Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Steve Greenberg | -- | 2091 | 2024-02-15 15:02:09 | | | |
| 2 | Mona Zou | -1 word(s) | 2090 | 2024-02-19 10:40:58 | | | | |
| 3 | Mona Zou | -1 word(s) | 2089 | 2024-02-26 10:19:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Blum, F.E.; Locke, A.R.; Nathan, N.; Katz, J.; Bissing, D.; Minhaj, M.; Greenberg, S.B. Residual Neuromuscular Block. Encyclopedia. Available online: https://encyclopedia.pub/entry/55073 (accessed on 08 February 2026).
Blum FE, Locke AR, Nathan N, Katz J, Bissing D, Minhaj M, et al. Residual Neuromuscular Block. Encyclopedia. Available at: https://encyclopedia.pub/entry/55073. Accessed February 08, 2026.
Blum, Franziska Elisabeth, Andrew R. Locke, Naveen Nathan, Jeffrey Katz, David Bissing, Mohammed Minhaj, Steven B. Greenberg. "Residual Neuromuscular Block" Encyclopedia, https://encyclopedia.pub/entry/55073 (accessed February 08, 2026).
Blum, F.E., Locke, A.R., Nathan, N., Katz, J., Bissing, D., Minhaj, M., & Greenberg, S.B. (2024, February 15). Residual Neuromuscular Block. In Encyclopedia. https://encyclopedia.pub/entry/55073
Blum, Franziska Elisabeth, et al. "Residual Neuromuscular Block." Encyclopedia. Web. 15 February, 2024.
Copy Citation
Residual neuromuscular block (RNMB) remains a significant safety concern for patients throughout the perioperative period and is still widely under-recognized by perioperative healthcare professionals. Current literature suggests an association between RNMB and an increased risk of postoperative pulmonary complications, a prolonged length of stay in the post anesthesia care unit (PACU), and decreased patient satisfaction. The 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade provide guidance for the use of quantitative neuromuscular monitoring coupled with neuromuscular reversal to recognize and reduce the incidence of RNMB. Using sugammadex for the reversal of neuromuscular block as well as quantitative neuromuscular monitoring to quantify the degree of neuromuscular block may significantly reduce the risk of RNMB among patients undergoing general anesthesia. Studies are forthcoming to investigate how using neuromuscular blocking agent reversal with quantitative monitoring of the neuromuscular block may further improve perioperative patient safety.
anesthesia
perioperative care
residual neuromuscular block
quantitative monitoring
neuromuscular blocking agent
1. Introduction
Neuromuscular block (NMB) is commonly used in perioperative care for a variety of circumstances. It can optimize surgical conditions [1], facilitate tracheal intubation, and be used in the intensive care unit (ICU) to improve chest wall compliance, reduce abdominal pressure, prevent and treat shivering, and reduce elevation in intracranial pressure from the airway reactivity [2][3]. In the emergency department (ED) and areas outside of the operating room and procedural areas, NMB can be used for rapid sequence induction and intubation (RSII), a technique used by healthcare professionals to minimize pulmonary aspiration [4]. There are two common types of neuromuscular block: depolarizing and nondepolarizing. The only commercially available depolarizing muscle relaxant, suxamethonium chloride or succinylcholine, binds to acetylcholine receptors and causes temporary depolarization. It produces muscle fasciculations followed by flaccid paralysis and is frequently used for urgent or emergent intubation in and outside of the operating room (OR) [4]. Nondepolarizing muscle relaxants are used more frequently in the operating room and provide competitive antagonistic activity of the nicotinic receptor and therefore block the action of acetylcholine (Figure 1) [5]. The nondepolarizing neuromuscular blockers can be further divided into two groups: benzylisoquinolins (or benzylisoquinoliniums) and aminosteroids. Types of drugs within these two classifications have their own unique pharmacokinetic makeup (Figure 2).
