You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Savino Spadaro | -- | 2459 | 2024-02-15 09:15:40 | | | |
| 2 | Lindsay Dong | Meta information modification | 2459 | 2024-02-18 01:46:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spadaro, S.; Jimenez-Santana, J.D.; La Rosa, R.; Spinazzola, G.; Argente Navarro, P.; Volta, C.A.; Scaramuzzo, G. Prone Positioning in COVID and Non-COVID ARDS. Encyclopedia. Available online: https://encyclopedia.pub/entry/55062 (accessed on 29 December 2025).
Spadaro S, Jimenez-Santana JD, La Rosa R, Spinazzola G, Argente Navarro P, Volta CA, et al. Prone Positioning in COVID and Non-COVID ARDS. Encyclopedia. Available at: https://encyclopedia.pub/entry/55062. Accessed December 29, 2025.
Spadaro, Savino, Jose Daniel Jimenez-Santana, Riccardo La Rosa, Giorgia Spinazzola, Pilar Argente Navarro, Carlo Alberto Volta, Gaetano Scaramuzzo. "Prone Positioning in COVID and Non-COVID ARDS" Encyclopedia, https://encyclopedia.pub/entry/55062 (accessed December 29, 2025).
Spadaro, S., Jimenez-Santana, J.D., La Rosa, R., Spinazzola, G., Argente Navarro, P., Volta, C.A., & Scaramuzzo, G. (2024, February 15). Prone Positioning in COVID and Non-COVID ARDS. In Encyclopedia. https://encyclopedia.pub/entry/55062
Spadaro, Savino, et al. "Prone Positioning in COVID and Non-COVID ARDS." Encyclopedia. Web. 15 February, 2024.
Copy Citation
Prone positioning (PP) represents a therapeutic intervention with the proven capacity of ameliorating gas exchanges and ventilatory mechanics indicated in acute respiratory distress syndrome (ARDS). When PP is selectively applied to moderate-severe cases of ARDS, it sensitively affects clinical outcomes, including mortality.
prone position
biomarkers
ARDS
COVID-19
1. Background
Prone positioning (PP) represents a therapeutic strategy originally described in critically ill patients in 1976 by Margaret Piehl [1]. In almost fifty years, research has extensively focused on understanding the physiological effects of this maneuver. Prone positioning indeed mitigates the pathological alterations of acute respiratory distress syndrome (ARDS), affecting the outcome in moderate-to-severe cases. After initial reports of oxygenation improvement [2][3], cornerstone studies such as the PROSEVA trial demonstrated that PP could reduce mortality in ARDS [4].
Physiologically, the prone position takes advantage of the anatomical features of the chest wall and of the inhomogeneous ventro-dorsal distribution of lung disease, permitting the optimization of ventilation/perfusion distribution [5]. Additionally, hemodynamic changes, such as the recruitment of intrathoracic vasculature and better right ventricle function, may contribute to the overall benefits of PP [6].
To date, a consensus exists on considering a PaO2/FiO2 ratio < 150 mmHg with positive end-expiratory pressure (PEEP) > 5 cm H2O as a strong indication to initiate PP [7][8]. These conditions were recently included in the latest European guidelines on ARDS management [9]. Evidence also exists on the timing to start PP, pointing towards a greater benefit in the earlier initiation of the maneuver [10]. An earlier initiation of PP may lie in the optimization of ventilation to effectively overcome the regional pulmonary stress and strain before the occurrence of fibrotic alterations in the later stage of the disease [8][11][12].
Despite the PROSEVA trial remaining the reference for PP interruption criteria (i.e., achieving PaO2/FiO2 > 150 mmHg with FiO2 < 0.6 and PEEP < 10 cm H2O in the supine position) [4], many aspects are still subject to debate. For example, the duration of a prone position to maximize its benefit is still controversial. Evidence supports that PP should be maintained for 12–16 h to obtain clinically relevant improvement in oxygenation and respiratory mechanics [13][14]. Karlis et al. [15] prospectively studied the differences between standard and prolonged PP in COVID-19 ARDS (C-ARDS) patients, finding no significant differences in respiratory mechanics improvement nor in 28-day mortality.
According to the most recent guidelines [9], cessation of the prone position is still guided by oxygenation improvement only. A recommendation exists on prolonging PP over 16 h of duration, when possible, to achieve the most benefit on clinical outcomes. However, prolonged PP also carries a higher risk for pressure injuries and damage to the nervous plexes and may also require deeper sedation and hamper enteral feeding [16]. It is possible that PaO2/FiO2 improvement does not fully reflect the changes in the lung when exposed to prolonged PP.
