
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Petr M. Korusenko | -- | 3068 | 2024-02-16 21:42:44 | | | |
| 2 | Jessie Wu | Meta information modification | 3068 | 2024-02-18 04:21:53 | | | | |
| 3 | Jessie Wu | -4 word(s) | 3064 | 2024-02-18 04:23:13 | | |
Video Upload Options
The polymers of square–planar complexes of 3d metal (M) atoms with tetradentate N2O2 Schiff base ligands, the so-called salen complexes ([M(Salen)]), are characterized by high redox conductivity, electrochromic behavior, and selective catalytic activity in heterogeneous reactions (including electrocatalysis). An important advantage of these polymers is also their high thermal stability (up to 350 °C) compared with monomer complexes due to their conductive polymer matrix. It is also expected that the synthesis of nanocomposites based on poly-[M(Salen)] and various forms of carbon (mesoporous and activated carbon), including nanostructured ones (carbon nanotubes, graphene, and nanoglobular carbon), will lead to the development of materials with improved energetic, catalytic, and other characteristics. This quality improvement is achieved due to the uniform distribution of the polymer on the surface of the carbon component of the composite material, which has a high specific surface area, electrical conductivity, and mechanical properties (strength, elasticity).
1. Structure and Methods for Preparing [M(Salen)] Complexes and Their Polymers
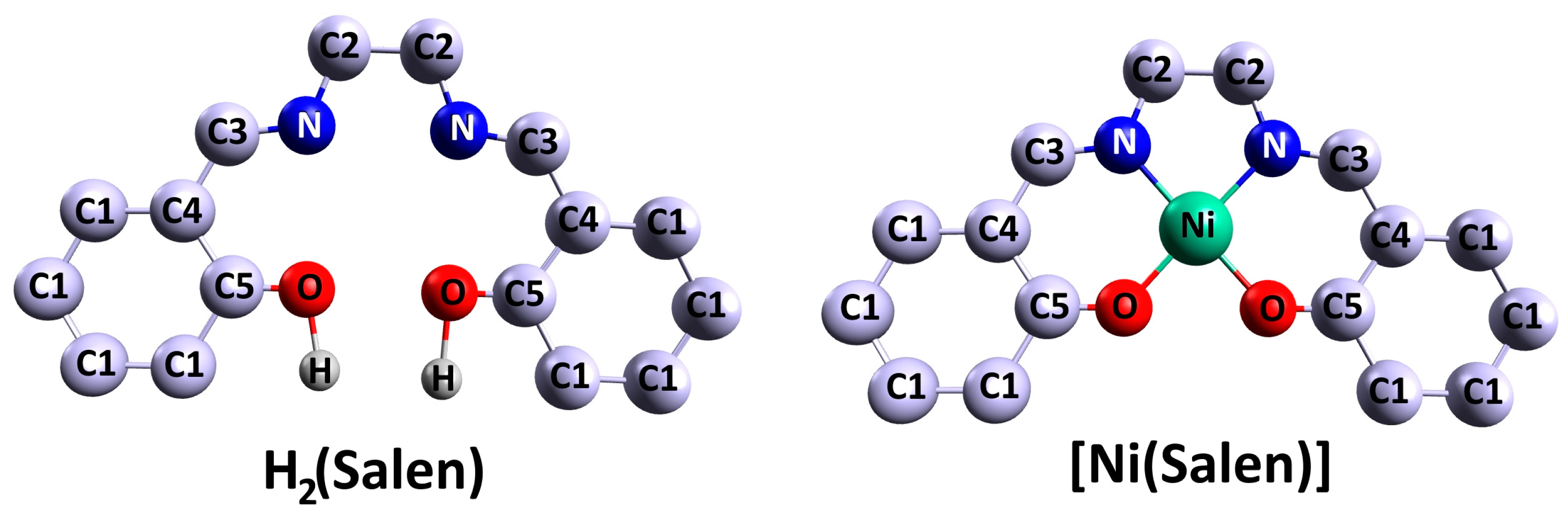
1.1. Monomeric Complexes
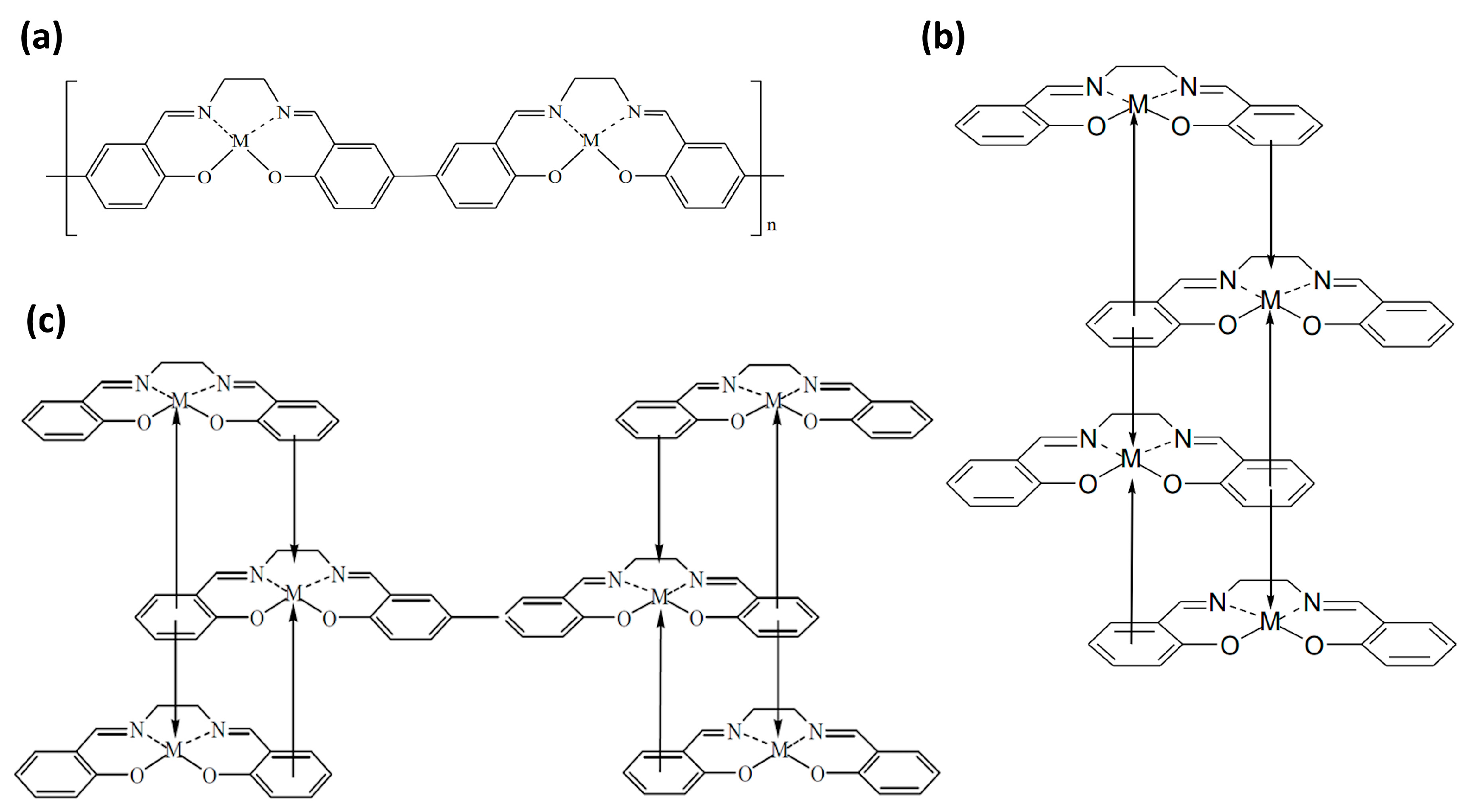
1.2. Polymers Prepared by Electropolymerization of [M(Salen)] Monomeric Molecules