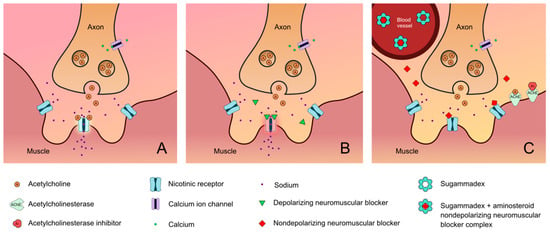
Figure 1. Neuromuscular junction: (A) normal; (B) activity of depolarizing neuromuscular blocking agent; (C) activity of nondepolarizing neuromuscular blocking agent as well as the activity of both neuromuscular blockade reversal agents (i.e., sugammadex and acetylcholinesterase inhibitors) [6][7][8][9][10].
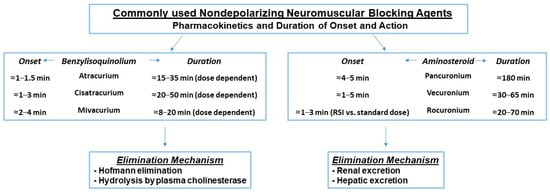
Figure 2. Pharmacokinetics of nondepolarizing agents. Factors that increase the duration of these agents include dosing, age >65 years, hepatic and renal disease, hypothermia, inhaled anesthetics, and medications including but not limited to antibiotics, antidepressants, magnesium, and antiepileptics. The ranges provided for onset and duration times are exclusionary of outliers [11][12][13][14][15][16][17][18][19][20][21][22][23].
Residual neuromuscular block (RNMB) is the unwanted presence of signs and symptoms of muscle weakness in the perioperative period and outside of the OR after the administration of neuromuscular blocking agents and is defined by a combination of quantitative and qualitative measures [4][24][25]. To quantify RNMB, a train-of-four ratio (TOFR) recorded on a quantitative neuromuscular monitor was used. The TOFR represents four supramaximal stimuli delivered every 0.5 s (2 Hz) and the muscle response to the fourth stimulus is compared to the first stimulus (Figure 3) [24][26]. A ratio of the fourth to the first response of TOF (train of four) less than 0.90 (90%) defines the RNMB. Although the definition of RNMB has changed over time due to clinical observations of weakness at lower TOFRs, the TOFR < 0.9 is currently the most accurate quantitative definition of residual neuromuscular block.
A quantitative TOFR < 0.9 has been associated with various unwanted symptoms of weakness that include an inability to breathe normally and maintain a patent airway, swallow dysfunction, lack of a strong cough, and even an inability to smile or talk [24][27][28][29][30][31][32]. These undesirable outcomes may be prevented if anesthesia professionals ensure the return of TOFR ≥ 0.9 in the perioperative space [33]. As a result, the widely recommended and accepted TOFR associated with higher rates of full clinical recovery from neuromuscular block is ≥0.9 measured at the adductor pollicis [24][33][34].
While the pharmacodynamic and pharmacokinetic profiles of NMB have improved over the past six decades, along with the monitoring capabilities, nondepolarizing neuromuscular blocking agent (NMBA) use may still result in residual neuromuscular block leading to a variety of deleterious outcomes [24]. Studies have suggested that postoperative pulmonary complications (PPCs) such as pneumonia, respiratory failure, atelectasis, and upper airway obstruction result in increased postoperative mortality and are associated with RNMB [35]. RNMB () has been further associated with prolonged lengths of stay in the post anesthesia care unit (PACU) and decreased patient satisfaction [24][36]. RNMB remains a significant issue in perioperative care and is frequently unrecognized, despite more perioperative provider acknowledgment that this phenomenon exists and the increase in literature on this topic that is now available. The presence of poor recognition of RNMB among anesthesia professionals, the monitoring modalities available, and the special populations that are at highest risk of RNMB will be discussed. In addition, obstacles to the implementation of quantitative monitoring and the reversal agents that may reduce the incidence of RNMB will be discussed.
