You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhaoliang Cui | -- | 2423 | 2024-02-17 01:54:45 | | | |
| 2 | Camila Xu | Meta information modification | 2423 | 2024-02-18 02:57:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liao, Z.; Wang, Q.; Zhou, Q.; Cui, Z.; Wang, Z.; Drioli, E. Fabrication of Ethylene-Chlorotrifluoroethylene Copolymer Membrane. Encyclopedia. Available online: https://encyclopedia.pub/entry/55103 (accessed on 05 January 2026).
Liao Z, Wang Q, Zhou Q, Cui Z, Wang Z, Drioli E. Fabrication of Ethylene-Chlorotrifluoroethylene Copolymer Membrane. Encyclopedia. Available at: https://encyclopedia.pub/entry/55103. Accessed January 05, 2026.
Liao, Zhangbin, Qian Wang, Qiuyueming Zhou, Zhaoliang Cui, Zhaohui Wang, Enrico Drioli. "Fabrication of Ethylene-Chlorotrifluoroethylene Copolymer Membrane" Encyclopedia, https://encyclopedia.pub/entry/55103 (accessed January 05, 2026).
Liao, Z., Wang, Q., Zhou, Q., Cui, Z., Wang, Z., & Drioli, E. (2024, February 17). Fabrication of Ethylene-Chlorotrifluoroethylene Copolymer Membrane. In Encyclopedia. https://encyclopedia.pub/entry/55103
Liao, Zhangbin, et al. "Fabrication of Ethylene-Chlorotrifluoroethylene Copolymer Membrane." Encyclopedia. Web. 17 February, 2024.
Copy Citation
Ethylene-chlorotrifluoroethylene (ECTFE) was first commercialized by DuPont in 1974. Its unique chemical structure gives it high heat resistance, mechanical strength, and corrosion resistance. But also due to these properties, it is difficult to prepare a membrane from it by the nonsolvent-induced phase separation (NIPS) method.
ethylene-chlorotrifluoroethylene
membrane
grafting
modification
1. Introduction
Ethylene-chlorotrifluoroethylene (ECTFE) is a copolymer composed of alternating monomer units of ethylene and chlorotrifluoroethylene. Its chemical structure is shown in Figure 1. The F atom has strong electronegativity and low polarization, and the C-F bond exhibits one of the strongest chemical bond energies within the structure, reaching up to 485 kJ/mol. At the same time, multiple F atoms are connected to the C atom, which further strengthens the C-F bond energy and creates its excellent performance [1]. The regular pattern of these monomer units results in a well-defined structure, which gives ECTFE its consistent and predictable properties.
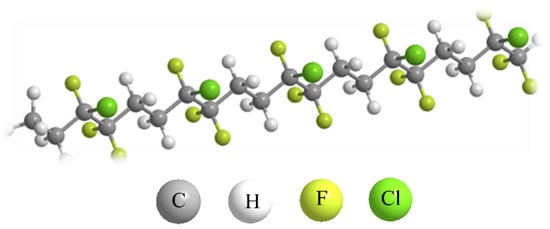
Figure 1. ECTFE molecular structure.
This unique type of fluorinated elastomer was first commercialized by DuPont in 1974 under the trade name Halar®. In 1986, Applied Chemical Organization transferred ECTFE products and production technology to Ausimont USA Inc. (Morristown, DE, USA). In 2001, the Solvay Group of Belgium acquired Ausimont, thereby becoming the sole producer of ECTFE. Figure 2 shows ECTFE particles from the Solvay company (Brussels, Belgium), demonstrating their uniform size and smooth surface.

Figure 2. ECTFE particles from the Solvay company.
ECTFE exhibits high chemical corrosion resistance, mechanical strength, and thermal resistance; exhibits low capacitance, flammability, refractive index, and surface energy (neither oil-wet nor water-wet); is especially inactive to various solvents, including hydrocarbons and various acids and bases, with no solvent able to attack it below 120 °C [2][3][4][5]. In terms of resistance to strong alkalis and acids, high-temperature resistance, and chemical resistance, ECTFE is even superior to other fluorinated materials, such as polytetrafluoroethylene (PTFE) [6][7] and poly (vinylidene fluoride) (PVDF) [8][9], and is an ideal material for preparing high-performance porous membranes.
