achieving high SSM rates and extents. Although internal and surface diffusion are theoretically different, they are related and influenced by similar parameters. This is because both internal and surface diffusion eliminates concentration gradients of by-products in the reaction zone. This eliminates depolymerization and promotes a shift in the reaction equilibrium towards the desired products [22].
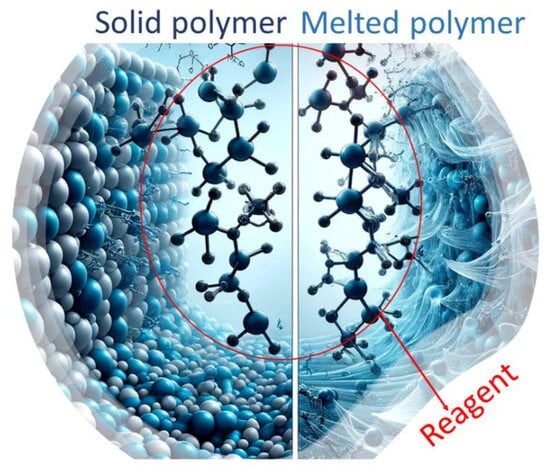
Figure 2. Schematic representation of how the same reagent can modify a polymer both in its melted and solid-state forms.
In SSM processes, the removal of by-products is accomplished by either applying a vacuum or through the convection caused by an inert gas at atmospheric pressure. The oligomers that are formed during the reaction can sublimate into the gas phase along with the condensate. This process can also involve heating the mixture under a continuous flow of inert gas (open system), which promotes the removal of by-products. During SSM (Solid State Polymerization) processes, the elimination of by-products is achieved either by using a vacuum or the convection created by an inert gas at atmospheric pressure. The oligomers that are produced during the reaction can vaporize into the gas phase alongside the condensate. The primary objectives of using an inert gas are to remove the condensate, prevent polymer oxidation by excluding oxygen from the reactor atmosphere, and to heat the reaction mixture. The most used inert gases include nitrogen, carbon dioxide, helium, superheated steam, and supercritical carbon dioxide. This process can also involve heating the mixture under a continuous flow of inert gas (open system), which helps to remove by-products.
3. Parameters That Affect the Solid-State Modification Technique
Solid-state modification is a complex process, and several parameters can be controlled to attain the desired polymer material. These parameters include temperature, gas flow, vacuum applied, crystallinity, catalysts, and others. The factors that impact the SSM technique are listed below [23].
3.1. Temperature
The temperature at which the SSM process occurs is a crucial factor that impacts almost all other process stages [22]. The reaction temperature mainly affects the reaction kinetics [25]. Maintaining a temperature range optimal for maximizing the reaction rate while avoiding undesired reactions such as partial melting, sticking, or cyclization is crucial. However, if the monomer has a high melting temperature, the temperature range can be broader, and the impact of temperature on the process rate is minimized. It is not feasible to carry out SSM on polymers with a melting temperature that is too low [26]. Raising the temperature of a process quickens its overall rate, the chemical reaction, and the movement of terminal functional groups and mass transport [22][25][26]. However, the temperature should not be too close to the melting temperature to avoid particle agglomeration. In summary, the reaction should be conducted above the glass transition temperature and between 10 °C to 40 °C below the melting temperature of the crystalline phase of the prepolymer (Figure 3) to prevent particle agglomeration [24][25][27].
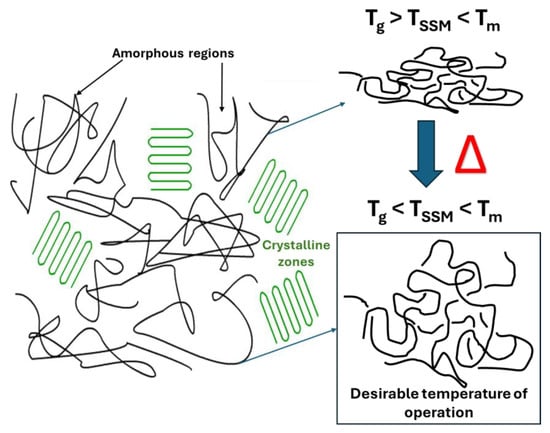
Figure 3. Schematic representation of desirable temperature to carry out the SSM.
3.2. Atmosphere
SSM can be carried out using either a vacuum or an inert gas, such as nitrogen. The main objectives of these reaction conditions are to remove by-products, inhibit polymer oxidation by excluding oxygen from the reactor atmosphere, and heat the reaction mass (in the case of gas flow only). The difference between these two methods is that for vacuum, the rapid removal of by-products is affected by the applied pressure, while for gas flow, it depends on the flow rate of the inert gas used. In SSM processes, the commonly used inert gases include nitrogen, carbon dioxide, helium, and superheated steam [28]. When performing the solid-state modification process, it is important to take into account the characteristics of the inert gas being used, since they may have an impact on the process. SSM can be carried out in two different ways: either under a continuous flow of inert gas, which is referred to as an open system and is primarily focused on removing by-products, or under a stagnant inert gas atmosphere, which is known as a closed system and helps to limit the loss of monomers and oligomers [29][30].