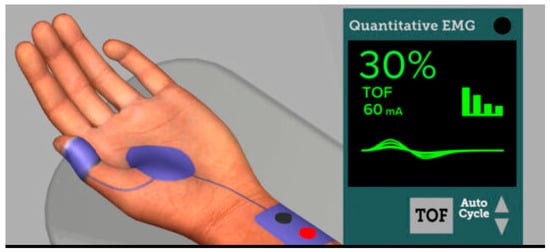
Figure 3. Online simulation course depiction of a quantitative EMG neuromuscular monitor (Adapted with permission from Ref. [37] 2023, APSF).
Poor Recognition of Residual NMB
Despite the rather high reported incidence of RNMB (as high as 65%, in elective abdominal surgeries [38]), many anesthesia professionals are unaware of the clinical significance of RNMB [39][40][41]. Poor recognition of residual neuromuscular block is a driving factor behind this phenomenon [42]. Several international surveys have suggested that anesthesia professionals do not routinely monitor for RNMB as well as underestimating the true incidence of RNMB [27][39]. The main reason for the survey results above may be rooted in the overconfidence of anesthesia professionals in their perceived knowledge of the relevance of neuromuscular monitoring to all aspects of medical practice [27].
2. Prevention of RNMB
Preventing RNMB is paramount to reducing unwanted patient adverse events in the perioperative period. First, a thorough review of the patient’s history, medications, and treatment of reversible conditions could help to prevent RNMB. Second, the patient should also be evaluated for the actual need for NMB. Third, using a shorter-acting NMB while using the minimal amount of NMB necessary to achieve the desired response may reduce RNMB [43]. This approach may lend itself to a higher probability of complete reversal at the end of a surgical case [44]. Lastly, recent literature suggests that sugammadex is superior compared to neostigmine in reducing RNMB [33] and particularly at moderate to deep forms of neuromuscular block. The American Society of Anesthesiologists 2023 Practice Guidelines suggest a systematic approach to implementation that may include quantitative monitoring in all anesthesia locations, individual and departmental education efforts, and performance feedback [33]. Use of the guidelines suggests that complete recovery from RNMB, as monitored through quantitative monitoring, may increase patient satisfaction, decrease PACU length of stay, pulmonary complications, and mortality [33].
2.1. Reversal of Neuromuscular Block
2.1.1. Neostigmine
Neostigmine is an acetylcholinesterase inhibitor that has been used for decades to reverse nondepolarizing neuromuscular blocking agents. Its advantages include that it can be used to reverse both classes of NMB, is generally cost-friendly in many countries in the world, and has a very low allergenicity rate [45]. However, it has several disadvantages. First, it requires concomitant use of anticholinergic agents such as glycopyrrolate or atropine to counteract neostigmine’s muscarinic activity that includes bronchospasm, increased gut motility, and bradycardia. Second, according to a recent ASA Practice Guideline it should be used to reverse NMB in the setting of minimal depth of neuromuscular block as defined by a TOFC of equal to 4 without fade (TOFR 0.4–0.9) [33]. It has been shown to be less effective with moderate to deep degrees of block. Third, it takes approximately 10 min to reach maximal effect and, therefore, needs to be administered with this duration kept in mind. Fourth, increasing the neostigmine dose does not always result in enhancing its reversal action due to its ceiling effect. Increasing the dose may also result in a depolarizing block given the increased availability of acetylcholine at the neuromuscular junction. Lastly, it may have a prolonged effect on patients with renal insufficiency [46].
2.1.2. Sugammadex
Sugammadex is a gamma-cyclodextrin that encapsulates aminosteroidal NMB in a 1:1 ratio. It was primarily developed to achieve a more complete recovery from aminosteroidal NMB. The sugammadex structure of eight outer tails that have a negative charge attract the positive charge on the aminosteroid molecule, facilitating an irreversible hold of the NMB in its lipophilic core. The aminosteroid-sugammadex complex is then excreted in the urine [46].