The polymer’s low polarizability and strong electronegativity also contribute to its excellent thermal stability and low coefficient of friction, allowing it to be used in high-temperature and wear-resistant applications. Additionally, due to the presence of chlorine atoms, ECTFE has stronger resistance to water vapor, hydrogen chloride, and chlorine gas than ordinary fluoropolymers. Its chlorine permeability is the best among all fluoropolymers, so it is widely used in some harsh environments exposed to chlorine. Even if ECTFE is exposed to ultraviolet light for a long time, its performance remains unchanged and can be used in the construction industry, such as in anti-UV coatings. It is commonly used as a protective coating, including for pipeline protection and corrosion prevention [10][11]. For example, ECTFE is frequently used as a coating for stainless steel exhaust pipes in a range of industrial applications (including clean rooms) to protect them from the corrosive effects of various airflows. When compared to PTFE coating, the adhesion and hardness of an induced draft fan impeller coated with ECTFE are twice as good when exposed to hydrofluoric acid containing special corrosive substances. Furthermore, ECTFE’s exceptional properties also make it an excellent material for use as an anticorrosion membrane resin on the surface of solar photovoltaic modules. In these applications, ECTFE resin has demonstrated exceptional corrosion resistance, weather resistance, and chemical resistance. This makes it an excellent long-term solution for protecting photovoltaic modules from environmental degradation and corrosion. However, it should be noted that the research on ECTFE as a porous membrane is still in its early stages. Despite its potential for a range of practical applications, further research is required to optimize its performance and explore its full potential.
2. Fabrication of ECTFE Membrane
Microfiltration membranes are usually prepared using thermally induced phase separation (TIPS) [12][13][14][15][16] or nonsolvent-induced phase separation (NIPS) [17][18][19][20][21][22][23].
TIPS is a relatively new method of preparing polymer microporous membranes proposed and patented by Castro in 1981. Its process and principle are above the melting point of the polymer; the polymer will be dissolved in a high-boiling-point and low-volatility diluent to form a homogeneous solution. It is then cooled down. During cooling, the system undergoes phase separation. This process is divided into two categories, solid–liquid phase separation (referred to as S-L phase separation) and liquid–liquid phase separation (L-L phase separation). The appropriate process conditions are controlled, and after phase separation, the system forms a two-phase structure with the polymer as the continuous phase and the diluent as the dispersed phase. At this point, the appropriate volatile reagent (i.e., extractant) is selected to extract the diluent in order to obtain a certain structure shape of the polymer porous membrane.
Using NIPS, the polymer is dissolved in a solvent to form a homogeneous solution, and then a reagent that is more soluble with the solvent (known as the extractant) is slowly added to extract the solvent, forming a two-phase structure with the polymer as the continuous phase and the solvent as the dispersed phase; then the solvent is removed to obtain polymers with a certain pore structure.
Compared with NIPS, TIPS has many advantages: (1) TIPS promotes phase separation of polymer solutions through faster heat exchange rather than slow solvent–nonsolvent exchange. (2) TIPS avoids the disadvantage of NIPS, that is, a low porosity due to the existence of the solvent–nonsolvent exchange, which leads to part of solvent participating in polymer gelation during the membrane-forming process. (3) TIPS can be used for the preparation of crystalline polymer microporous membranes that are difficult to prepare with NIPS. (4) TIPS has fewer influencing factors than NIPS and is easier to be controlled. (5) A variety of microstructures can be obtained by TIPS such as open-pore, closed-pore, isotropic, anisotropic, and asymmetric, etc.
To date, no organic solvent is able to dissolve ECTFE at room temperature. Therefore, some common membrane preparation methods, such as NIPS [12][13][14][15][16][17][18], cannot be used to prepare ECTFE membranes. However, with increasing temperature, especially when the temperature is above the melting point of ECTFE, ECTFE can form homogeneous solutions with some diluents, which provides the basis for the preparation of ECTFE membranes by TIPS [4][12][13][14][15][16]. One can control the pore size of ECTFE membranes when using TIPS by adjusting the cooling temperature and selecting the appropriate solvent. The main problem in the preparation of microfiltration membranes by the TIPS method is the selection of diluents.
