1. ToF-SIMS
ToF-SIMS is an ideal choice for material analysis due to its parallel ion detection, high transmission, and high mass resolution. Therefore, fields that call for understanding small-scale interactions to comprehend behaviors, such as corrosion behavior, film characterization, and biomedical alloy, are all excellent examples of understanding what ToF-SIMS can perform for material analysis.
1.1. Corrosion Behavior
Localized corrosion is known across many fields, specifically as a cause of failure for metal and alloyed components. With the atomic scale interactions that guide corrosion behavior, SIMS, as a highly sensitive technique, allows for insight into both the in-depth chemical structure and elemental distribution within samples to determine the effects of environment and additives on corrosion behavior.
For instance, Li et al. applied ToF-SIMS 3D imaging to investigate granular corrosion as a precursor to stress corrosion cracking for Al–Cu–Li alloys
[1]. SIMS’s sensitivity allowed for the analysis of sub-ppm to ppb levels of lighter mass elemental distributions, such as Li. Al–Cu–Fe–Mn intermetallic particles were shown to be preferential sites for corrosion in Al alloys. Two-dimensional (2D) images showed higher intensities of alloying elements Cu, Fe, and Mn and lower intensities of Al within the boundaries of the intermetallic particle region of interest. Lighter mass elements were observed to not be present within the intermetallic particles where localized corrosion occurred. In another study, Esmaily et al. demonstrated ToF-SIMS’s surface sensitivity to study the corrosion mechanisms of Mg alloys at sub-zero temperatures
[2]. Two-dimensional imaging of selected ionic species, including Cl
− and AlO
−, demonstrated that the redistribution of light atoms was temperature-dependent. At higher temperatures, AlO
− was found a distance away from the anodic sites, and it had not migrated toward the cathodic sites at sub-zero temperatures. Seyeux et al. have shown how ToF-SIMS can provide insight into corroded layers or anti-corrosion films upon engineered surfaces
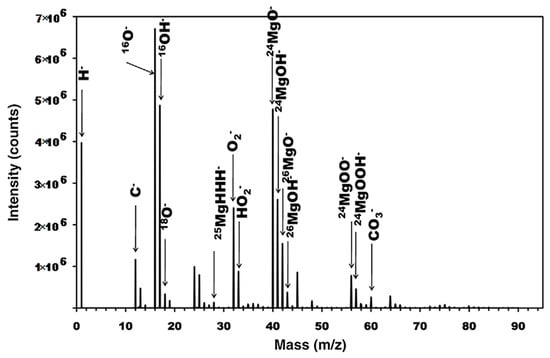
[3]. SIMS spectral analysis and depth profiling were performed to investigate the presence of MgH
2 and confirm its formation in submerged Mg. SIMS’s high sensitivity makes it ideal for validation of this process. In a previous study, MgH
2 formation was identified from weak XRD data on submerged pure Mg
[4]. Spectral analysis revealed the presence of both MgOH
− and MgO
−, as well as MgH
2, which can be seen in dept profiling (
Figure 1), indicating that the surface layer was split into a hydroxide dominated outer layer and oxide dominated inner layer. MgH
2 was at a much lower intensity than either the oxide or hydroxide signals and decreased from the film’s surface, which hinted that further work could illustrate its possible role in corrosion mechanics.
Figure 1. ToF-SIMS Spectrum of pure Mg after submersion in pure water. Reproduced with permission from Ref.
[3]. Copyright 2009 Elsevier.