3.3. Crystallinity
Crystallinity plays a crucial role in SSM as it impacts the movement of chain end groups and the diffusion of by-products. The effect of crystallinity on the rate of SSM is two-fold. Higher crystallinity levels result in an increased concentration of end groups released into the amorphous phase, which in turn leads to an acceleration of the reaction rate [24]. The polymer chains become less mobile as the reaction progresses due to increased crystallinity. This decrease in mobility slows down the rate of SSM. When reactions are limited by by-product diffusion, high crystallinity also reduces the rate of SSM. However, in processes controlled by chemical reactions, high crystallinity actually increases the rate of SSM due to the concentration of end groups in the amorphous phase [31]. In order to prevent particles from sticking together, it is necessary for them to have a high level of crystallinity. In particle agglomeration, particles must have sufficiently high crystallinity [32]. Furthermore, it has been shown for many years that semi-crystalline polymers can be described using a three-phase model. This means that they contain a crystalline phase and an amorphous phase. However, the amorphous phase is divided into two zones: a mobile and a rigid amorphous zone, as shown in Figure 4 [33]. The exchange reactions should occur in the more mobile zones that can better tolerate changes [30].
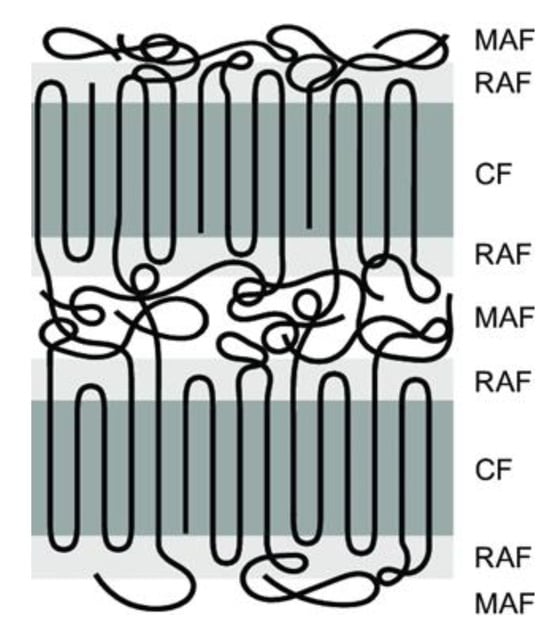
Figure 4. Schematic representation of three-phase model, i.e., mobile amorphous fraction (MAF), the rigid amorphous fraction (RAF) and the crystalline fraction (CF).
3.4. Catalysts
Catalysts are crucial for the rate of polymerization and modification reactions. Their role extends beyond simply accelerating reactions. They also contribute to the quality and properties of the final polymer product by influencing the chain initiation and growth, as well as the interactions among the polymer constituents. Additionally, catalysts can lead to complete conversions of specific groups, significantly impacting the polymerization rate, as observed with phase-transfer catalysts [34]. This ability to control reaction rates is crucial, especially in processes where removing by-products like water is necessary for the desired reaction outcomes [22]. The incorporation of catalysts into the polymerization process can be done at different stages. They can be added during the production of the prepolymer or while melting the prepolymer, providing flexibility in controlling the polymerization process. This versatility allows for a tailored approach to achieve specific properties and characteristics in the final thermoplastic polymer product [29].
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 25–29.
- Zabihzadeh Khajavi, M.; Mohammadi, R.; Ahmadi, S.; Farhoodi, M.; Yousefi, M. Strategies for Controlling Release of Plastic Compounds into Foodstuffs Based on Application of Nanoparticles and Its Potential Health Issues. Trends Food Sci. Technol. 2019, 90, 1–12.
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of Aging on Environmental Behavior of Plastic Additives: Migration, Leaching, and Ecotoxicity. Sci. Total Environ. 2022, 849, 157951.
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and Chemical Modifications of Poly(Vinyl Chloride) Materials to Prevent Plasticizer Migration—Still on the Run. React. Funct. Polym. 2020, 147, 104458.
- Hou, R.; Lin, L.; Li, H.; Liu, S.; Xu, X.; Xu, Y.; Jin, X.; Yuan, Y.; Wang, Z. Occurrence, Bioaccumulation, Fate, and Risk Assessment of Novel Brominated Flame Retardants (NBFRs) in Aquatic Environments—A Critical Review. Water Res. 2021, 198, 117168.
- Hales, B.F.; Robaire, B. Effects of Brominated and Organophosphate Ester Flame Retardants on Male Reproduction. Andrology 2020, 8, 915–923.
- Peng, Y.Y.; Srinivas, S.; Narain, R. Modification of Polymers. In Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 95–104. ISBN 9780128168066.
- Pillay, V.; Seedat, A.; Choonara, Y.E.; Du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K. A Review of Polymeric Refabrication Techniques to Modify Polymer Properties for Biomedical and Drug Delivery Applications. Aaps PharmSciTech 2013, 14, 692–711.