During TIPS, the polymer is dissolved in a diluent at a temperature above its melting point to form a homogeneous solution [24][25][26][27][28][29][30]. By ensuring the uniform distribution of the polymer in the solution, a high-quality membrane can be made. The homogeneous solution is subsequently cooled to induce the phase separation. The main steps of preparing a microfiltration membrane by TIPS include solution preparation (continuous or intermittent preparation), membrane configuration (plate or hollow-fiber as shown in Figure 3), and post-treatment [31][32]. The specific steps are as follows: (1) Mix the polymer with a high-boiling-point and low-molecular-weight liquid or solid diluent at a high temperature to form a homogeneous solution. (2) Cast the solution into the desired shape (flat, hollow, or tubular). (3) Cool the solution to induce phase separation. (4) Remove the diluent (cosolvent extraction). (5) Remove the extractant (by evaporation) to obtain the porous structure.
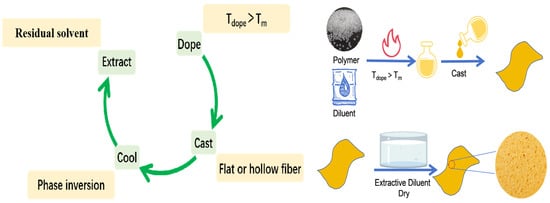
Figure 3. The procedure for preparing ECTFE membranes by TIPS. (Tdope is the temperature of casting solution, Tm is the temperature of melting temperature).
Table 1 lists several studies on the production and applications of porous ECTFE membranes. It is crucial to select an appropriate diluent for the ECTFE polymer, as this process can be challenging. The polymers must be dissolved in solvents at a temperature of 250 °C, which requires the use of solvents with high boiling points, flash points, and melting points that are higher than the melting point of the polymer. Moreover, it is paramount that these solvents are safe for human use and the environment. These constraints hinder the advancement and industrial application of ECTFE polymers in the membrane industry.
Table 1. ECTFE membrane fabrication by TIPS method and their applications.
| Author | Polymer Type | Diluent Type | Diluent Boiling Point (°C) |
Membrane Type |
Membrane Process | Year |
|---|---|---|---|---|---|---|
| Ramaswamy [11] et al. | HALAR®901 | Dibutyl phthalate (DBP) |
337 | Flat | - | 2002 |
| Roh [12] et al. | HALAR®901 | Dibutyl phthalate (DBP) |
337 | Flat | - | 2010 |
| Simone [15] et al. | HALAR®901 | 1-methyl-2-pyrrolidinone (NMP) | 202 | Flat | Pervaporation | 2012 |
| Drioli [33] et al. | HALAR®901 | Glyceryl triacetate (GTA) |
258 | Flat | Membrane condenser | 2014 |
| Pan [34] et al. | HALAR®902 | Diethyl adipate (DEHA)/diethyl phthalate (DEP) | 247/294 | Flat | Membrane distillation | 2015 |
| Abdel-Hady [35] et al. | Commercial ECTFE membrane | Grafting of vinyl pyrrolidone (NVP)/styrene | - | Flat | Fuel cell | 2015 |
| Zhou [14] et al. | HALAR®902 | Dibenzylidene sorbitol (DBS)/triphenyl phosphate (TPP) | 549/412 | Hollow fiber | - | 2012 |
| Matsuyama [36] et al. | HALAR®901 | Diethyl phthalate (DEP) and glyceryl triacetate (GTA) | 294/258 | Hollow fiber | - | 2016 |
| Ursino [37] et al. | LMPECTFE | Diethyl phthalate (DEP) | 294 | Flat | Organic-solvent filtration separation | 2016 |
| Pan [38] et al. | HALAR®902 | Diethyl adipate (DEHA)/diethyl phthalate (DEP) | 247/294 | Flat | Oil/water separation | 2017 |
| Abdel-Hamed [39] et al. | ECTFE-g-PSSA | - | - | Flat | Proton exchange membrane fuel cells (PEMFC) | 2018 |
| Xu [5] et al. | HALAR®902 | Acetyl tributyl citrate (ATBC) |
327 | Flat | Membrane distillation | 2019 |
| Liu [40] et al. | HALAR®902 | Trioctyl trimellitate (TOTM) |
414 | Flat | Membrane distillation | 2020 |