2. Preparation of Composites Based on [M(Salen)] and Carbon Nanomaterials
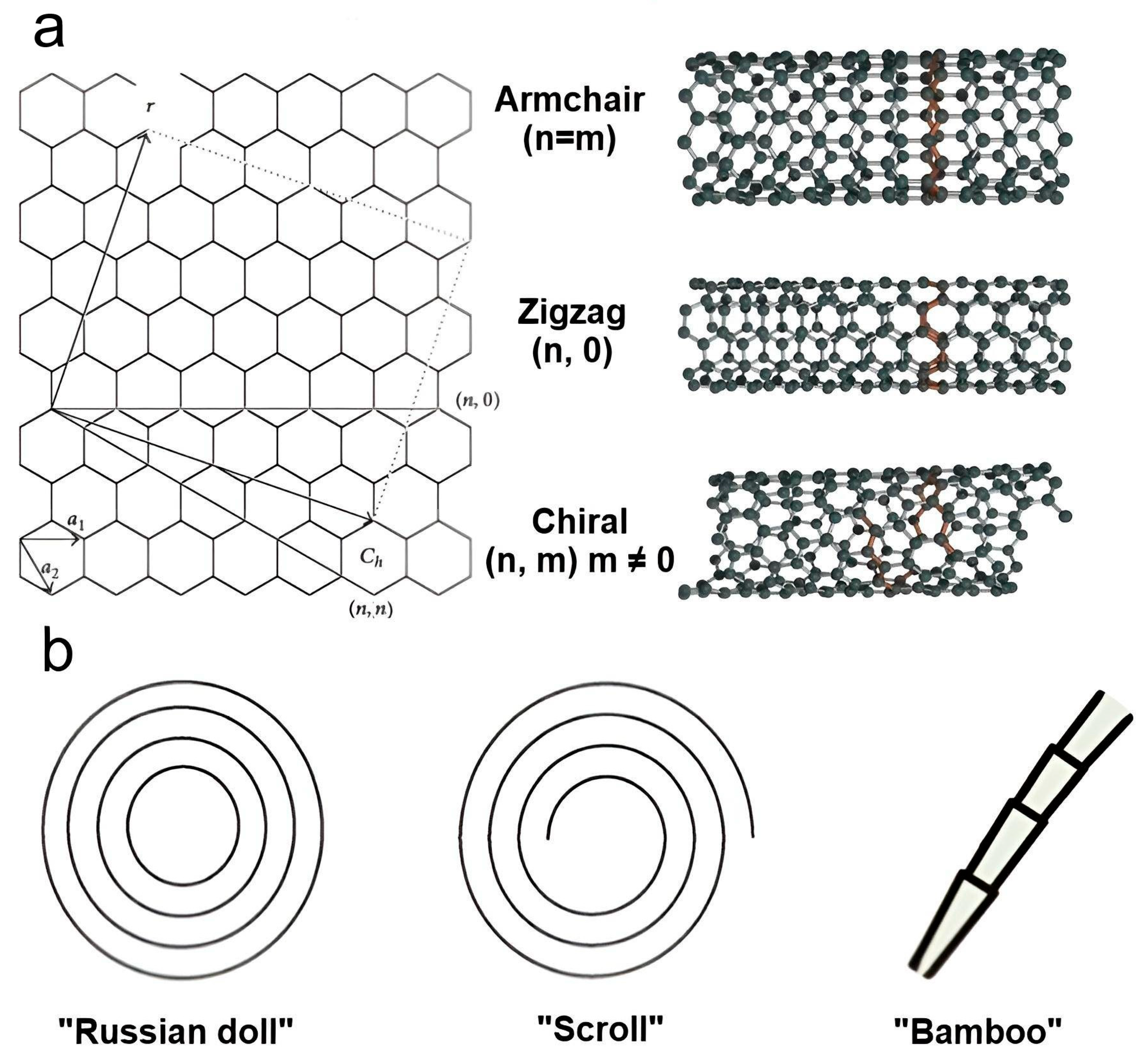
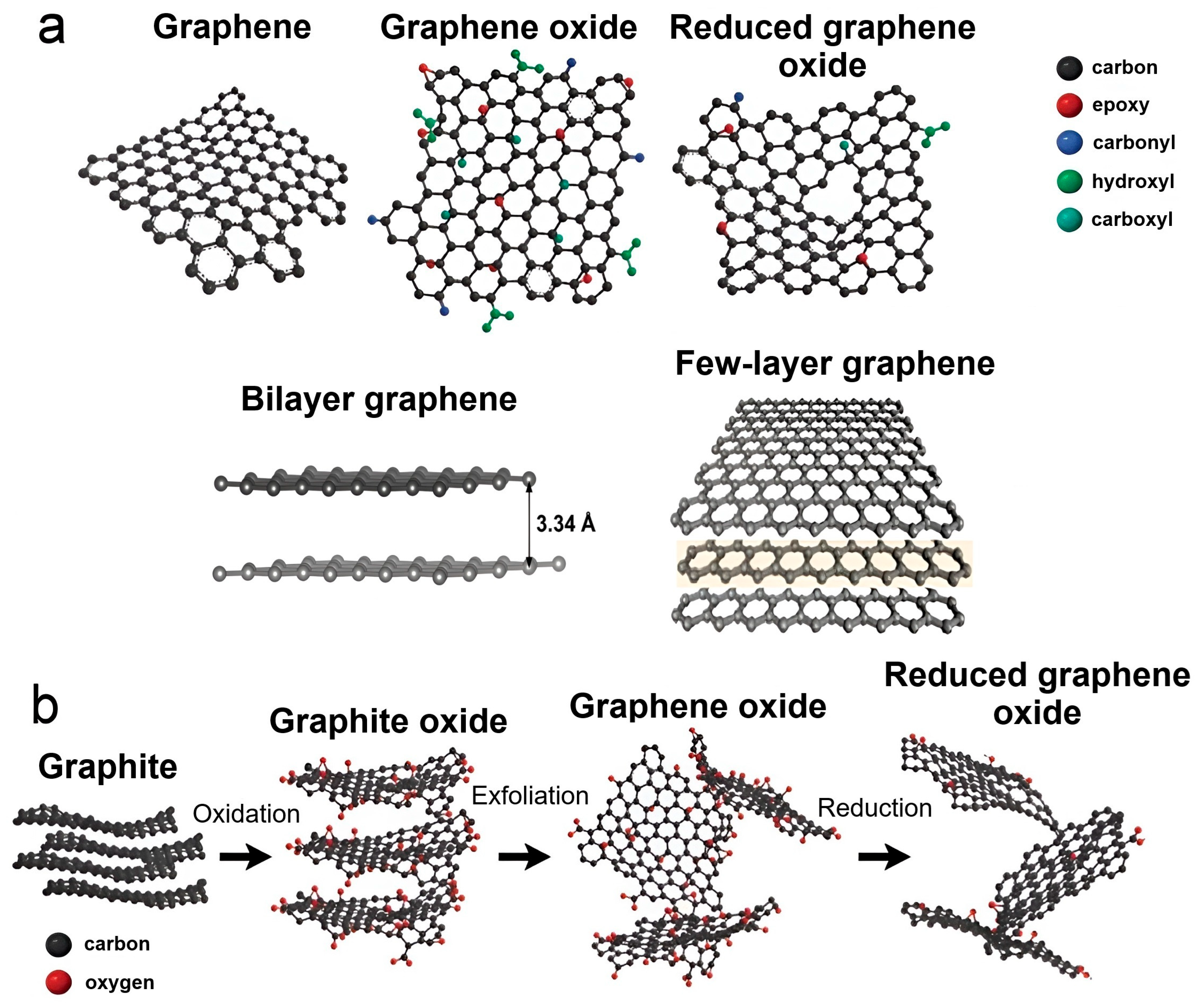
2.1. Nanotubes and Graphene as a Promising Basis for Composites
| Material | Specific Surface Area, m2g−1 | Young’s Modulus, TPa | Thermal Conductivity (at Room Temperature), Wm−1K−1 | Refs. |
|---|---|---|---|---|
| SWCNTs | 400–900 | ~1 | 3000 | [47] |
| MWCNTs | 200–400 | 0.3–1 | 3000 | [47] |
| Monolayer graphene | up to 2630 | 1 | 5000 | [48][49][50] |
| rGO sheet | up to 713 | 0.25 | 46.1 to 118.7 | [48][49][51] |
| Freestanding GO | up to 456 | 0.7 ± 0.015 | 72 (oxidation degree of 0.35); 670 (oxidation degree of 0.05) |
[48][49][52] |
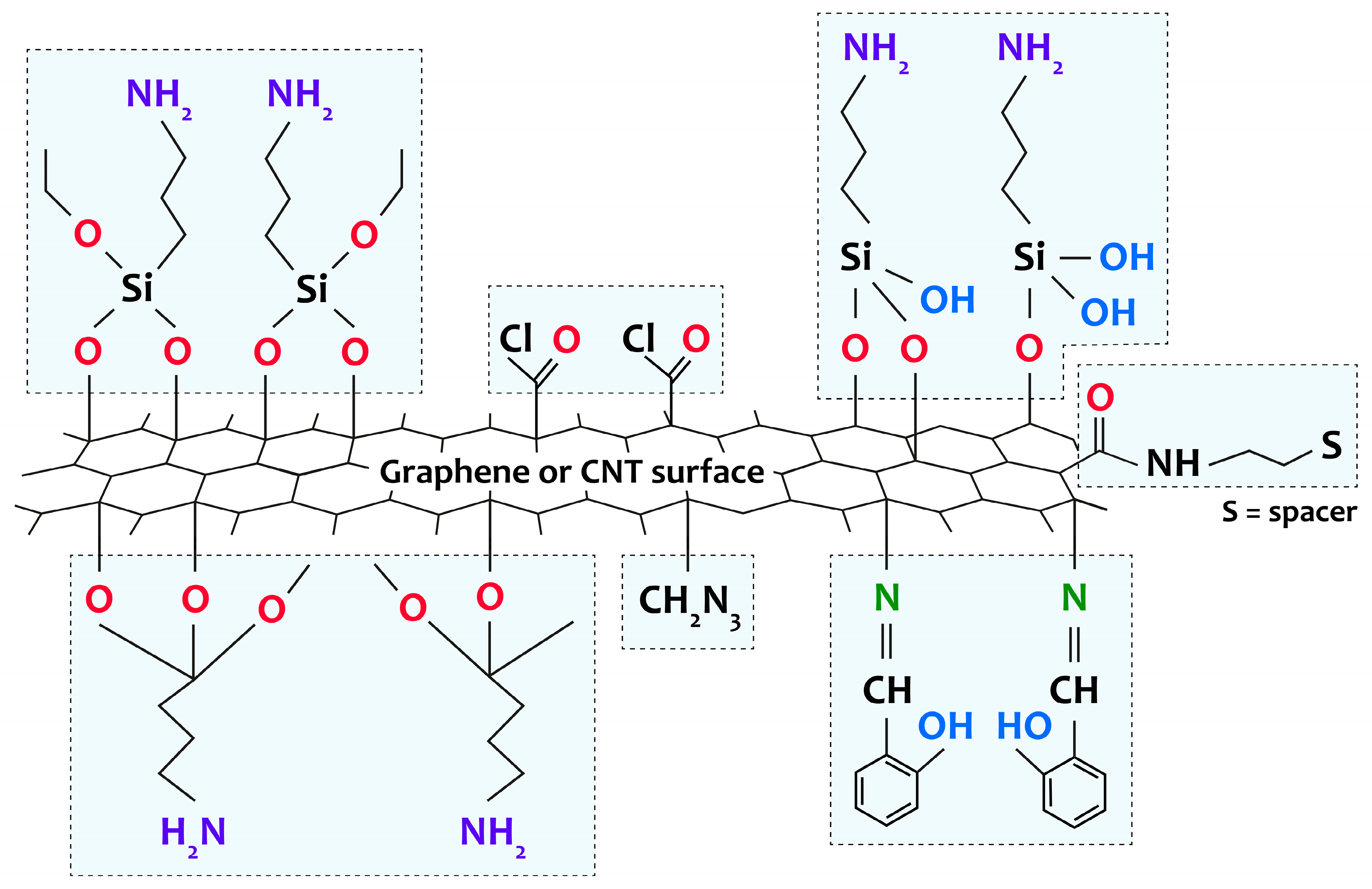
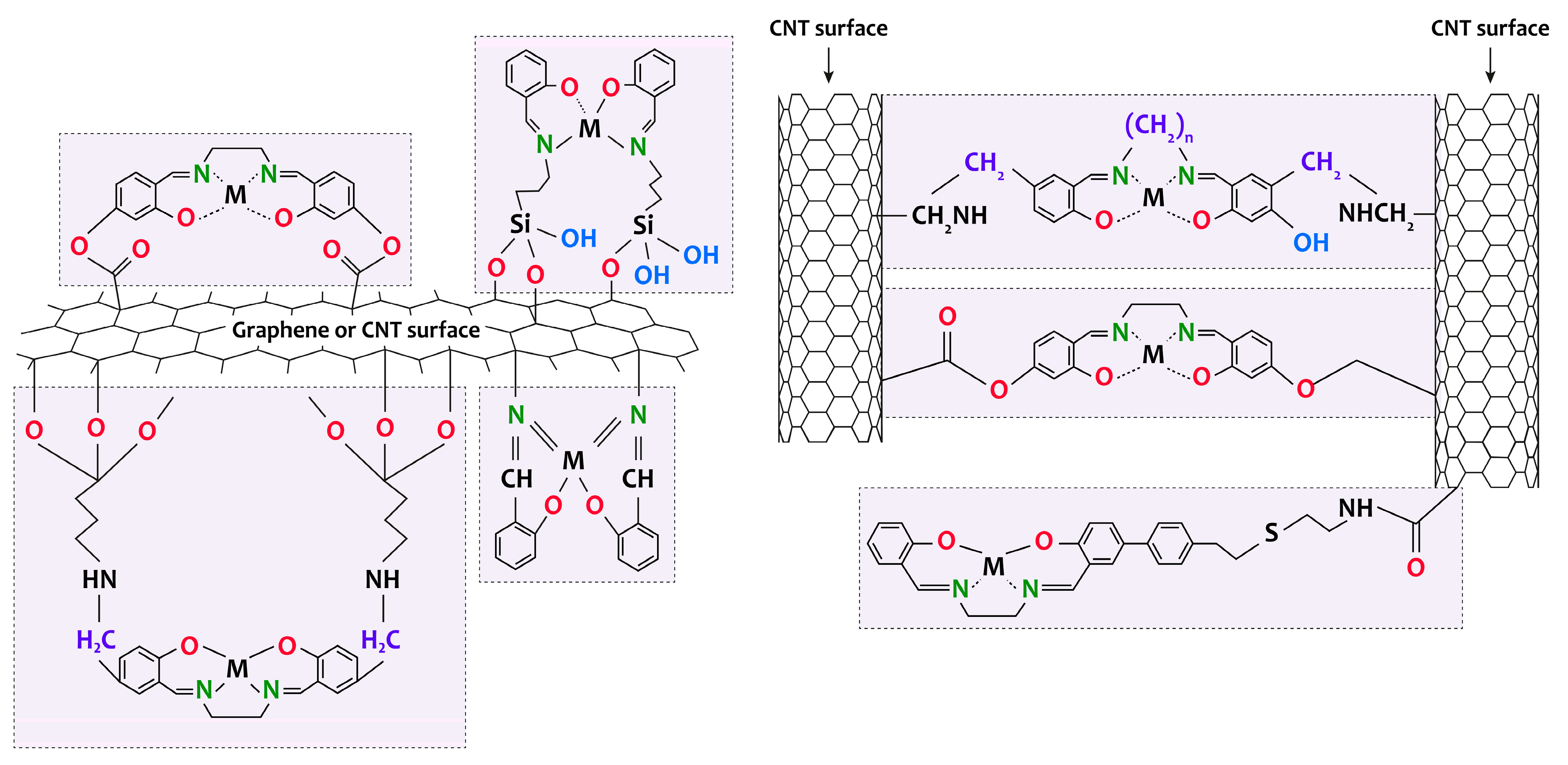
2.2. Features of the Formation of [M(Salen)]/Carbon Nanotubes and [M(Salen)]/Graphene Composites
References
- Sliznev, V.V.; Girichev, G.V. A Theoretical Study of Keto-Enol Isomerism and Internal Rotation in the H2Salen Molecule, N,N′-Ethylene-Bis(Salicylidenimine)—Schiff Base. J. Struct. Chem. 2011, 52, 16–26.
- Korusenko, P.M.; Petrova, O.V.; Vereshchagin, A.A.; Katin, K.P.; Levin, O.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Vinogradov, A.S. A Comparative XPS, UV PES, NEXAFS, and DFT Study of the Electronic Structure of the Salen Ligand in the H2(Salen) Molecule and the Complex. Int. J. Mol. Sci. 2023, 24, 9868.
- Girichev, G.V.; Giricheva, N.I.; Kuzmina, N.P.; Levina, Y.S.; Rogachev, A.Y. Molecular Structure of NiO2N2C16H14 from Gas-Phase Electron Diffraction and Quantum Chemical Data. J. Struct. Chem. 2005, 46, 813–823.