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247.
- Meer, M.; Mohd, S.; Itam, Z.; Beddu, S.; Zahari, N.M.; Liyana, N.; Kamal, M.; Mohamad, D.; Zulkepli, N.A.; Shafiq, M.D.; et al. Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers 2022, 14, 2911.
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166.
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199.
- Kemmlein, S.; Herzke, D.; Law, R.J. Brominated Flame Retardants in the European Chemicals Policy of REACH-Regulation and Determination in Materials. J. Chromatogr. A 2009, 1216, 320–333.
- European Commission Laying down Ecodesign Requirements for Electronic Displays Pursuant to Directive 2009/125/EC of the European Parliament and of the Council, Amending Commission Regulation (EC) No 1275/2008 and Repealing Commission Regulation (EC) No642/2009. Off. J. Eur. Union 2019, 241–266.
- Shea, K.M. Pediatric Exposure and Potential Toxicity of Phthalate Plasticizers. Pediatrics 2003, 111, 1467–1474.
- Gerbehaye, C.; Bernaerts, K.V.; Mincheva, R.; Raquez, J.-M. Solid-State Modification of Poly(Butylene Terephthalate): Design of Process from Calorimetric Methods for Catalyst Investigation to Reactive Extrusion. Eur. Polym. J. 2022, 166, 111010.
- Mohammed, M.; Jawad, A.J.M.; Mohammed, A.M.; Oleiwi, J.K.; Adam, T.; Osman, A.F.; Dahham, O.S.; Betar, B.O.; Gopinath, S.C.B.; Jaafar, M. Challenges and Advancement in Water Absorption of Natural Fiber-Reinforced Polymer Composites. Polym. Test. 2023, 124, 108083.
- Boey, J.Y.; Lee, C.K.; Tay, G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers 2022, 14, 3737.
- Zhao, H.B.; Wang, Y.Z. Design and Synthesis of PET-Based Copolyesters with Flame-Retardant and Antidripping Performance. Macromol. Rapid Commun. 2017, 38, 1700451.
- Mincheva, R.; Guemiza, H.; Hidan, C.; Moins, S.; Coulembier, O.; Dubois, P.; Laoutid, F. Development of Inherently Flame-Retardant Phosphorylated Pla by Combination of Ring-Opening Polymerization and Reactive Extrusion. Materials 2020, 13, 13.
- Papaspyrides, C.D.; Vouyiouka, S.N. Fundamentals of Solid State Polymerization. In Solid State Polymerization; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2009; pp. 1–37. ISBN 9780470451830.
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid State Polymerization. Prog. Polym. Sci. 2005, 30, 10–37.
- Chang, S.; Sheu, M.-F.; Chen, S.-M. Solid-state Polymerization of Poly(Ethylene Terephthalate). J. Appl. Polym. Sci. 1983, 28, 3289–3300.
- Yoon, K.H.; Kwon, K.H.; Jeon, M.H.; Park, O.O. Diffusion of Ethylene Glycol in Solid State Poly(Ethylene Terephthalate). Polym. J. 1993, 25, 219–226.
- Bamford, C.H.; Wayne, R.P. Polymerization in the Solid Phase: A Polycondensation Reaction. Polymer 1969, 10, 661–681.
- Wang, X.Q.; Deng, D.C. A Comprehensive Model for Solid-State Polycondensation of Poly(Ethylene Terephthalate): Combining Kinetics with Crystallization and Diffusion of Acetaldehyde. J. Appl. Polym. Sci. 2002, 83, 3133–3144.
- Culbert, B.; Christel, A. Continuous Solid-State Polycondensation of Polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2003; pp. 143–194. ISBN 9780470090688.
- Steinborn-Rogulska, I.; Rokicki, G. Solid-State Polycondensation (SSP) as a Method to Obtain High Molecular Weight Polymers: Part II. Synthesis of Polylactide and Polyglycolide via SSP. Polimery 2013, 58, 85–92.
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-State Polymerization of PET: Influence of Nitrogen Sweep and High Vacuum. Polymer 2003, 44, 4085–4096.
- Gantillon, B.; Spitz, R.; McKenna, T.F. The Solid State Postcondensation of PET, 1: A Review of the Physical and Chemical Processes Taking Place in the Solid State. Macromol. Mater. Eng. 2004, 289, 88–105.
- Yao, Z.; Harmon Ray, W. Modelling and Analysis of New Processes for Polyester and Nylon Production. AIChE J. 2001, 47, 401–412.
- Heidrich, D.; Gehde, M. The 3-Phase Structure of Polyesters (PBT, PET) after Isothermal and Non-Isothermal Crystallization. Polymers 2022, 14, 793.
- N'Guyen, T.D.; Deffieux, A.; Boileau, S. Phase-Transfer Catalysis in the Chemical Modification of Polymers: 1. Polymer 1978, 19, 423–426.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
538
Revisions:
2 times
(View History)
Update Date:
19 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