The ECTFE membrane was first prepared by the TIPS method in 2002 by Ramaswamm et al. In that study, a surface-dense ECTFE membrane was successfully prepared with DBP as a single dilution. The dense layer was successfully eliminated and the porosity was improved [11]. In 2010, Roh et al. improved the membrane preparation process and conducted a detailed study on the effects of casting fluid composition, quenching temperature, membrane thickness, bore fluid composition, and traction force on the structure and performance of an ECTFE membrane when preparing the membrane using the TIPS process [12]. However, the cross-sectional structure of the ECTFE membrane prepared with DBP as the diluent was incomplete. Fundamentally, this structure was obtained using DBP because the system had a relatively narrow liquid–liquid (L-L) phase separation region, resulting in the short L-L phase separation process, resulting in the formation of a disordered spherulite structure. The effect of the interaction parameters between ECTFE and different solvents on the L-L phase separation region of the ECTFE solvent system was studied by Zhou et al. [14]. The Flory–Huggins interaction parameter reflects the change in the interaction energy of the polymer molecules during mixing with the solvent and is denoted by χ. From the polymer solution thermodynamic theory for derivation, it can be seen that the value of the polymer–solvent interaction parameter χ can be used as a semiquantitative judgment of the solvent superiority. If χ is greater than 0.5, the polymer is generally insoluble; if χ is less than 0.5, the polymer can be dissolved, that is, the smaller the value, the better the solvent’s ability to dissolve. Therefore, the value can be used as a basis for determining whether a polymer and a solvent system are miscible. Increasing interaction parameters change the cross-sectional structure of the membrane from a spherulite structure to a cellular structure or a continuous structure. The larger the interaction parameter χ, the less the compatibility of the polymer with the solvent, indicating the wider the L-L phase separation area. At appropriate cooling rates, the ECTFE cross section will present a double continuous structure.
However, in the published papers, the cross sections of ECTFE membranes prepared using a single diluent in the TIPS process present either closed cellular structures with good mechanical properties but almost no pores or spherulite structures with poor mechanical properties, which is not sufficient for practical applications.
The effect of mixed solvent in ECTFE membranes prepared by the TIPS method on the microfiltration membrane structure and properties was also thoroughly investigated by Zhou et al. [41][42]. The investigators also studied the effects of different nonsolvents on the crystallization trends of the ECTFE/DBS systems. The solubility parameter distances between ECTFE and each nonsolvent are as follows: χ(ECTFE-TEG) > χ(ECTFE-TPP) > χ(ECTFE-OA) > χ(ECTFE-HDC) (TEG is triethylene glycol, TPP is triphenyl phosphate, OA is an oxidized amine, HDC is hexamethylene diamino carbamate). The solubility parameters of DBS are relatively close to those of TPP and OA, indicating that DBS has good compatibility with TPP and OA. In both cases, the ECTFE membrane exhibits a bicontinuous structure. The other two remain microporous cellular structures because when the solvent compatibility is poor, the polymer is cooled first during the binary diluent cooling process, thus forming an irregular cross-sectional structure. Zhou’s work [41] offers us profound inspiration. The use of a single solvent in the preparation of polymer membranes often has numerous limitations, making it challenging to expand the polymer–solvent system. By applying the theory of the solubility parameter, the L-L separation area can be separated according to the theory of the solubility parameter, adjusting the interaction between polymers and solvents. This approach enables us to find suitable solvents and additives.
To prepare high-performance ECTFE membranes, most studies are limited to a temperature of around 250 °C, which is very close to the decomposition temperature of ECTFE. At this temperature, ECTFE will be rapidly oxidized and degrade, and it will also cause diluent volatilization, resulting in harm to the environment and researchers. Before ECTFE membranes were prepared at relatively low temperatures by Simone [15] et al. and Drioli [33] et al., researchers gradually turned their attention to how to prepare ECTFE membranes in a more environmentally friendly manner. In Simone et al.’s work, they found that N-methylpyrrolidone (NMP) can dissolve ECTFE at 180 °C [15].