ARDS severity may progress to the point of being refractory to improvement with protective ventilation and a prone position. In these cases, extracorporeal support with veno-venous ECMO (vv-ECMO) may represent a life-saving therapeutic approach. Criteria for initiation of vv-ECMO in ARDS of different etiologies mirror the criteria designed for the EOLIA trial [16], which represents one of the cornerstone trials on this matter. Ultra-protective ventilation and minimization of lung injury could potentially mediate the effects of vv-ECMO on lung parenchyma [7][17].
The pandemic outbreak of COVID-19 represented an overwhelming burden for healthcare workers worldwide and challenged the approach to ARDS treatment, including the use of prone positioning. Before the pandemics, prone positioning was employed in up to 7.9% of ARDS cases (16.3% in severe ARDS) according to the LUNG SAFE study [18] and over 13.7% (32.9% in severe cases) in the APRONET study [19]. After the COVID-19 outbreak, clinical application of PP has reached 60% of treated cases, according to the PRoVENT-COVID study [20]. This outstanding increase has been linked to specific pathological changes pertaining to COVID-19 ARDS [21][22].
Biological markers have been extensively described for both COVID and non-COVID ARDS [23]. Biomarker expression patterns may reflect different stages and phenotypes of ARDS. These patterns may also reveal different responses to clinical interventions, including prone positioning.
2. Oxygenation and Ventilatory Mechanics in Prone Position
Improvement in gas exchange, as reported by the PaO2 increase, was the first therapeutic benefit described for the prone position [1]. The PaO2/FiO2 ratio continues to be a pivotal criterion in the starting prone position [9][24]. However, increased alveolar oxygen diffusion may be the result of several macro- and microscopic physiological modifications that the lung parenchyma undergoes during prone positioning [25]. Optimization of ventilatory/perfusion matching (V/Q ratio) and reduction of intrapulmonary shunt represent key elements of the process [26][27] (Figure 1).
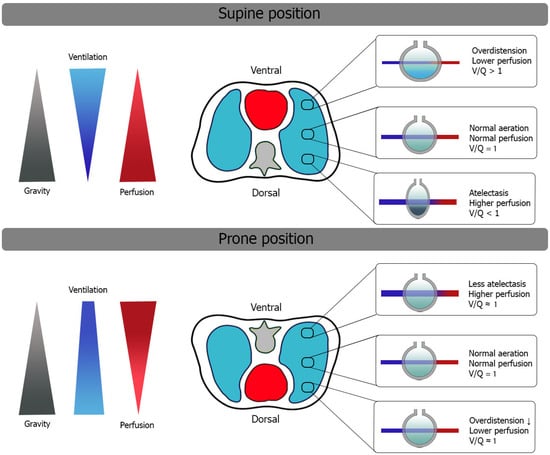
Figure 1. Changes in V/Q ratio after initiation of PP in mechanically ventilated patients with ARDS. Redistribution of ventilation more homogeneously helps the overall reduction of V/Q mismatch. See the text for details.
The human rib cage consists of a ventral area composed of ribs and sternum and a dorsal area, including the spine and scapular surfaces, which is also less compliant with its intrinsic structure. Cranially, the thoracic cage is vertex-shaped, whereas caudally, it is limited by the diaphragm that separates the abdominal and thoracic cavities. Alveolar and vascular distributions are also inhomogeneous within the thorax; dependent regions contain higher alveolar and vascular densities with a limited contribution of gravity to the overall V/Q ratio [28][29][30][31].
In the supine position, gravity impacts the distribution of inflammatory edema, increasing its content in the dependent regions and redistributing ventilation preferentially to non-dependent areas, where transpulmonary pressure is lower and the ventral rib cage has greater compliance [24].
These macroscopic modifications ultimately lead to dorsal collapse, which is evident on CT imaging of ARDS [32][33]. (Figure 2) Lung protective ventilation strategies with lower tidal volume and a moderate-to-high PEEP level demonstrated significant benefit in this setting [34]. However, in patients with moderate-severe ARDS, disease progression makes lung protective ventilation insufficient, requiring damaging pressures to maintain sufficient gas exchange, possibly meeting the criteria for starting in the prone position.