Sugammadex’s drug profile and pharmacokinetics make it an ideal reversal agent for aminosteroid NMBs. First, it does not require an additional agent like an antimuscarinic to counteract its side effects. Second, its fast onset of action can allow rapid reversal even with moderate-deep depths of block within 2 min. With larger doses (4–16 mg/kg), sugammadex can also reverse much deeper forms of NMB than neostigmine. In fact, with the appropriate dosing, sugammadex can reverse any depth of NMB created by rocuronium and vecuronium. Therefore, in situations where profound depths of NMB are used, sugammadex might be the preferred agent when using aminosteroid NMBs [46].
While sugammadex has facilitated a more predictable reversal, especially with moderate to deep depths of NMB, it also has its disadvantages. First, in a variety of countries, the drug may not be available or is too costly for hospitals to purchase. Yet, sugammadex is already released in a generic form in some countries, reducing its cost. Furthermore, cost analyses suggest that when considering the potential for reducing operating room time and adverse effects from RNMB when using sugammadex, especially in the settings of moderate to deeper depths of block, it may either be comparable to neostigmine or reduce overall hospital costs [46]. Second, with any drug shortages, the cost of glycopyrrolate and neostigmine may rival that of sugammadex. Third, it is important to consider all adverse effects of sugammadex, albeit it carries a reasonably low risk. For instance, the risk of anaphylaxis delayed the FDA approval of sugammadex in the US. The incidence has been reported to be anywhere from 0.012–0.0016% [46]. The clinical signs are hypotension, flushing, and bronchospasm. In addition, reports of profound bradycardia leading to cardiac arrest were reported in the literature that seemed not to be connected to anaphylaxis [46].
2.1.3. Perioperative Outcomes Associated with Neostigmine vs. Sugammadex
Several randomized studies have been conducted to date (n − 10) that suggest sugammadex use is associated with a lower incidence of RNMB vs. neostigmine [33]. In addition, several studies suggest that acceptable recovery to a TOF ≥ 0.9 occurs in a shorter period of time with the use of sugammadex vs. neostigmine and in the presence of deep, moderate, and shallow forms of block [33]. However, further studies are required to clearly delineate the benefit with regards to efficacy, safety, and cost when choosing sugammadex vs. neostigmine, particularly in the setting of shallow depths of block. Low strength of evidence suggested a lower incidence of pneumonia with sugammadex vs. neostigmine use [33]. However, when pooling seven non-randomized and six randomized studies, the evidence to date does not suggest a clear difference in overall pulmonary complications (including respiratory failure, hypoxia, infection atelectasis, aspiration pneumonia, bronchospasm or pulmonary edema) with the use of sugammadex vs. neostigmine [33]. Similarly, overall significant differences were not detected among the current studies available that evaluated the presence of tachycardia, bradycardia, hypertension, or arrhythmias when using sugammadex vs. neostigmine (with concomitant use of an antimuscarinic agent). Similarly, there was not an overall significant difference in the incidence of postoperative nausea and vomiting when using sugammadex vs. neostigmine [33].
Given the available evidence, the American Society of Anesthesiologists Practice Guideline was recently released and provides the following overall recommendations for anesthesia professionals with regard to reversal of NMB. A strong recommendation with a moderate level of evidence was provided for the statement that sugammadex should be used over neostigmine to avoid residual neuromuscular block at deep, moderate, and shallow depths of neuromuscular block. With a conditional recommendation with a low level of evidence, the ASA Practice Guideline suggested that neostigmine is a reasonable alternative to sugammadex at minimal depths of NMB. Patients who have achieved a qTOFR ≥ 0.9 do not require any reversal agents. Further studies are forthcoming with regards to comparing patient outcomes with sugammadex and neostigmine at shallow depths of block. In addition, studies should further evaluate the routine avoidance of reversal in patients with qTOFR ≥ 0.9. Therefore, it is suggested that patients who receive neuromuscular blockade during surgery should be reversed unless they achieve the TOF ≥ 0.9. Lastly, further trials investigating the association of RNMB and postoperative pulmonary complications are required and especially in high-risk surgical populations such as those with sleep apnea, morbid obesity, and chronic pulmonary disease [33].