- Svirskiy, G.I.; Generalov, A.V.; Vinogradov, N.A.; Brykalova, X.O.; Vereshchagin, A.V.; Levin, O.V.; Lyalin, A.G.; Preobrajenski, A.B.; Vinogradov, A.S. Electronic Structure of the Complex Studied by Core-Level Spectroscopies. Phys. Chem. Chem. Phys. 2021, 23, 11015–11027.
- Freire, C.; Nunes, M.; Pereira, C.; Fernandes, D.M.; Peixoto, A.F.; Rocha, M. Metallo(Salen) Complexes as Versatile Building Blocks for the Fabrication of Molecular Materials and Devices with Tuned Properties. Coord. Chem. Rev. 2019, 394, 104–134.
- Clarke, R.M.; Herasymchuk, K.; Storr, T. Electronic Structure Elucidation in Oxidized Metal–Salen Complexes. Coord. Chem. Rev. 2017, 352, 67–82.
- Deng, F.; Li, X.; Ding, F.; Niu, B.; Li, J. Pseudocapacitive Energy Storage in Schiff Base Polymer with Salphen-Type Ligands. J. Phys. Chem. C 2018, 122, 5325–5333.
- Burness, J.H.; Dillard, J.G.; Taylor, L.T. An X-ray Photoelectron Spectroscopic Study of Cobalt(II) Schiff Base Complexes and Their Oxygenation Products. J. Am. Chem. Soc. 1975, 97, 6080–6088.
- Dillard, J.G.; Taylor, L.T. X-ray Photoelectron Spectroscopic Study of Schiff Base Metal Complexes. J. Electron. Spectros Relat. Phenom. 1974, 3, 455–460.
- Vilas-Boas, M.; Henderson, M.J.; Freire, C.; Hillman, A.R.; Vieil, E. A Combined Electrochemical Quartz-Crystal Microbalance Probe Beam Deflection (EQCM-PBD) Study of Solvent and Ion Transfers at a Poly-Modified Electrode during Redox Switching. Chem.-Eur. J. 2000, 6, 1160–1167.
- Vilas-Boas, M.; Freire, C.; de Castro, B.; Christensen, P.A.; Hillman, A.R. Spectroelectrochemical Characterisation of Poly-Modified Electrodes. Chemistry 2001, 7, 139–150.
- Semenistaya, T.V.; Shagisultanova, G.A. Synthesis of New Cu(II), Ni(II), Pd(II), and Pt(II) Complexes and Conducting Photosensitive Polymers Based on Them. Russ. J. Inorg. Chem. 2003, 48, 520–527.
- Tedim, J.; Bessada, R.; Patrício, S.; Magalhães, A.L.; Freire, C.; Gorman, S.J.; Hillman, A.R. Unusual Coordination Environment for Barium Cations in Ion Recognition Conducting Poly Films. Langmuir 2008, 24, 8998–9005.
- Tedim, J.; Patrício, S.; Fonseca, J.; Magalhães, A.L.; Moura, C.; Hillman, A.R.; Freire, C. Modulating Spectroelectrochemical Properties of Polymeric Films at Molecular Level. Synth. Met. 2011, 161, 680–691.
- Sizov, V.V.; Novozhilova, M.V.; Alekseeva, E.V.; Karushev, M.P.; Timonov, A.M.; Eliseeva, S.N.; Vanin, A.A.; Malev, V.V.; Levin, O.V. Redox Transformations in Electroactive Polymer Films Derived from Complexes of Nickel with SalEn-Type Ligands: Computational, EQCM, and Spectroelectrochemical Study. J. Solid State Electrochem. 2015, 19, 453–468.
- Nunes, M.; Araújo, M.; Fonseca, J.; Moura, C.; Hillman, R.; Freire, C. High-Performance Electrochromic Devices Based on Poly-Type Polymer Films. ACS Appl. Mater. Interfaces 2016, 8, 14231–14243.
- Goldsby, K.A.; Blaho, J.K.; Hoferkamp, L.A. Oxidation of Nickel(II) Bis(Salicylaldimine) Complexes: Solvent Control of the Ultimate Redox Site. Polyhedron 1989, 8, 113–115.
- Hoferkamp, L.A.; Goldsby, K.A. Surface-Modified Electrodes Based on Nickel(II) and Copper(II) Bis(Salicylaldimine) Complexes. Chem. Mater. 1989, 1, 348–352.
- Soos, Z.; Mukhopadhyay, D.; Painelli, A.; Girlando, A. Handbook of Conducting Polymers, 2nd ed.; Revised and Expanded; CRC Press: Boca Raton, FL, USA, 1998; pp. 165–208. ISBN 0824700503.
- Leung, A.C.W.; MacLachlan, M.J. Schiff Base Complexes in Macromolecules. J. Inorg. Organomet. Polym. Mater. 2007, 17, 57–89.
- Vasil’eva, S.V.; Balashev, K.P.; Timonov, A.M. Effects of the Nature of the Ligand and Solvent on the Electrooxidation of Complexes Formed by Nickel and Schiff’s Bases. Russ. J. Electrochem. 1998, 34, 978–983.
- Vilas-Boas, M.; Santos, I.C.; Henderson, M.J.; Freire, C.; Hillman, A.R.; Vieil, E. Electrochemical Behavior of a New Precursor for the Design of Poly-Based Modified Electrodes. Langmuir 2003, 19, 7460–7468.
- Hamnett, A.; Abel, J.; Eameaim, J.; Christensen, P.; Timonov, A.; Vasilyeva, S. A Study of the Polymerisation and Electrochemical Cycling of Pd Methoxy- Salen Derivatives Using Fast Ellipsometry and FT-Infrared Spectroscopy. Phys. Chem. Chem. Phys. 1999, 1, 5147–5156.
- Krasikova, S.A.; Besedina, M.A.; Karushev, M.P.; Dmitrieva, E.A.; Timonov, A.M. In Situ Electrochemical Microbalance Studies of Polymerization and Redox Processes in Polymeric Complexes of Transition Metals with Schiff Bases. Russ. J. Electrochem. 2010, 46, 218–226.
- Dmitrieva, E.A.; Logvinov, S.A.; Kurdakova, V.V.; Kondrat’ev, V.V.; Malev, V.V.; Timonov, A.M. Redox Polymer Poly-N,N′-2,3-Dimethylbutane-2,3-Diyl-Bis(Salicylideniminato)Nickel: An Impedance Spectroscopy Study. Russ. J. Electrochem. 2005, 41, 381–387.