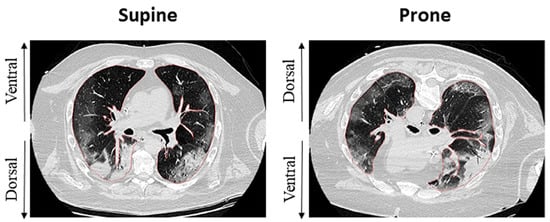
Figure 2. Effects of the prone position on recruitment and ventilation distribution in a patient with C-ARDS. Inhomogeneity of dorsal regions appears reduced after shift to PP, whereas ventral regions appear less recruited, possibly by means of reduced overdistention. Adapted from Fossali T. et al. [35].
In the prone position, the inversion of the ventral and dorsal lungs redistributes the mechanical characteristics of the chest wall, decreasing its overall compliance [23] and changing its interaction with the lungs. Inversion of gravitational forces on parenchyma has been demonstrated on CT studies [24], and cardiac compression on lung parenchyma is also released. Changes in respiratory system compliance depend not only on the opening of previously unrecruited parenchyma but also on the improved mechanical behavior of already-opened alveoli [33][36]. When these changes occur together, the respiratory system moves towards a more favorable position, and total stress and strain may redistribute more homogeneously [30]. The reduction of airway plateau pressure after prone positioning thus acts as an indirect indicator of improved respiratory system compliance [36]. Pleural pressures also show a more homogeneous gravitational gradient in the prone position than in the supine position, possibly as a result of regional improvements in ventilation distribution [36][37].
Therefore, regional ventilation redistribution acts as the primary mechanism for V/Q homogeneization in the prone position.
The redistribution of ventilation also plays an important role in the distribution of airway pressures. Therefore, in the prone position, the application of positive end-expiratory pressure (PEEP) has a lower probability of causing regional hyperinflation [38]. Protective ventilation is thus enhanced during prone positioning, providing better protection from ventilator-induced lung injury (VILI) [25][26][28]. Homogenization of ventilation may also reduce the alveolar hypoxemic reflex and may favor nitric oxide production in the capillaries of the posterior and inferior lung regions [29].
Hemodynamic changes also play an important role in the prone position [39]. Beneficial hemodynamic changes may indeed counteract some of the pathologic features of ARDS. Huang et al. [40] have reported the results of an echocardiographic assessment after an observational study of a large cohort of patients with COVID-19. They reflect the high incidence of pathologic echocardiographic findings in patients with moderate-severe C-ARDS. Huang et al. postulate that in C-ARDS, the heart suffers direct insult by the systemic inflammatory state (i.e., septic cardiomyopathy with LV systolic dysfunction), but also indirect damage caused by distress on the pulmonary vasculature (i.e., RV dysfunction, RV failure, and acute cor pulmonale (ACP)). In the post-hoc analysis of the ECHO-COVID study [41], they analyzed the different types of right ventricular involvement, concluding that of the wide variety of RV pathologies due to C-ARDS, ACP was associated with worse outcomes.
Contradictory results have been reported regarding PaCO2 changes during PP. By homogenizing ventilation distribution, a reduction in alveolar dead space and arterial CO2 is expected. However, the reduction in rib cage compliance may lead to a lower Vt in the case of pressure-controlled ventilation, producing lower minute ventilation. On the other hand, in the case of volume-controlled ventilation, this reduction in rib cage compliance can lead to an increase in pleural pressure that hinders venous return and thus produces an increase in dead space due to reduced pulmonary regional perfusion [42]. The value of PaCO2 reduction as an indicator of net lung recruitment is such that it has been directly related to a decrease in 28-day mortality [32][34][43][44].
In conclusion, the prone position ameliorates gas exchange, ventilatory mechanics, and protective mechanical ventilation in moderate-severe ARDS. At the tissue level, this translates into less stress and strain on the lung parenchyma, potentially modulating inflammatory stimuli. Understanding these macroscopic changes in ventilatory mechanics helps understand the microscopic effects of PP, which may be reflected by the expression of different biomarkers.
3. Biomarkers in COVID ARDS and Non-COVID ARDS
ARDS is characterized by pathological alteration of the alveolar-capillary membrane (epithelial and endothelial damage) leading to pulmonary edema with protein-rich fluids (unfit for the alveolar and interstitial space), cell migration, and disseminated compressive atelectasis with lung collapse [45][46][47]. The inflammatory response that takes place in lung parenchyma arises from a primary insult and amplifies over time. Mechanical ventilation can potentially contribute to parenchymal stress and overall biotrauma [48].