References
- Blobner, M.; Frick, C.G.; Stauble, R.B.; Feussner, H.; Schaller, S.J.; Unterbuchner, C.; Lingg, C.; Geisler, M.; Fink, H. Neuromuscular blockade improves surgical conditions (NISCO). Surg. Endosc. 2015, 29, 627–636.
- Geller, B.J.; Maciel, C.B.; May, T.L.; Jentzer, J.C. Sedation and shivering management after cardiac arrest. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 518–524.
- Tezcan, B.; Turan, S.; Ozgok, A. Current Use of Neuromuscular Blocking Agents in Intensive Care Units. Turk. J. Anaesthesiol. Reanim. 2019, 47, 273–281.
- Harlan, S.S.; Philpott, C.D.; Foertsch, M.J.; Takieddine, S.C.; Harger Dykes, N.J. Sugammadex Efficacy and Dosing for Rocuronium Reversal Outside of Perioperative Settings. Hosp. Pharm. 2023, 58, 194–199.
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Shear, T.; Vender, J.S.; Gray, J.; Landry, E. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth. Analg. 2013, 117, 133–141.
- Brull, S.J.; Murphy, G.S. Residual neuromuscular block: Lessons unlearned. Part II: Methods to reduce the risk of residual weakness. Anesth. Analg. 2010, 111, 129–140.
- Finkel, R.; Clark, M.A.; Cubeddu, L.X. Pharmacology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA; London, UK,, 2009; p. 564.
- Jung, K.T.; An, T.H. Updated review of resistance to neuromuscular blocking agents. Anesth. Pain Med. 2018, 13, 122–127.
- Boon, M.; Martini, C.; Dahan, A. Recent advances in neuromuscular block during anesthesia. F1000Research 2018, 7, 167.
- Sturgill, E.L.; Campbell, N.F. Neuromuscular Blocking and Reversal Agents. In Basic Clinical Anesthesia; Sikka, P.K., Beaman, S.T., Street, J.A., Eds.; Springer: New York, NY, USA, 2015; pp. 151–158.
- D’Souza, R.S.; Porter, B.R.; Johnson, R.L. Nondepolarizing Paralytics. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519510/ (accessed on 5 September 2023).
- Brull, S.J. Neuromuscular blocking agents. In Clinical Anesthesia, 8th ed.; Barash, P.G., Cullen, B.F., Stoelting, R.K., Ortega, R.A., Cahalan, M.K., Holt, N.F., Stock, M.C., Sharar, S.R., Eds.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2017; p. 527.
- Yamauchi, M.; Takahashi, H.; Iwasaki, H.; Namiki, A. Respiratory acidosis prolongs, while alkalosis shortens, the duration and recovery time of vecuronium in humans. J. Clin. Anesth. 2002, 14, 98–101.
- Katz, B.; Miledi, R. The Effect of Calcium on Acetylcholine Release from Motor Nerve Terminals. Proc. R. Soc. Lond. B Biol. Sci. 1965, 161, 496–503.
- Weber, V.; Abbott, T.E.F.; Ackland, G.L. Reducing the dose of neuromuscular blocking agents with adjuncts: A systematic review and meta-analysis. Br. J. Anaesth. 2021, 126, 608–621.
- Hill, G.E.; Wong, K.C.; Shaw, C.L.; Blatnick, R.A. Acute and chronic changes in intra- and extracellular potassium and responses to neuromuscular blocking agents. Anesth. Analg. 1978, 57, 417–421.