What is more, the solvents for dissolving ECTFE mostly use aromatic compounds with high boiling points, which are generally toxic and will cause harm to human health and the natural environment. Therefore, the development of environmentally friendly solvents for the TIPS method has attracted more and more attention from researchers [43][44]. Xu [5] et al. and Liu [40] et al., respectively, selected the environmentally friendly diluents ATBC and TOTM as a single solvent to prepare the ECTFE membrane. Liu et al. chose TOTM as the solvent, and a significant L-L phase separation was observed for the bicontinuous structure when the polymer concentration was 15%. As the polymer concentration increased, the ECTFE membrane structure changed from a bicontinuous structure to a stacked-block structure. During the 30 h membrane distillation process, the ECTFE membrane at 15% TOTM achieved a retention of 23.09 kg·m−2·h−1 and a 99.9% rejection rate. The ECTFE membrane maintained great salt protection at a running-feed solution concentration ratio of 3.49 (i.e., 12.1 Wt.% concentration).
As can be seen from the development of ECTFE membranes prepared by the TIPS method, researchers have begun to pay attention to the preparation of high-performance membranes in a safe and environmentally friendly way. The choice of diluent has also changed from toxic phthalic acid reagents to green solvents, such as ATBC and TOTM, etc. Finding ways to successfully use green solvents to prepare high-performance ECTFE membranes under low-temperature conditions to effectively excavate the potential of this high-performance partial-crystalline fluorine-containing material in the field of the membrane is in line with the development concept of the green chemical industry.
References
- Gryta, M. The study of performance of polyethylene chlorinetrifluoroethylene membranes used for brine desalination by membrane distillation. Desalination 2016, 398, 52–63.
- Muller, H. A new solvent resistant membrane based on ECTFE. Desalination 2006, 199, 191–192.
- Khayet, M.; Mengual, J.I.; Matsuura, T. Porous hydrophobic/hydrophilic composite membranes—Application in desalination using direct contact membrane distillation. J. Membr. Sci. 2005, 252, 101–113.
- Cui, Z.L.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198.
- Xu, K.; Cai, Y.C.; Hassankiadeh, N.T.; Cheng, Y.M.; Li, X.; Wang, X.Z.; Wang, Z.H.; Drioli, E.; Cui, Z.L. ECTFE membrane fabrication via TIPS method using ATBC diluent for vacuum membrane distillation. Desalination 2019, 456, 13–22.
- Goessi, M.; Tervoort, T.; Smith, P. Melt-spun poly(tetrafluoroethylene) fibers. J. Mater. Sci. 2007, 42, 7983–7990.
- Hou, D.Y.; Wang, J.; Sun, X.C.; Ji, Z.G.; Luan, Z.K. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 405, 185–200.
- Ji, G.L.; Zhu, L.P.; Zhu, B.K.; Zhang, C.F.; Xu, Y.Y. Structure formation and characterization of PVDF hollow fiber membrane prepared via TIPS with diluent mixture. J. Membr. Sci. 2008, 319, 264–270.
- Toniolo, P.; Carella, S. Halar (R) High Clarity ECTFE film—An highly transparent film for new buildings structures. Procedia Eng. 2016, 155, 28–37.
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13–22.
- Ramaswamy, S.; Greenberg, A.R.; Krantz, W.B. Fabrication of poly (ECTFE) membranes via thermally induced phase separation. J. Membr. Sci. 2002, 210, 175–180.
- Roh, I.J.; Ramaswamy, S.; Krantz, W.B.; Greenberg, A.R. Poly(ethylene chlorotrifluoroethylene) membrane formation via thermally induced phase separation (TIPS). J. Membr. Sci. 2010, 362, 211–220.
- Randova, A.; Bartovska, L.; Pilnacek, K.; Lanc, M.; Vopicka, O.; Matejka, P.; Izak, P.; Karaszova, M.; Macedonio, F.; Figoli, A.; et al. Sorption of organic liquids in poly (ethylene chlorotrifluoroethylene) Halar((R)) 901: Experimental and theoretical analysis. Polym. Test. 2017, 58, 199–207.
- Zhou, B.; Lin, Y.K.; Ma, W.Z.; Tang, Y.H.; Tian, Y.; Wang, X.L. Preparation of Ethylene Chlorotrifluoroethylene Co-polymer Membranes via Thermally Induced Phase Separation. Chem. J. Chin. Univ. 2012, 33, 2585–2590.