The soluble receptor for advanced glycation end-products (sRAGE) represents an interesting example of a soluble plasma biomarker characterizing the acute inflammatory phase of ARDS [49]. This receptor is highly expressed by alveolar type-I cells and acts as an intercellular signaling molecule, mediating inflammatory response propagation. Increasing levels of sRAGE correlate with the severity of ARDS, potentially representing a marker of lung epithelial damage regardless of etiology [50][51].
Similarly to sRAGE, Surfactant Protein D (SP-D) behaves as a tissue-specific biomarker in ARDS, with an acute rise as epithelial damage occurs [52]. Interestingly, SP-D increases during acute lung injury and seems attenuated when protective mechanical ventilation is guaranteed, thus acting as indirect information on ventilator-induced lung injury (VILI) [53].
Markers of the inflammatory cascade represent another valuable source of information for ARDS patients. Interleukin (IL) signaling plays a key role in inflammatory modulation. As a result, cytokines such as IL-8, IL-6, and IL-1B have been studied in different ARDS models [49]. IL-8 has been isolated in BALF of patients at risk for ARDS, and its levels correlate with disease severity [54][55]. IL-8 holds diagnostic potential for ARDS, especially when combined with other biomarkers such as SP-D and sRAGE [56].
A dysregulated inflammatory response is also a hallmark alteration in severe COVID-19 cases, especially when causing ARDS [57][58][59]. IL-6 elevation in plasma has been extensively studied in C-ARDS, showing a direct correlation with disease severity [57]. IL-6 also represents a key example of a biomarker becoming a pharmacological target [60].
Systemic immune dysregulation has also been investigated as a potential therapeutic target for hemopurification (HP) strategies. Different HP techniques exist and can be tailored to patients’ needs [61][62].
C-ARDS represents nowadays a distinct subphenotype of ARDS with specific pathogenic patterns. Indeed, Sars-COV-2 has tropisms for molecules such as Angiotensin Converting Enzyme-2 (ACE2) and Transmembrane Serine Proteases (TMPRSS2) [63][64]. This mainly affects type-II alveolar cells, decreasing pulmonary surfactant production and hindering regeneration of the epithelium [58].
Endothelial damage may, therefore, be a characteristic feature of C-ARDS. Indeed, the predominance of biomarker-measured endothelial damage over alveolar epithelial damage has been demonstrated with differential levels of biomarkers such as ANG-2 and ICAM-1 (intercellular soluble adhesion molecule-1) [58].
At the molecular level, recent research has also focused on how Sars-Cov2 may trigger an exaggerated inflammatory response and how this affects cellular functioning. Indeed, evidence points towards the possibility that Sars-Cov2 may activate multiple pro-apoptotic signaling pathways, including lipid peroxidation and altered iron metabolism (i.e., ferroptosis) [65]. Radical oxygen species (ROS) are increasingly produced intracellularly in dysregulated inflammatory responses.
4. Prone Positioning and Biomarkers in COVID-19 and Non-COVID-19 ARDS
An ideal biomarker should give us relevant information about the onset and course of the pathology, should have a sensitivity and specificity close to 100%, and should provide information during the course of the disease on the response to treatment [66]. In ARDS, the goal is to find biomarkers that allow us to assess severity, the progression to more severe phenotypes, and predict outcomes.
The fundamental characteristic of biomarkers is that they reflect the pathophysiology of the disease. In-depth knowledge of the molecular aspects of non-COVID ARDS and C-ARDS is what allows us to understand the role of biomarkers. In turn, the discovery of certain biomarkers in relation to each disease has helped in understanding and learning about these pathologies [66][67].
As mentioned, prone positioning is especially indicated in the diffuse alveolar damage phase of ARDS, when there is still lung parenchymal recruitability before fibrosis is reached. The prone position can allow the use of lower FiO2 due to better oxygenation, less pulmonary regional stress and strain, and better vascularization of the ventilated alveoli [68][69]. These improvements would presumably lead to a lower inflammatory state compared to the supine position, with less alveolar endothelial and epithelial damage.