- Caldwell, J.E.; Heier, T.; Wright, P.M.; Lin, S.; McCarthy, G.; Szenohradszky, J.; Sharma, M.L.; Hing, J.P.; Schroeder, M.; Sessler, D.I. Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology 2000, 92, 84–93.
- Rupp, S.M.; Miller, R.D.; Gencarelli, P.J. Vecuronium-induced neuromuscular blockade during enflurane, isoflurane, and halothane anesthesia in humans. Anesthesiology 1984, 60, 102–105.
- Burkett, L.; Bikhazi, G.B.; Thomas, K.C., Jr.; Rosenthal, D.A.; Wirta, M.G.; Foldes, F.F. Mutual potentiation of the neuromuscular effects of antibiotics and relaxants. Anesth. Analg. 1979, 58, 107–115.
- Muller, T.C.; Rocha, J.B.; Morsch, V.M.; Neis, R.T.; Schetinger, M.R. Antidepressants inhibit human acetylcholinesterase and butyrylcholinesterase activity. Biochim. Biophys. Acta 2002, 1587, 92–98.
- Usubiaga, J.E.; Standaert, F. The effects of local anesthetics on motor nerve terminals. J. Pharmacol. Exp. Ther. 1968, 159, 353–361.
- Bash, L.D.; Turzhitsky, V.; Black, W.; Urman, R.D. Neuromuscular Blockade and Reversal Agent Practice Variability in the US Inpatient Surgical Settings. Adv. Ther. 2021, 38, 4736–4755.
- Bevan, D.R.; Donati, F. Muscle Relaxants. In Clinical Anesthesia, 4th ed.; Barash, P.G., Cullen, B.F., Stoelting, R.K., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 419–447.
- Murphy, G.S.; Brull, S.J. Residual neuromuscular block: Lessons unlearned. Part I: Definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth. Analg. 2010, 111, 120–128.
- Hile, G.B.; Ostinowsky, M.E.; Sandusky, N.P.; Howington, G.T. Evaluation of Sugammadex Dosing for Neurological Examination in the Emergency Department. J. Pharm. Pract. 2023. online ahead of print.
- Ali, H.H.; Utting, J.E.; Gray, C. Stimulus frequency in the detection of neuromuscular block in humans. Br. J. Anaesth. 1970, 42, 967–978.
- Naguib, M.; Kopman, A.F.; Lien, C.A.; Hunter, J.M.; Lopez, A.; Brull, S.J. A survey of current management of neuromuscular block in the United States and Europe. Anesth. Analg. 2010, 111, 110–119.
- Kopman, A.F.; Yee, P.S.; Neuman, G.G. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology 1997, 86, 765–771.
- Heier, T.; Caldwell, J.E.; Feiner, J.R.; Liu, L.; Ward, T.; Wright, P.M. Relationship between normalized adductor pollicis train-of-four ratio and manifestations of residual neuromuscular block: A study using acceleromyography during near steady-state concentrations of mivacurium. Anesthesiology 2010, 113, 825–832.
- Eriksson, L.I.; Sato, M.; Severinghaus, J.W. Effect of a vecuronium-induced partial neuromuscular block on hypoxic ventilatory response. Anesthesiology 1993, 78, 693–699.
- Eriksson, L.I.; Sundman, E.; Olsson, R.; Nilsson, L.; Witt, H.; Ekberg, O.; Kuylenstierna, R. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: Simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology 1997, 87, 1035–1043.
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Marymont, J.H.; Vender, J.S.; Gray, J.; Landry, E.; Gupta, D.K. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology 2011, 115, 946–954.
- Thilen, S.R.; Weigel, W.A.; Todd, M.M.; Dutton, R.P.; Lien, C.A.; Grant, S.A.; Szokol, J.W.; Eriksson, L.I.; Yaster, M.; Grant, M.D.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A Report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023, 138, 13–41.
