1. Substitution of Fe for Co
SmFe
5 is a metastable compound and is not present in the Sm–Fe phase diagram. The initial focus on partially replacing Co with Fe was due to the obvious fact that Fe is more abundant and thus cheaper. Fe is the second-most abundant metal in the Earth’s crust after aluminum. This could reduce the price of raw materials needed for applications and potentially improve magnetization. Equilibrium solubility of Fe in SmCo system is limited, making this specific substitution difficult. The formation of metastable Sm (TM)
5 (TM = Fe-Co) compounds and their magnetic properties has been reported on by Miyazaki et al. since 1988
[1][2]. In that work, the rapidly quenched Sm (Co
xFe
1−x)
5 alloy ribbons were investigated. These ribbons were prepared at a velocity of 41.9 m/s and found to exhibit amorphous microstructure in the stoichiometric range 0 ≤ x ≤ 0.2 for Sm (Fe
1−xCo
x)
5.
It was depicted that the structure is retained in the range 0.6 ≤ x ≤ 1.0 and single-phase alloys were produced by quenching. The single phase Sm(TM)5 compound exhibits large coercivity of about 5 to 14 kOe. Despite large coercivity values, the performance of the materials was hindered by the low values of magnetization, which were measured at 16 kOe, lower than expected. It is well known that Fe has the highest atomic magnetic moment, but the coercivity of the ribbons with more 50% replacement of Co for Fe decreased.
Similar works to those mentioned above are also reported in
[3][4], where the investigation of structural and magnetic properties of alloys with SmCo
5−xFe
x (
x = 0–4) stoichiometry produced in the form of ribbons by melt-spinning. XRD and thermomagnetic studies revealed that the hexagonal Sm(Co, Fe)
5 phase can be retained with replacement up to
x = 2 and that the large substitution of Fe for Co in the alloys will produce the appearance of other intermetallic phases like Sm(Co, Fe)
7 and Sm
2(Co, Fe)
7. Heat treatment of the materials increases the remanence up to
x = 3, and further increase in Fe content will reduce both coercivity and remanence. This heavy dependence of structural and magnetic properties in the system on Fe content is the common ground in all similar studies. Annealed SmCo
4Fe alloys exhibited a relatively high coercivity of 10.2 kOe combined with a remanence of 60 Am/kg, while thermally treated SmCo
2Fe
3 alloys were utilized in a nanocomposite magnet with a high remanence of 100 emu/g, but a rather low coercivity of 2.9 kOe
[3].
Although these results did not encourage the intensification of studying substitution of Co only by Fe, the efforts continued with the addition of theoretical tools
[4][5][6]. Most of the findings are consistent with previous research. The origin of magnetocrystalline anisotropy and the effect of Si was described in
[5]. In this case, the substitution scheme included both magnetic and non-magnetic atoms (Fe/Si). The magnetic hardness is mostly due to the atoms in 2c positions, the layers that also contain the rare-earth atoms. The Sm 4f electrons that belong to the highly asymmetric and localized orbital play a vital role. When Co atoms are replaced in 2c positions, the 4f orbitals are broadened and shifted towards the Fermi level, and this has a negative effect on the anisotropy. In the other layer of the structure, which is occupied by Co in 3g positions, the main contribution to the total magnetization can be traced; the presence of Fe atoms in these positions increases the magnetic moment to 14.02 μ
B in total and has a positive effect in the anisotropy. Si substitution favors thermodynamic stability in high-Fe content, but has a negative effect on the magnetic properties, both magnetization and anisotropy.
2. Substitution of Cu and Other Transition or Non-Transition Elements for Co on SmCo5 Alloys
The replacement of cobalt with copper was also reported in several studies in the early eighties. In some cases, they result in solid materials with significant permanent magnet properties. In magnetic materials with R(Co, Cu)
5 stoichiometry, large coercivity values have already been reported in alloys in the as-cast form; these properties can be further enhanced by thermal treatment. Coercive forces in the vicinity of 28–30 kOe have been obtained in annealed samples
[6][7]. Some research on SmCo
1−xCu
x, as cast, annealed alloys, or single crystal, was published in the 1970s and 1980s
[8][9][10][11][12][13][14].
According to Oesterreicher et al.