Evaluating the effect of atelectasis on proteomics is fundamental to understanding the biological alterations generated by ARDS. Rashid et al. [70] studied proteomics following a model of acute lung damage generated with lipopolysaccharide (LPS) and endotoxin. They were able to reflect how atelectasis in these cases has a clear effect on increasing inflammation and dysfunction of the alveolar-capillary membrane. They detected a high expression of a wide range of pro-inflammatory interleukins (IL-6 and IL-20), signaling molecules (MAPK12 and STAT 1), inflammatory mediators (MPO, BTK, and RAGE), and chemokines (CXCL11 and CCL5), as well as increased leukocyte migration. As markers of alveolar-capillary dysfunction, they found extracellular matrix glycoproteins, vascular endothelial growth factor, and fibrinogen independently of the presence of systemic endotoxemia
5. Conclusions
In conclusion, the prone position ameliorates gas exchange and ventilatory mechanics, enhancing protective mechanical ventilation in moderate-severe ARDS. At the tissue level, this translates into less stress and strain on the lung parenchyma, potentially modulating inflammatory stimuli. Biomarker expression patterns may reflect different stages and phenotypes of ARDS. These patterns may also reveal different responses to clinical interventions, including prone positioning. The evidence available to date only confirms IL-6 as a useful marker for predicting short-term mortality in the prone position.
References
- Piehl, M.A.; Brown, R.S. Use of extreme position changes in acute respiratory failure. Crit. Care Med. 1976, 4, 13–14.
- Gattinoni, L.; Tognoni, G.; Pesenti, A.; Taccone, P.; Mascheroni, D.; Labarta, V.; Malacrida, R.; Di Giulio, P.; Fumagalli, R.; Pelosi, P.; et al. Effect of Prone Positioning on the Survival of Patients with Acute Respiratory Failure. N. Engl. J. Med. 2001, 345, 568–573.
- Guerin, C.; Gaillard, S.; Lemasson, S.; Ayzac, L.; Girard, R.; Beuret, P.; Palmier, B.; Le, Q.V.; Sirodot, M.; Rosselli, S.; et al. Effects of Systematic Prone Positioning in Hypoxemic Acute Respiratory Failure: A Randomized Controlled Trial. JAMA 2004, 292, 2379–2387.
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168.
- Gattinoni, L.; Brusatori, S.; D’albo, R.; Maj, R.; Velati, M.; Zinnato, C.; Gattarello, S.; Lombardo, F.; Fratti, I.; Romitti, F.; et al. Prone position: How understanding and clinical application of a technique progress with time. Anesthesiol. Perioper. Sci. 2023, 1, 3.
- Jozwiak, M.; Teboul, J.-L.; Anguel, N.; Persichini, R.; Silva, S.; Chemla, D.; Richard, C.; Monnet, X. Beneficial Hemodynamic Effects of Prone Positioning in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2013, 188, 1428–1433.
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69.
- Gattinoni, L.; Busana, M.; Giosa, L.; Macrì, M.M.; Quintel, M. Prone Positioning in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 094–100.
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759.
- Camporota, L.; Sanderson, B.B.; Chiumello, D.; Terzi, N.; Argaud, L.; Rimmelé, T.; Metuor, R.; Verstraete, A.; Cour, M.; Bohé, J.; et al. Prone Position in COVID-19 and -COVID-19 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. Crit. Care Med. 2021, 50, 633–643.
- Johannigman, J.A.; Davis, K.; Miller, S.L.; Campbell, R.S.; Luchette, F.A.; Frame, S.B.; Branson, R.D. Prone positioning for acute respiratory distress syndrome in the surgical intensive care unit: Who, when, and how long? Surgery 2000, 128, 708–716.
- Pelosi, P.; Brazzi, L.; Gattinoni, L. Prone position in acute respiratory distress syndrome. Eur. Respir. J. 2002, 20, 1017–1028.
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288.
- Spadaro, S.; Scaramuzzo, G.; Volta, C.A. Prone the Lung and Keep It Prone! Chest 2023, 163, 469–470.
- Karlis, G.; Markantonaki, D.; Kakavas, S.; Bakali, D.; Katsagani, G.; Katsarou, T.; Kyritsis, C.; Karaouli, V.; Athanasiou, P.; Daganou, M. Prone Position Ventilation in Severe ARDS due to COVID-19: Comparison between Prolonged and Intermittent Strategies. J. Clin. Med. 2023, 12, 3526.
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975.
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363.
- Bellani, G.; On behalf of the LUNG SAFE Investigators and the ESICM Trials Group; Laffey, J.G.; Pham, T.; Fan, E. The LUNG SAFE study: A presentation of the prevalence of ARDS according to the Berlin Definition! Crit. Care 2016, 20, 268.