- Naguib, M.; Brull, S.J.; Kopman, A.F.; Hunter, J.M.; Fulesdi, B.; Arkes, H.R.; Elstein, A.; Todd, M.M.; Johnson, K.B. Consensus Statement on Perioperative Use of Neuromuscular Monitoring. Anesth. Analg. 2018, 127, 71–80.
- Liu, H.-M.; Yu, H.; Zuo, Y.-D.; Liang, P. Postoperative pulmonary complications after sugammadex reversal of neuromuscular blockade: A systematic review and meta-analysis with trial sequential analysis. BMC Anesthesiol. 2023, 23, 130.
- Kopman, A.F.; Brull, S.J. Is Postoperative Residual Neuromuscular Block Associated with Adverse Clinical Outcomes? What Is the Evidence? Curr. Anesthesiol. Rep. 2013, 3, 114–121.
- Caruso, L.; Faulk, D.; Lampotang, S.; Lizdas, D.E.; Gravenstein, N. Quantitative Neuromuscular Blockade. APSF Technology Education Initiative (TEI): Quantitative Neuromuscular Monitoring (QNM); published by the Anesthesia Patient Safety Foundation (APSF) in collaboration with the American Society of Anesthesiologists (ASA). Online Educational Simulation Environment. 2023. Available online: https://apsf.org/tei/qnm (accessed on 22 November 2023).
- Saager, L.; Maiese, E.M.; Bash, L.D.; Meyer, T.A.; Minkowitz, H.; Groudine, S.; Philip, B.K.; Tanaka, P.; Gan, T.J.; Rodriguez-Blanco, Y.; et al. Incidence, risk factors, and consequences of residual neuromuscular block in the United States: The prospective, observational, multicenter RECITE-US study. J. Clin. Anesth. 2019, 55, 33–41.
- Grayling, M.; Sweeney, B.P. Recovery from neuromuscular blockade: A survey of practice. Anaesthesia 2007, 62, 806–809.
- Naguib, M.; Kopman, A.F.; Ensor, J.E. Neuromuscular monitoring and postoperative residual curarisation: A meta-analysis. Br. J. Anaesth. 2007, 98, 302–316.
- Baillard, C.; Gehan, G.; Reboul-Marty, J.; Larmignat, P.; Samama, C.M.; Cupa, M. Residual curarization in the recovery room after vecuronium. Br. J. Anaesth. 2000, 84, 394–395.
- Naguib, M.; Brull, S.J.; Hunter, J.M.; Kopman, A.F.; Fülesdi, B.; Johnson, K.B.; Arkes, H.R. Anesthesiologists’ Overconfidence in Their Perceived Knowledge of Neuromuscular Monitoring and Its Relevance to All Aspects of Medical Practice: An International Survey. Anesth. Analg. 2019, 128, 1118–1126.
- Thilen, S.R.; Bhananker, S.M. Qualitative Neuromuscular Monitoring: How to Optimize the Use of a Peripheral Nerve Stimulator to Reduce the Risk of Residual Neuromuscular Blockade. Curr. Anesthesiol. Rep. 2016, 6, 164–169.
- Brull, S.J.; Kopman, A.F.; Naguib, M. Management Principles to Reduce the Risk of Residual Neuromuscular Blockade. Curr. Anesthesiol. Rep. 2013, 3, 130–138.
- Ji, W.; Zhang, X.; Liu, J.; Sun, G.; Wang, X.; Bo, L.; Deng, X. Efficacy and safety of neostigmine for neuromuscular blockade reversal in patients under general anesthesia: A systematic review and meta-analysis. Ann. Transl. Med. 2021, 9, 1691.
- Hunter, J.M. Reversal of neuromuscular block. Br. J. Anaesth. 2020, 20, 259–265.
More
Information
Subjects:
Anesthesiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
803
Revisions:
3 times
(View History)
Update Date:
26 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