[13] the magnetic hardness in pseudo-binaries like SmCo
5−xCu
x may already be an intrinsic property in nature and does not dependent on mechanisms such as pinning by other grain boundary phases. Under this scheme, coercivity is affected by temperature following a typical model based on thermally driven propagation of domain walls.
Metallographic and microprobe analysis of SmCo
1−xCu
x indicates the presence of spinodal decomposition in the metastable as-cast material
[8][9][10]. This relates to the increase in coercivity after heat treatment in Sm(Co,Cu)
5 alloys, even under relatively low temperatures in the area of 300–500 °C; this has been correlated with spinodal decomposition into both Co- and Cu-rich Sm(Co,Cu)
5 phases
[8][10]. Curie temperature determination and further DTA analysis have been depicted in a hypothetical Sm–Co–Cu ternary phase diagram. The critical initial amount of copper leads to decomposition within the range 24 to 40 at. %
[10]. Resulting components retain the hexagonal CaCu
5-type structure but exhibit a disorder in the composition concerning the transition metal. Subsequent annealing at elevated temperatures in the area of 1073–1273 K settles the stoichiometry of the materials. It seems that annealing affects the coercivity mechanism. The dominant domain-wall nucleation in the as-cast samples with relatively weak pinning at the grain boundaries changes towards a stronger domain-wall pinning process in the annealed alloys. Electron microscopy analysis of as-cast samples with moderate Cu content provided evidence for the existence of three phases: two of them retained the hexagonal CaCu
5-type structure but were Co-rich and Cu-rich, and an intergranular Cu-rich Ce
5Co
19-type phase
[11][12].
Later in the 1990s, the properties of Sm(Co,Cu)
5 magnets were systematically studied over the full stoichiometry range and in a large temperature area, as reported by Blanco et al.
[3][4][5][6][7][8][9][10][11][12][13][14][15]. It seems that Cu atoms act like local defects enhancing the coercive field. In particular, they also measured the hysteresis loops in SmCo
5−xCu
x as-cast and annealed magnets (1 <
x ≤ 3) at room temperature using a pulsed-field magnetometer and a static vibrating sample magnetometer and noticed a giant magnetic viscosity effect, proportional to copper content
[16].
The internal mechanism that produces the large coercive fields in the Sm(Co
1−xCu
x)
5 (0 < x ≤ 1) compounds has been also studied by focusing on structural parameters, namely, the coherence between unit cell parameters of Sm(Co, Cu)
5 and hcp Co with coercivity
[17]. In this work, it was suggested that cobalt precipitates along grain boundaries may affect the coercivity in Sm(Co,Cu)
5 alloys. It was also mapped that intrinsic coercivity is optimized within the area between 60% and 80% replacement of Co by Cu. In all these cases, elevated thermal treatment was applied. Tellez-Blanco et al. annealed Sm(Co, Cu)
5 samples for 504 h (21 days) at 1000 °C
[15][16]. Nishida et al. used a lower annealing temperature above 800 °C for 160 h
[18]. Gabay et al. used a more complex approach: thermal treatment of the Sm(Co, Cu)
5 magnets for 100 h in two steps. The first lasted 50 h at 1050 °C and then another 50 h in a temperature range of 350–450 °C
[19]. As such, the synthesis of Sm–Co–Cu ternary alloys must be considered a high-energy-consumption process.