- For the Investigators of the APRONET Study Group; The REVA Network; The Réseau Recherche de la Société Française d’Anesthésie-Réanimation (SFAR-Recherche); The ESICM Trials Group; Guérin, C.; Beuret, P.; Constantin, J.M.; Bellani, G.; Garcia-Olivares, P.; Roca, O.; et al. A prospective international observational prevalence study on prone positioning of ARDS patients: The APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2017, 44, 22–37.
- Stilma, W.; van Meenen, D.M.P.; Valk, C.M.A.; de Bruin, H.; Paulus, F.; Neto, A.S.; Schultz, M.J.; on behalf of the PRoVENT-COVID Collaborative Group. Incidence and Practice of Early Prone Positioning in Invasively Ventilated COVID-19 Patients—Insights from the PRoVENT-COVID Observational Study. J. Clin. Med. 2021, 10, 4783.
- Schultz, M.J.; Neto, A.S.; Paulus, F. Battling COVID-19-related mortality: From a fight for ventilators to a cry for oxygen. Lancet Respir. Med. 2021, 9, 939–941.
- Constantin, J.-M.; Jabaudon, M.; Lefrant, J.-Y.; Jaber, S.; Quenot, J.-P.; Langeron, O.; Ferrandière, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880.
- Bain, W.; Yang, H.; Shah, F.A.; Suber, T.; Drohan, C.; Al-Yousif, N.; DeSensi, R.S.; Bensen, N.; Schaefer, C.; Rosborough, B.R.; et al. COVID-19 versus Non–COVID-19 Acute Respiratory Distress Syndrome: Comparison of Demographics, Physiologic Parameters, Inflammatory Biomarkers, and Clinical Outcomes. Ann. Am. Thorac. Soc. 2021, 18, 1202–1210.
- Gattinoni, L.; Taccone, P.; Carlesso, E.; Marini, J.J. Prone Position in Acute Respiratory Distress Syndrome. Rationale, Indications, and Limits. Am. J. Respir. Crit. Care Med. 2013, 188, 1286–1293.
- Lamm, W.J.; Graham, M.M.; Albert, R.K. Mechanism by which the prone position improves oxygenation in acute lung injury. Am. J. Respir. Crit. Care Med. 1994, 150, 184–193.
- Nyrén, S.; Mure, M.; Jacobsson, H.; Larsson, S.A.; Lindahl, S.G.E.; Henderson, A.C.; Sá, R.C.; Theilmann, R.J.; Buxton, R.B.; Prisk, G.K.; et al. Pulmonary perfusion is more uniform in the prone than in the supine position: Scintigraphy in healthy humans. J. Appl. Physiol. 1999, 86, 1135–1141.
- Yuan, X.; Zhao, Z.; Chao, Y.; Chen, D.; Chen, H.; Zhang, R.; Liu, S.; Xie, J.; Yang, Y.; Qiu, H.; et al. Effects of early versus delayed application of prone position on ventilation–perfusion mismatch in patients with acute respiratory distress syndrome: A prospective observational study. Crit. Care 2023, 27, 462.
- Albert, R.K.; Hubmayr, R.D. The Prone Position Eliminates Compression of the Lungs by the Heart. Am. J. Respir. Crit. Care Med. 2000, 161, 1660–1665.
- Rimeika, D.; Nyrén, S.; Wiklund, N.P.; Koskela, L.R.; Tørring, A.; Gustafsson, L.E.; Larsson, S.A.; Jacobsson, H.; Lindahl, S.G.E.; Wiklund, C.U. Regulation of Regional Lung Perfusion by Nitric Oxide. Am. J. Respir. Crit. Care Med. 2004, 170, 450–455.
- Scaramuzzo, G.; Ball, L.; Pino, F.; Ricci, L.; Larsson, A.; Guérin, C.; Pelosi, P.; Perchiazzi, G. Influence of Positive End-Expiratory Pressure Titration on the Effects of Pronation in Acute Respiratory Distress Syndrome: A Comprehensive Experimental Study. Front. Physiol. 2020, 11, 179.
- Scaramuzzo, G.; Broche, L.; Pellegrini, M.; Porra, L.; Derosa, S.; Tannoia, A.P.; Marzullo, A.; Borges, J.B.; Bayat, S.; Bravin, A.; et al. Regional Behavior of Airspaces During Positive Pressure Reduction Assessed by Synchrotron Radiation Computed Tomography. Front. Physiol. 2019, 10, 719.