- Simone, S.; Figoli, A.; Santoro, S.; Galiano, F.; Alfadul, S.M.; Al-Harbi, O.A.; Drioli, E. Preparation and characterization of ECTFE solvent resistant membranes and their application in pervaporation of toluene/water mixtures. Sep. Purif. Technol. 2012, 90, 147–161.
- Pan, J.; Chen, K.; Cui, Z.L.; Bamaga, O.; Albeirutty, M.; Alsaiari, A.O.; Macedonio, F.; Drioli, E. Preparation of ECTFE Porous Membrane for Dehumidification of Gaseous Streams through Membrane Condenser. Membranes 2022, 12, 65–68.
- Yu, H.R.; Shangguan, S.Y.; Yang, H.Y.; Rong, H.W.; Qu, F.S. Chemical cleaning and membrane aging of poly(vinylidene fluoride) (PVDF) membranes fabricated via non-solvent induced phase separation (NIPS) and thermally induced phase separation (TIPS). Sep. Purif. Technol. 2023, 313, 123488–123490.
- Peng, H.; Li, K. Nanostructured membranes with interconnected pores via a combination of phase inversion and solvent crystallisation approach. J. Membr. Sci. 2023, 680, 121738–121741.
- Jiang, S.H.; Qian, H.; Zhang, P.Y.; Xu, C.; Li, K.K. Facile membrane preparation strategy to reinforce permeability of polyethersulfone (PES) micro-ultrafiltration membrane for drinking water treatment. J. Mater. Res. 2023, 38, 2369–2378.
- Liu, T.; Miao, J.P.; Wang, L.L.; Hu, Y.X. Structure Design and Tailoring Strategy of Polymeric Materials for Fabrication of Nanofiltration Membranes via Phase Inversion. Prog. Chem. 2023, 35, 1199–1213.
- Kleinberg, M.N.; Thamaraiselvan, C.; Powell, C.D.; Arnusch, C.J. Preserved subsurface morphology in NIPS and VIPS laser-induced graphene membranes affects electrically-dependent microbial decontamination. J. Membr. Sci. 2023, 673, 121481–121484.
- Kong, X.; Shu, G.M.; Lu, X.L.; Wu, C.R.; Gai, Y. Manipulating membrane surface porosity via deep insight into surfactants during nonsolvent induced phase separation. J. Membr. Sci. 2020, 611, 118358–118363.
- Aissou, K.; Mumtaz, M.; Hermida-Merino, D.; Solano, E.; Cot, D.; Tarek Benkhaled, B.; Quémener, D.; Roualdes, S.; Fleury, G.; Hadziioannou, G. Square arrays of vertically aligned nanoporous cylinders from a linear ABC triblock terpolymer. J. Polym. Sci. 2023, 61, 1259–1269.
- Zuo, J.H.; Wei, C.; Cheng, P.; Yan, X.; Chen, Y.; Lang, W.Z. Breakthrough the upperbond of permeability vs. tensile strength of TIPS-prepared PVDF membranes. J. Membr. Sci. 2020, 604, 118089–118095.
- Han, J.C.; Xing, X.Y.; Wang, J.; Wu, Q.Y. Preparation and Properties of Thin-Film Composite Forward Osmosis Membranes Supported by Cellulose Triacetate Porous Substrate via a Nonsolvent-Thermally Induced Phase Separation Process. Membranes 2022, 12, 412–415.
- Takao, S.; Rajabzadeh, S.; Shibata, M.; Otsubo, C.; Hamada, T.; Kato, N.; Nakagawa, K.; Kitagawa, T.; Matsuyama, H.; Yoshioka, T. Preparation of Chemically Resistant Cellulose Benzoate Hollow Fiber Membrane via Thermally Induced Phase Separation Method. Membranes 2022, 12, 1199–1205.
- Basko, A.V.; Pochivalov, K.V.; Yurov, M.Y.; Lebedeva, T.N.; Yushkin, A.A.; Volkov, A.V. Materials. Preparation of thermostable polypropylene membranes with a controlled structure by nonsolvent thermally induced phase separation. Polym. Plast. Technol. Mater. 2023, 62, 247–259.
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of combined nonsolvent and thermally induced phase separation (N-TIPS) method for fabricating novel PVDF hollow fiber membranes using mixed diluents. J. Membr. Sci. 2019, 572, 210–222.