A more recent work deals with these problems using reduction diffusion to synthesis, with improved energy and time efficiency compared to the previous metallurgical methods
[20]. In this study, Haider et al. applied an optimized chemical method that is more energy-efficient for the synthesis of Cu-substituted SmCo
5 compounds. Following this scheme, chemical precursors including samarium, cobalt, copper and potassium chlorides (SmCl
3, CoCl
2, CuCl
2, KCl) were utilized for the synthesis of Sm(Co,Cu)
5 particles by adjusting the molar ratios of the reagents in order to keep Cu introduction in moderate amounts. Final products were thermally treated at 900 °C for only 2 h, a much shorter annealing time than traditional approaches, reducing overall preparation cost. Cu introduction in 2c positions in the hexagonal SmCo
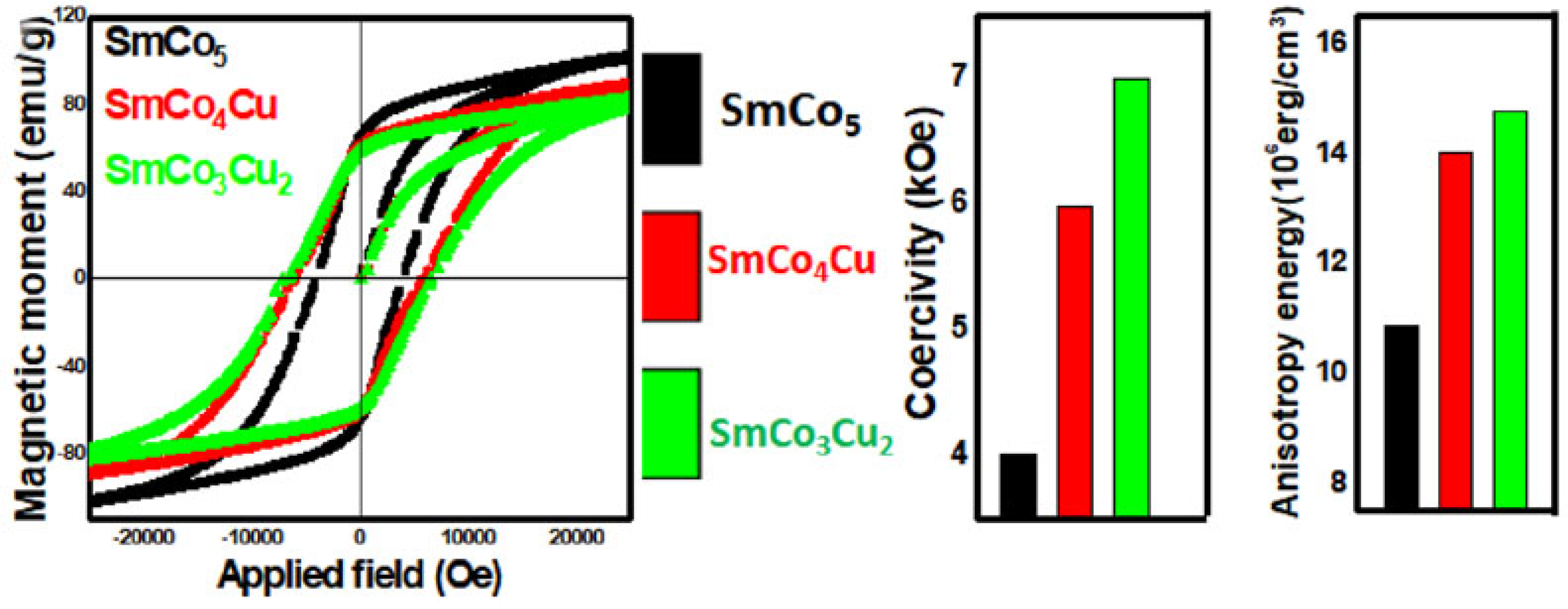
5 crystal structure leads to weakening of the coupling in the surroundings. The resultant decoupling obviously affects all the magnetic properties of moment, magnetocrystalline anisotropy and coercive field (
Figure 1). The magnetic moment was profoundly reduced, but coercivity and anisotropy were enhanced as a result of Cu substitution for Co. Enhancement of the magnetocrystalline anisotropy energy also affected the coercivity positively, and values of 4.50, 5.97 and 6.99 kOe for SmCo
5, SmCo
4Cu and SmCo
3Cu
2, respectively, were measured (
Figure 1).
Figure 1. Magnetic hysteresis loops of SmCo
5 and SmCo
5−xCu
x. Variations in magnetic moment, Hc, and anisotropy energy, before and after Cu substitution, obtained from
[20], which is published as an open-access article and distributed under the terms of the Creative Commons CC BY license.
In addition to Fe and Cu substitution materials for Co in SmCo
5, alloys with substitution of other elements, such as Ni, Pt, Cu, Ag, Al, In, Si and Sn (transition or non-transition elements), have been synthesized
[21]. The enthalpy of formation for some doping elements when they are introduced in 2c or 3g positions of alloys with nominal SmCo
4.5M
0.5 stoichiometry. Magnetic properties have been investigated at liquid helium temperature. In general, substitution of Co by non-magnetic elements like Al and Si weakens magnetic properties, especially saturation magnetization; however, when Al and Si replace Co in SmCo
5, the result is an outstanding increase in coercivity at values in the range of 30–50 kOe, even in bulk materials. Most of the other chemical combinations yielded unsatisfactory results.