- Vollenberg, R.; Matern, P.; Nowacki, T.; Fuhrmann, V.; Padberg, J.-S.; Ochs, K.; Schütte-Nütgen, K.; Strauß, M.; Schmidt, H.; Tepasse, P.-R. Prone Position in Mechanically Ventilated COVID-19 Patients: A Multicenter Study. J. Clin. Med. 2021, 10, 1046.
- Pelosi, P.; D’Andrea, L.; Vitale, G.; Pesenti, A.; Gattinoni, L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 149, 8–13.
- Nuckton, T.J.; Alonso, J.A.; Kallet, R.H.; Daniel, B.M.; Pittet, J.-F.; Isner, M.D.E.; Matthay, M.A. Pulmonary Dead-Space Fraction as a Risk Factor for Death in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2002, 346, 1281–1286.
- Fossali, T.; Pavlovsky, B.; Ottolina, D.; Colombo, R.; Basile, M.C.; Castelli, A.; Rech, R.; Borghi, B.; Ianniello, A.; Flor, N.; et al. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit. Care Med. 2022, 50, 723–732.
- Chiumello, D.; Marino, A.; Brioni, M.; Cigada, I.; Menga, F.; Colombo, A.; Crimella, F.; Algieri, I.; Cressoni, M.; Carlesso, E.; et al. Lung Recruitment Assessed by Respiratory Mechanics and Computed Tomography in Patients with Acute Respiratory Distress Syndrome. What Is the Relationship? Am. J. Respir. Crit. Care Med. 2016, 193, 1254–1263.
- Scaramuzzo, G.; Spadaro, S.; Waldmann, A.D.; Böhm, S.H.; Ragazzi, R.; Marangoni, E.; Alvisi, V.; Spinelli, E.; Mauri, T.; Volta, C.A. Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit. Care 2019, 23, 119.
- Cornejo, R.A.; Díaz, J.C.; Tobar, E.A.; Bruhn, A.R.; Ramos, C.A.; González, R.A.; Repetto, C.A.; Romero, C.M.; Gálvez, L.R.; Llanos, O.; et al. Effects of Prone Positioning on Lung Protection in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2013, 188, 440–448.
- Hering, R.; Wrigge, H.; Vorwerk, R.; Brensing, K.A.; Schröder, S.; Zinserling, J.; Hoeft, A.; Spiegel, T.V.; Putensen, C. The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth. Analg. 2001, 92, 1226–1231.
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678.
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Dessap, A.M.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956.
- Guérin, C.; Albert, R.K.; Beitler, J.; Gattinoni, L.; Jaber, S.; Marini, J.J.; Munshi, L.; Papazian, L.; Pesenti, A.; Vieillard-Baron, A.; et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020, 46, 2385–2396.
- Gattinoni, L.; Vagginelli, F.; Carlesso, E.; Taccone, P.; Conte, V.; Chiumello, D.; Valenza, F.; Caironi, P.; Pesenti, A. Decrease in Paco2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit. Care Med. 2003, 31, 2727–2733.
- Protti, A.; Chiumello, D.; Cressoni, M.; Carlesso, E.; Mietto, C.; Berto, V.; Lazzerini, M.; Quintel, M.; Gattinoni, L. Relationship between gas exchange response to prone position and lung recruitability during acute respiratory failure. Intensive Care Med. 2009, 35, 1011–1017.
- Tomashefski, J.F., Jr. Pulmonary Pathology of Acute Respiratory Distress Syndrome. Clin. Chest Med. 2000, 21, 435–466.
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572.
- van der Zee, P.; Rietdijk, W.; Somhorst, P.; Endeman, H.; Gommers, D. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit. Care 2020, 24, 243.
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury. Chest 2016, 150, 1109–1117.
- García-Laorden, M.I.; Lorente, J.A.; Flores, C.; Slutsky, A.S.; Villar, J. Biomarkers for the acute respiratory distress syndrome: How to make the diagnosis more precise. Ann. Transl. Med. 2017, 5, 283.
- Calfee, C.S.; Ware, L.B.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Wickersham, N.; Matthay, M.A.; Network, T.N.A. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008, 63, 1083–1089.
- Jabaudon, M.; Blondonnet, R.; Pereira, B.; Cartin-Ceba, R.; Lichtenstern, C.; Mauri, T.; Determann, R.M.; Drabek, T.; Hubmayr, R.D.; Gajic, O.; et al. Plasma sRAGE is independently associated with increased mortality in ARDS: A meta-analysis of individual patient data. Intensive Care Med. 2018, 44, 1388–1399.