- Vasil’ev, V.; Popeko, I.E.; Timonov, A.; Shagisultanova, G.A. The Electrochemical Behavior of Palladium(II) Complexes with Schift Bases and the Synthesis of a Mixed-Valence Pd(II)-Pd(IV) Complex. Russ. J. Inorg. Chem. 1990, 35, 523–525.
- Audebert, P.; Capdevielle, P.; Maumy, M. Redox and Conducting Polymers Based on Salen-Type Metal Units; Electrochemical Study and Some Characteristics. New J. Chem. 1992, 16, 697–703.
- Vilas-Boas, M.; Freire, C.; De Castro, B.; Christensen, P.A.; Hillman, A.R. New Insights into the Structure and Properties of Electroactive Polymer Films Derived from . Inorg. Chem. 1997, 36, 4919–4929.
- Tedim, J.; Carneiro, A.; Bessada, R.; Patrício, S.; Magalhães, A.L.; Freire, C.; Gurman, S.J.; Hillman, A.R. Correlating Structure and Ion Recognition Properties of -Based Polymer Films. J. Electroanal. Chem. 2007, 610, 46–56.
- Mukherjee, P.; Biswas, C.; Drew, M.G.B.; Ghosh, A. Structural Variations in Ni(II) Complexes of Salen Type Di-Schiff Base Ligands. Polyhedron 2007, 26, 3121–3128.
- Storr, T.; Wasinger, E.C.; Pratt, R.C.; Stack, T.D.P. The Geometric and Electronic Structure of a One-Electron-Oxidized Nickel(II) Bis(Salicylidene)Diamine Complex. Angew. Chem.-Int. Ed. 2007, 46, 5198–5201.
- Shimazaki, Y.; Arai, N.; Dunn, T.J.; Yajima, T.; Tani, F.; Ramogida, C.F.; Storr, T. Influence of the Chelate Effect on the Electronic Structure of One-Electron Oxidized Group 10 Metal(Ii)-(Disalicylidene)Diamine Complexes. Dalton Trans. 2011, 40, 2469–2479.
- Levin, O.V.; Karushev, M.P.; Timonov, A.M.; Alekseeva, E.V.; Zhang, S.; Malev, V.V. Charge Transfer Processes on Electrodes Modified by Polymer Films of Metal Complexes with Schiff Bases. Electrochim. Acta 2013, 109, 153–161.
- Fonseca, J.; Tedim, J.; Biernacki, K.; Magalhães, A.L.; Gurman, S.J.; Freire, C.; Hillman, A.R. Structural and Electrochemical Characterisation of -Type Conducting Polymer Films. Electrochim. Acta 2010, 55, 7726–7736.
- Dmitrieva, E.; Rosenkranz, M.; Danilova, J.S.; Smirnova, E.A.; Karushev, M.P.; Chepurnaya, I.A.; Timonov, A.M. Radical Formation in Polymeric Nickel Complexes with N2O2 Schiff Base Ligands: An in Situ ESR and UV–Vis–NIR Spectroelectrochemical Study. Electrochim. Acta 2018, 283, 1742–1752.
- Chen, C.; Li, X.; Deng, F.; Li, J. Electropolymerization and Electrochemical Behavior of Nickel Schiff Base Complexes with Different Groups between Imine Linkages. RSC Adv. 2016, 6, 79894–79899.
- Alekseeva, E.V.; Vereshchagin, A.A.; Novozhilova, M.V.; Panjwani, N.A.; Novoselova, J.V.; Lukyanov, D.A.; Beletskii, E.V.; Behrends, J.; Sizov, V.V.; Levin, O.V. Uncovering the Mechanism of Water-Promoted Electrochemical Degradation of NiSalen Polymers. J. Electroanal. Chem. 2023, 935, 117310:1–117310:16.
- Sunday Nworie, F. Bis(Salicylidene)Ethylenediamine(Salen) and Bis(Salicylidene)Ethylenediamine-Metal Complexes: From Structure to Biological Activity. J. Anal. Pharm. Res. 2016, 3, 00076.
- Dahm, C.E.; Peters, D.G.; Simonet, J. Electrochemical and Spectroscopic Characterization of Anodically Formed Nickel Salen Polymer Films on Glassy Carbon, Platinum, and Optically Transparent Tin Oxide Electrodes in Acetonitrile Containing Tetramethylammonium Tetrafluoroborate. J. Electroanal. Chem. 1996, 410, 163–171.
- Chepurnaya, I.A.; Karushev, M.P.; Alekseeva, E.V.; Lukyanov, D.A.; Levin, O.V. Redox-Conducting Polymers Based on Metal- Salen Complexes for Energy Storage Applications. Pure Appl. Chem. 2020, 92, 1239–1258.
- Shagisultanova, G.A.; Ardasheva, L.P. Electrochemical Synthesis of Thin Films of Polymers Derived from and . Russ. J. Appl. Chem. 2003, 76, 1626–1630.
- Egbedina, A.O.; Bolade, O.P.; Ewuzie, U.; Lima, E.C. Emerging Trends in the Application of Carbon-Based Materials: A Review. J. Environ. Chem. Eng. 2022, 10, 107260.
- Ouyang, J. Applications of Carbon Nanotubes and Graphene for Third-Generation Solar Cells and Fuel Cells. Nano Mater. Sci. 2019, 1, 77–90.
- Yuan, W.; Zhang, Y.; Cheng, L.; Wu, H.; Zheng, L.; Zhao, D. The Applications of Carbon Nanotubes and Graphene in Advanced Rechargeable Lithium Batteries. J. Mater. Chem. A Mater. 2016, 4, 8932–8951.
- Gopiraman, M.; Soo Kim, I. Carbon Nanocomposites: Preparation and Its Application in Catalytic Organic Transformations. In Nanocomposites—Recent Evolutions; IntechOpen: Rijeka, Croatia, 2019; pp. 17–43.
- Zhu, Z. An Overview of Carbon Nanotubes and Graphene for Biosensing Applications. Nanomicro Lett. 2017, 9, 25.
- Onyancha, R.B.; Aigbe, U.O.; Ukhurebor, K.E.; Muchiri, P.W. Facile Synthesis and Applications of Carbon Nanotubes in Heavy-Metal Remediation and Biomedical Fields: A Comprehensive Review. J. Mol. Struct. 2021, 1238, 130462.
- Albers, P.W.; Leich, V.; Ramirez-Cuesta, A.J.; Cheng, Y.; Hönig, J.; Parker, S.F. The Characterisation of Commercial 2D Carbons: Graphene, Graphene Oxide and Reduced Graphene Oxide. Mater. Adv. 2022, 3, 2810–2826.