- Ge, W.Z.; Wu, Y.; Wang, D.K.; Valix, M. Ammonia removal using thermally induced phase separation PVDF hollow fibre membrane contactors. Sep. Purif. Technol. 2023, 307, 122780–122785.
- Umakoshi, K.; Gonzales, R.R.; Kato, N.; Zhang, P.F.; Ono, T.; Matsuyama, H. Effect of polymer-solvent compatibility on polyamide hollow fiber membranes prepared via thermally induced phase separation. Colloids Surf. A 2022, 642, 128704–128707.
- Pan, J.; Ma, W.Y.; Huang, L.L.; Li, R.Z.; Huang, Q.L.; Xiao, C.F.; Jiang, Z.H. Fabrication and characterization of ECTFE hollow fiber membranes via low-temperature thermally induced phase separation (L-TIPS). J. Membr. Sci. 2021, 634, 119429–119433.
- Pan, J.; Xiao, C.F.; Huang, Q.L.; Wang, C.; Liu, H.L. Fabrication and properties of poly(ethylene chlorotrifluoroethylene) membranes via thermally induced phase separation (TIPS). Rsc. Adv. 2015, 5, 45249–45257.
- Drioli, E.; Santoro, S.; Simone, S.; Barbieri, G.; Brunetti, A.; Macedonio, F.; Figoli, A. ECTFE membrane preparation for recovery of humidified gas streams using membrane condenser. React. Funct. Polym. 2014, 79, 1–7.
- Pan, J.; Xiao, C.F.; Huang, Q.L.; Liu, H.L.; Hu, J. ECTFE porous membranes with conveniently controlled microstructures for vacuum membrane distillation. J. Mater. Chem. A 2015, 3, 23549–23559.
- Abdel-Hady, E.E.; El-Toony, M.M. Grafting of Vinyl Pyrrolidone/Styrene onto Ethylene/Chlorotrifluoroethylene Membrane for Proton ExchangeMembrane Fuel Cell. Electrochim. Acta 2015, 176, 472–479.
- Karkhanechi, H.; Rajabzadeh, S.; Di Nicolo, E.; Usuda, H.; Shaikh, A.R.; Matsuyama, H. Preparation and characterization of ECTFE hollow fiber membranes via thermally induced phase separation (TIPS). Polymer 2016, 97, 515–524.
- Ursino, C.; Simone, S.; Donato, L.; Santoro, S.; De Santo, M.P.; Drioli, E.; Di Nicolo, E.; Figoli, A. ECTFE membranes produced by non-toxic diluents for organic solvent filtration separation. RSC Adv. 2016, 6, 81001–81012.
- Pan, J.; Xiao, C.F.; Huang, Q.L.; Liu, H.L.; Zhang, T. ECTFE hybrid porous membrane with hierarchical micro/nano-structural surface for efficient oil/water separation. J. Membr. Sci. 2017, 524, 623–630.
- Abdel-Hamed, M.O. Styrene grafted ethylene chlorotrifluoroethylene (ECTFE-g-PSSA) protonic membranes: Preparation, characterization, and transport mechanism. Polym. Adv. Technol. 2018, 29, 658–667.
- Liu, G.; Pan, J.; Xu, X.L.; Wang, Z.H.; Cui, Z.L. Preparation of ECTFE porous membrane with a green diluent TOTM and performance in VMD process. J. Membr. Sci. 2020, 612, 118735–118739.
- Zhou, B.; Lin, Y.K.; Ma, W.Z.; Tian, Y.; Wang, X.L. ECTFE membranes prepared viathermally induced phase separation-selection of mixed diluent. Membr. Sci. Technol. 2013, 33, 27–32.
- Zhou, B.; Li, Q.; Tang, Y.H.; Lin, Y.K.; Wang, X.L. Preparation of ECTFE membranes with bicontinuous structure via TIPS method by a binary diluent. Desalination Water Treat. 2016, 57, 17646–17657.
- Dai, Z.Z.; Liu, H.; Yu, S.Y.; Zhong, G.Y.; Zhang, Y.Z.; Gao, L.N. Investigation on Thermal Stability of ECTFE in Machining Process. Z. Chem. Ind. 2017, 48, 1–3.
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolo, E.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034–4059.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
555
Revisions:
2 times
(View History)
Update Date:
18 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