- Endo, S.; Sato, N.; Nakae, H.; Yamada, Y.; Makabe, H.; Abe, H.; Imai, S.; Wakabayashi, G.; Inada, K.; Sato, S. Surfactant protein A and D (SP-A, AP-D) levels in patients with septic ARDS. Res. Commun. Mol. Pathol. Pharmacol. 2002, 111, 245–251.
- Determann, R.M.; Royakkers, A.A.; Haitsma, J.J.; Zhang, H.; Slutsky, A.S.; Ranieri, V.M.; Schultz, M.J. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm. Med. 2010, 10, 6.
- Terpstra, M.L.; Aman, J.; van Nieuw, A.; Geerten, P.; Groeneveld, A.B.J. Plasma Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Crit. Care Med. 2014, 42, 691–700.
- Ware, L.B.; Koyama, T.; Billheimer, D.D.; Wu, W.; Bernard, G.R.; Thompson, B.T.; Brower, R.G.; Standiford, T.J.; Martin, T.R.; Matthay, M.A. Prognostic and Pathogenetic Value of Combining Clinical and Biochemical Indices in Patients with Acute Lung Injury. Chest 2010, 137, 288–296.
- Calfee, C.S.; Ware, L.B.; Glidden, D.V.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Matthay, M.A. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit. Care Med. 2011, 39, 711–717.
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244.
- Acosta, R.A.H.; Garrigos, Z.E.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 Pathogenesis and Clinical Manifestations. Infect. Dis. Clin. N. Am. 2022, 36, 231–249.
- Scaramuzzo, G.; Nucera, F.; Asmundo, A.; Messina, R.; Mari, M.; Montanaro, F.; Johansen, M.D.; Monaco, F.; Fadda, G.; Tuccari, G.; et al. Cellular and molecular features of COVID-19 associated ARDS: Therapeutic relevance. J. Inflamm. 2023, 20, 11.
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Domingo, P.; Mur, I.; Mateo, G.M.; Gutierrez, M.D.M.; Pomar, V.; de Benito, N.; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; et al. Association between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499–518.
- Sanfilippo, F.; Martucci, G.; La Via, L.; Cuttone, G.; Dimarco, G.; Pulizzi, C.; Arcadipane, A.; Astuto, M. Hemoperfusion and blood purification strategies in patients with COVID-19: A systematic review. Artif. Organs 2021, 45, 1466–1476.
- Ronco, C.; Bagshaw, S.M.; Bellomo, R.; Clark, W.R.; Husain-Syed, F.; Kellum, J.A.; Ricci, Z.; Rimmelé, T.; Reis, T.; Ostermann, M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2020, 50, 17–27.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8.
- Jovandaric, M.Z.; Dokic, M.; Babovic, I.R.; Milicevic, S.; Dotlic, J.; Milosevic, B.; Culjic, M.; Andric, L.; Dimic, N.; Mitrovic, O.; et al. The Significance of COVID-19 Diseases in Lipid Metabolism Pregnancy Women and Newborns. Int. J. Mol. Sci. 2022, 23, 15098.
- Villar, J.; Blanco, J.; Kacmarek, R.M. Acute respiratory distress syndrome definition: Do we need a change? Curr. Opin. Crit. Care 2011, 17, 13–17.
- Sega, F.V.D.; Fortini, F.; Spadaro, S.; Ronzoni, L.; Zucchetti, O.; Manfrini, M.; Mikus, E.; Fogagnolo, A.; Torsani, F.; Pavasini, R.; et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients: A pilot study. Clin. Transl. Med. 2021, 11, e283.
- Selickman, J.; Vrettou, C.S.; Mentzelopoulos, S.D.; Marini, J.J. COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J. Clin. Med. 2022, 11, 4896.
- Kallet, R.H. A Comprehensive Review of Prone Position in ARDS. Respir. Care 2015, 60, 1660–1687.
- Rashid, A.; Zeng, C.; Motta-Ribeiro, G.; Dillon, S.T.; Libermann, T.A.; Lessa, M.A.; Bagchi, A.; Hutchinson, J.; Melo, M.F.V. Proteomics of lung tissue reveals differences in inflammation and alveolar-capillary barrier response between atelectasis and aerated regions. Sci. Rep. 2022, 12, 7065.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
941
Revisions:
2 times
(View History)
Update Date:
18 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