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review on the Mechanical Properties of Polymer Composites Reinforced by Carbon Nanotubes and Graphene. Carbon. Lett. 2021, 31, 149–165.
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H. A Methodical Review on Carbon-Based Nanomaterials in Energy-Related Applications. Adsorpt. Sci. Technol. 2022, 2022, 1–21.
- Zeng, Y.; Li, T.; Yao, Y.; Li, T.; Hu, L.; Marconnet, A. Thermally Conductive Reduced Graphene Oxide Thin Films for Extreme Temperature Sensors. Adv. Funct. Mater. 2019, 29, 1901388.
- Chen, J.; Li, L. Thermal Conductivity of Graphene Oxide: A Molecular Dynamics Study. JETP Lett. 2020, 112, 117–121.
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58.
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095:1–115095:28.
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770.
- Moradi, O.; Yari, M.; Zare, K.; Mirza, B.; Najafi, F. Carbon Nanotubes: A Review of Chemistry Principles and Reactions. Fuller. Nanotub. Carbon Nanostructures 2012, 20, 138–151.
- Zhang, L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531.
- Kharissova, O.V.; Kharisov, B.I. Variations of Interlayer Spacing in Carbon Nanotubes. RSC Adv. 2014, 4, 30807–30815.
- Kumar Jagadeesan, A.; Thangavelu, K.; Dhananjeyan, V. Carbon Nanotubes: Synthesis, Properties and Applications. In 21st Century Surface Science—A Handbook; IntechOpen: Rijeka, Croatia, 2020.
- Jang, J.W. Direct Curvature Measurement of the Compartments in Bamboo-Shaped Multi-Walled Carbon Nanotubes via Scanning Probe Microscopy. Sci. Rep. 2021, 11, 701.
- Taib, N.-A.; Rahman, M.; Matin, M.; Uddin, J.; Bakri, M.K.; Khan, A. A Review on Carbon Nanotubes (CNT): Structure, Synthesis, Purification and Properties for Modern Day Applications. 2021.
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for Carbon Nanotubes Synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large Area Few-Layer Graphene with Scalable Preparation from Waste Biomass for High-Performance Supercapacitor. Sci. Rep. 2017, 7, 15239:1–15239:14.
- Zhang, J.; Liu, X.; Zhang, M.; Zhang, R.; Ta, H.Q.; Sun, J.; Wang, W.; Zhu, W.; Fang, T.; Jia, K.; et al. Fast Synthesis of Large-Area Bilayer Graphene Film on Cu. Nat. Commun. 2023, 14, 3199:1–3199:9.
- Podlivaev, A.I.; Grishakov, K.S.; Katin, K.P.; Maslov, M.M. Stone–Wales Bilayer Graphene: Structure, Stability, and Interlayer Heat Transfer. JETP Lett. 2021, 114, 143–149.
- Acik, M.; Chabal, Y.J. Nature of Graphene Edges: A Review. Jpn. J. Appl. Phys. 2011, 50, 070101.
- Yan, J.A.; Chou, M.Y. Oxidation Functional Groups on Graphene: Structural and Electronic Properties. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 125403.
- Suhaimin, N.S.; Hanifah, M.F.R.; wani Jusin, J.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Othman, M.H.D.; Abd Rahman, M.; Aziz, F.; Yusof, N.; et al. Tuning the Oxygen Functional Groups in Graphene Oxide Nanosheets by Optimizing the Oxidation Time. Phys. E Low. Dimens. Syst. Nanostr. 2021, 131, 114727.
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and Their Applications. J. Mater. Chem. A Mater. 2015, 3, 18753–18808.
- Ganesha, H.; Veeresh, S.; Nagaraju, Y.S.; Suresh, D.S.; Vijaykumar, S.P.; Sapna, S.; Khmis, A.; Devendrappa, H. Polymer Incorporated with Graphene Oxide Composite for High Electrochemical Performance. Mater. Today Proc. 2023.
- Bai, H.; Li, C.; Shi, G. Functional Composite Materials Based on Chemically Converted Graphene. Adv. Mater. 2011, 23, 1089–1115.
- Seok, D.; Jeong, Y.; Han, K.; Yoon, D.Y.; Sohn, H. Recent Progress of Electrochemical Energy Devices: Metal Oxide-Carbon Nanocomposites as Materials for next-Generation Chemical Storage for Renewable Energy. Sustainability 2019, 11, 3694.
- Kochaev, A.I.; Efimov, V.V.; Kaya, S.; Flores-Moreno, R.; Katin, K.P.; Maslov, M.M. On Point Perforating Defects in Bilayer Structures. Phys. Chem. Chem. Phys. 2023, 25, 30477–30487.
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Ullah Khan, A. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163.
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing Polymer Composite Materials: Carbon Nanotubes or Graphene? Adv. Mater. 2013, 25, 5153–5176.
- Saeed, K.; Khan, I.; Khan, I.; Ali, N.; Bilal, M.; Akhter, M.S. Graphene and Carbon Nanotubes-Based Polymer Nanocomposites. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–218.
- Wang, J.; Zhang, Y.; Liu, G.; Zhang, T.; Zhang, C.; Zhang, Y.; Feng, Y.; Chi, Q. Improvements in the Magnesium Ion Transport Properties of Graphene/CNT-Wrapped TiO2-B Nanoflowers by Nickel Doping. Small 2023, 2304969.
- Sonkar, P.K.; Ganesan, V.; Sen Gupta, S.K.; Yadav, D.K.; Gupta, R.; Yadav, M. Highly Dispersed Multiwalled Carbon Nanotubes Coupled Manganese Salen Nanostructure for Simultaneous Electrochemical Sensing of Vitamin B2 and B6. J. Electroanal. Chem. 2017, 807, 235–243.
- Lu, B.; Zhang, Z.; Hao, J.; Xu, G.; Zhang, B.; Tang, J. Electrochemical Sensing Platform Based on Schiff-Base Cobalt(Ii)/Single-Walled Carbon Nanohorns Complexes System. Anal. Methods 2012, 4, 3580–3585.
- Rajarao, R.; Kim, T.H.; Bhat, B.R. Multi-Walled Carbon Nanotube Bound Nickel Schiff-Base Complexes as Reusable Catalysts for Oxidation of Alcohols. J. Coord. Chem. 2012, 65, 2671–2682.