The origin of these hard magnetic properties is usually discussed in terms of domain-wall pinning by atomic dimension centers that act as obstacles. Additionally, first-principle calculations and statistical thermodynamics can be utilized for studying the composition-dependent structural stability and the relevant magnetic and electronic properties of alloys with mediocre substitution of Co by other transition metal elements, e.g., alloys with nominal composition of SmCo
4.5M
0.5, M = Ti, Zr, Hf, Mn and Cr
[22]. The addition of Ti, Zr, Hf and Mn was beneficial for the stability of the SmCo
5 phase, while doping of Cr was detrimental. It was found that the SmCo
4.5M
0.5 ternary alloys can be stabilized by Mn, Ti and Hf introduction over an even wider temperature range, while the overall magnetic moment of the SmCo
4.5M
0.5 system was usually weakened by non-magnetic element doping, as expected. On the contrary, doping with Mn increased the total magnetic moment.
3. Simultaneous Substitution of Two or More Transition Metals Like Fe and Cu for Co in SmCo5 Alloys
Gabay et al. studied the possibility of stabilizing compounds with nominal composition RCo
5−xCu
x (R = Y, Sm) with respect to phase separation
[19]. They utilized first-principle density functional calculations, and their observations imply that decomposition of the material into two separate phases with different copper content is energetically favorable. They also estimated that the magnetic state of the alloys is connected to parameters like the Cu content, which can be accommodated, and Cu atomic position preference. Thermal treatment of samples of alloys with nominal compositions of SmCo
4Cu
1, SmCo
3.5Cu
1.5, SmCo
3Cu
2 and SmCo
2.25Fe
0.75Cu
2 affects the application of important magnetic properties, notably the Curie temperature and coercivity. The latter increases significantly if the annealing temperature is 100–140 °C below the Curie temperature. For example, in the case of SmCo
2.25Fe
0.75Cu
2, room-temperature coercivity increases from 12.3 kOe to 37.3 kOe. A difference from other studies, especially
[7][8][9], these experimental results do not seem compatible with the theory of spinodal decomposition. Another possibility suggested is that the improvement in coercive force has to do with differences in the occupancies of the transition metal atomic positions.
Concerning the maximum energy product of the final magnets based on the SmCo
5 alloy, the main limiting factor is the remanent magnetization of the basic ingredient. Exploration of both Fe- and Cu-substituted alloys has been successfully applied in a SmCo
5 system
[23]. Additionally, partial substitution of Sm by Zr has been applied, as in the case of the related Sm
2Co
17 magnets: Zr can replace both transition metals and rare earths in relevant intermetallics
[24]. Cu accumulates in the grain boundary phase and is responsible for the enhancement of coercivity under the domain-wall pinning mechanism
[25]. The magnetic properties of these kinds of as-cast alloys depend strongly on the annealing temperature and cooling rate. The best prepared magnet with composition of SmFe
0.4Co
3.5Cu
1.1 has a maximum energy product of 13 MGOe. This alloy was annealed for 2 h at 1100 °C, then slowly cooled in argon atmosphere
[26]. Initial magnetization curves of as-spun SmCo
3.839Cu
0.48Fe
0.48 ribbons and the variation in magnetization versus wheel speed at 20 kOe were generated
[26]. Ribbons produced with higher wheel speed (≥30 m/s) exhibited single-phase SmCo
5-type structure. At higher wheel speed, the results were even more encouraging: a large value of coercivity of about 33 kOe was obtained in ribbons prepared at 50 m/s. This was attributed to the formation of single-phase Sm(CoCuFe)
5 crystallites, and in optimization of their size, grains of the high anisotropic phase are smaller due to the more intense cooling introduced by higher wheel speed.
It is worth mentioning the work of Zhu et al., which focused on the substitution of both Cu and Ti for Co Sm-Co magnets
[27], a new class of materials with promising potential for usage in permanent magnet applications. They achieved an appreciable high-temperature coercivity of 8.6 kOe at 500 °C. Thermomagnetic analysis and temperature-varied X-ray diffraction patterns revealed that their samples were two-phase mixtures of the crystallographically related 2:17 and 1:5 type structures, and their combination was the reason for these interesting properties.