- Salavati-Niasari, M.; Bazarganipour, M. Covalent Functionalization of Multi-Wall Carbon Nanotubes (MWNTs) by Nickel(II) Schiff-Base Complex: Synthesis, Characterization and Liquid Phase Oxidation of Phenol with Hydrogen Peroxide. Appl. Surf. Sci. 2008, 255, 2963–2970.
- Bai, L.; Li, M.; Guan, J. Co(II) or Cu(II) Schiff Base Complex Immobilized onto Carbon Nanotubes as a Synergistic Catalyst for the Oxygen Reduction Reaction. ChemistrySelect 2018, 3, 581–585.
- Li, H.; Zhong, M.; Li, C.; Ren, Y.; Chen, J.; Yang, Q. Synthesis of CNTs@POP-Salen Core-Shell Nanostructures for Catalytic Epoxides Hydration. ChemCatChem 2019, 11, 3952–3958.
- Zheng, W.; Tan, R.; Yin, S.; Zhang, Y.; Zhao, G.; Chen, Y.; Yin, D. Ionic Liquid-Functionalized Graphene Oxide as an Efficient Support for the Chiral Salen Mn(iii) Complex in Asymmetric Epoxidation of Unfunctionalized Olefins. Catal. Sci. Technol. 2015, 5, 2092–2102.
- Zhou, X.; Lu, X. Co(Salen) Supported on Graphene Oxide for Oxidation of Lignin. J. Appl. Polym. Sci. 2016, 133, 44133.
- Li, Z.; Wu, S.; Ding, H.; Lu, H.; Liu, J.; Huo, Q.; Guan, J.; Kan, Q. Oxovanadium(Iv) and Iron(Iii) Salen Complexes Immobilized on Amino-Functionalized Graphene Oxide for the Aerobic Epoxidation of Styrene. New J. Chem. 2013, 37, 4220–4229.
- Wei, W.-C.; Deng, C.; Huang, S.-C.; Wei, Y.-X.; Wang, Y.-Z. Nickel-Schiff Base Decorated Graphene for Simultaneously Enhancing the Electroconductivity, Fire Resistance, and Mechanical Properties of a Polyurethane Elastomer. J. Mater. Chem. A Mater. 2018, 6, 8643–8654.
- Li, Z.; Wu, S.; Ding, H.; Zheng, D.; Hu, J.; Wang, X.; Huo, Q.; Guan, J.; Kan, Q. Immobilized Cu(Ii) and Co(Ii) Salen Complexes on Graphene Oxide and Their Catalytic Activity for Aerobic Epoxidation of Styrene. New J. Chem. 2013, 37, 1561–1568.
- Zhao, Q.; Bai, C.; Zhang, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Catalytic Epoxidation of Olefins with Graphene Oxide Supported Copper (Salen) Complex. Ind. Eng. Chem. Res. 2014, 53, 4232–4238.
- Ghabdian, M.; Nasseri, M.A.; Allahresani, A.; Motavallizadehkakhky, A. Heterogenized Cu (II) Salen Complex Grafted on Graphene Oxide Nanosheets as a Precursing Catalyst for the Pd-free Sonogashira Coupling. Appl. Organomet. Chem. 2018, 32, 4545:1–4545:9.
- Zhu, Z.; Lu, J.; Li, X.; Xu, G.; Chen, C.; Li, J. Effects of Potential Modes on Performances of Electrodeposited Poly/MWCNTs Composite as Supercapacitor Electrode Material. Electrochemistry 2016, 84, 427–431.
- Li, J.L.; Gao, F.; Zhang, Y.K.; Kang, F.Y.; Wang, X.D.; Ye, F.; Yang, J. Electropolymerization of Ni(Salen) on Carbon Nanotube Carrier as a Capacitive Material by Pulse Potentiostatic Method. Sci. China Chem. 2012, 55, 1338–1344.
- Li, X.; Xu, G.; Deng, F.; Chen, C.; Li, J.; Kang, F. Understanding the Charge Storage Mechanism and Electrochemical Performance on the Poly-Modified Electrode Electropolymerized with Different Sweep Rate. Electrochemistry 2017, 85, 461–468.
- Araújo, M.P.; Nunes, M.; Fonseca, J.; Moura, C.; Hillman, R.; Freire, C. Graphene-Poly(Nickel Complex) as Novel Electrochromic Nanocomposite for the Fabrication of a Robust Solid-State Device. J. Colloid. Interface Sci. 2017, 504, 790–799.
- Chen, C.; Zhu, Z.; Li, X.; Li, J. Electropolymerization and Energy Storage of Poly/MWCNT Composite Materials for Supercapacitors. J. Appl. Polym. Sci. 2017, 134, 44464:1–44464:8.
- Chepurnaya, I.A.; Logvinov, S.A.; Karushev, M.P.; Timonov, A.M.; Malev, V.V. Modification of Supercapacitor Electrodes with Polymer Metallocomplexes: Methods and Results. Russ. J. Electrochem. 2012, 48, 538–544.
- Karushev, M.P.; Timonov, A.M. Adsorption-Electrochemical Modification of Nanoporous Carbon Materials by Nickel Complexes with Schiff Bases. Russ. J. Appl. Chem. 2012, 85, 914–920.
- Alekseeva, E.; Stelmashuk, T.; Ershov, V.; Levin, O. Low-Temperature Energy Storage Performance of NiSalen Type Polymer and It’s Composite with SWCNT. Electrochim. Acta 2021, 383, 138309:1–138309:10.
- Gao, F.; Li, J.; Kang, F.; Zhang, Y.; Wang, X.; Ye, F.; Yang, J. Preparation and Characterization of a Poly/Multiwalled Carbon Nanotube Composite by in situ Electropolymerization as a Capacitive Material. J. Phys. Chem. C 2011, 115, 11822–11829.
- Tedim, J.; Gonçalves, F.; Pereira, M.F.R.; Figueiredo, J.L.; Moura, C.; Freire, C.; Hillman, A.R. Preparation and Characterization of Poly/Multi-Walled Carbon Nanotube Composite Films. Electrochim. Acta 2008, 53, 6722–6731.
- Nunes, M.; Araújo, M.; Bacsa, R.; Ferreira, R.V.; Castillejos, E.; Serp, P.; Hillman, A.R.; Freire, C. N-Doped Few-Layered Graphene-PolyNi Complex Nanocomposite with Excellent Electrochromic Properties. Carbon 2017, 120, 32–43.