ToF-SIMS’s ability to discern both organic and inorganic species via depth profiling and imaging can facilitate the characterization of metals and alloys, a valuable tool for the analysis of cultural artifacts. Yin et al. analyzed a historical copper alloy, which contained Cu, Zn, Tn, and Pb, from a bronze lion and an official Seal from the Han dynasty
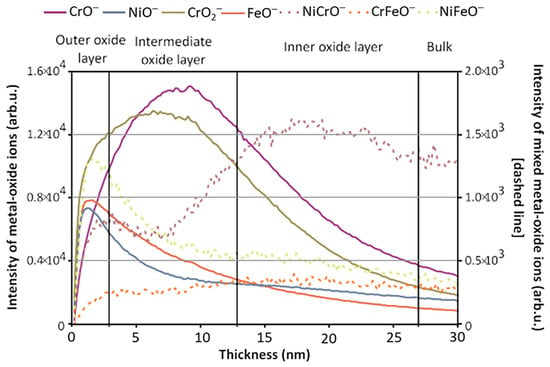
[5]. The cause of the artifact’s anticorrosive properties was dissected using static and dynamic SIMS to obtain surface composition and depth-resolved information, respectively, to observe the method the artisans utilized in its manufacture. The depth profile result showed that Ni enrichment was discovered at the surface of the artifact, which was mirrored across three of the four locations where analysis was performed. Mazenc et al. studied the behavior of thermally oxidized films formed on nickel-based 690 alloys in high-temperature water
[6]. The depth profile of a steam generator (SG) tube (alloy 690) was sputtered at 0.5 keV (30 nA), with key results shown in
Figure 2. The oxide layer can be discerned into three distinct parts. The outer layer is comprised of a mixed oxide layer rich in Ni and Fe, and the intermediate layer is predominantly composed of chromium oxide. The inner layer, marked by a pronounced NiCrO
− signal, corresponds to a spinel-rich NiCr
2O
4 portion. Trace elements such as gold and lead were also observed thanks to SIMS’s high sensitivity. Lead was seen to disrupt the distribution of major elements and a Ni/Zn alloy, which explained the anti-corrosion behavior exhibited by the artifact
[7].
Figure 2. ToF-SIMS depth profile (negative mode) of an SG tube (alloy 690) thermally treated in air for 4 h at 500 °C (Cs
+ 0.5 keV, current 30 nA). Reproduced with permission from Ref.
[6]. Copyright 2012 Wiley.
1.2. Thin Films and Oxide Layers
Oxides and other surface layers play key roles in nuclear and material science, from the engineering of material properties to environmental remediation in materials from steel to paint mediums
[8][9]. The characterization of these films and layers’ structures is therefore important in understanding the properties that they impart upon their host material.
The loss of Cr from stainless steels and Ni-base alloys has been reported to be a cause of significant loss of performance, as well as a poison of other elements of the work environment, such as CrO
3 poisoning solid-state battery cathodes
[10]. Thus, the growth and transport mechanism of chromium oxide layers is of immense importance. Poulain et al. investigated the oxidation of chromium at 300 °C to observe and determine the governing transport behavior within the oxide film at elevated temperatures. ToF-SIMS was used to analyze a polished sample to obtain spatially resolved depth profiles of the oxide layer. It was confirmed that oxygen diffused through the oxide layer to react with the metal at the oxide/metal interface to grow. The excellent mass resolution allowed for depth profiling to also reveal a second mechanism between
16O and
18O that takes place at the oxide surface as well as to separate the oxide into two regions. One layer is where Cr and
18O dominate, and an outer layer is where the
18O is exchanged for
16O, showing an inward diffusion of oxygen.
SIMS’s capacity to study surface layers is useful in optimization in addition to characterization. Byrne et al. studied copper retention in a thin film of SiO
2 [11]. Depth profiling was used to determine if the inclusion of Al to form a Cu–Al alloy would retain the Cu inside the film. The inward diffusion of Cu into the SiO
2 layer for both a pure Cu and a Cu–Al alloy layer served to evaluate the role in evaluating aluminum’s addition to the metal film. It was discovered that diffusion into the SiO2 substrate occurred in the pure Cu sample, while in the Cu–Al alloy sample, the Cu was retained in the surface alloy layer. These behaviors demonstrated the benefit of doping Cu surface films with Al for use as stabilized dielectric device structures. Jolanta et al. have similarly relied on the elemental distributions of Li to observe its mobility in V oxide films
[10]. In another study, positive and negative depth profiles were taken to measure the intercalation mechanism of Li for battery host material development. ToF-SIMS’s excellent sensitivity when measuring Li has been shown in studies of tungsten oxides
[12]. The distribution of the Li was measured and showed a maximum presence in the outer V2O5 layer but was also found at the oxide/metal substrate, indicating Li diffusion across to the inner oxide layers below via grain boundaries. ToF-SIMS was also used to study copper adsorption on pyrite. It was found that Cu
2+ ions could result in the activation of pyrite during separation in processing, thus lowering the grade of copper produced from the process
[13]. SIMS surface sensitivity revealed that pyrite surfaces were activated with high and low Cu concentrations at neutral pH, with the surface dominated by Cu and Fe hydroxides. At low pH, Cu(OH)
2 formed a layer on the surface of the pyrite.
ToF-SIMS was shown to be an effective tool in analyzing pigment and binder alteration processes in the paint layers of ‘Le Bonheur de vivre’ (1905–1906, The Barnes Foundation) by Henri Matisse due to its ability to image inorganic and organic elements at a µm spatial resolution
[14]. Imaging of elemental and molecular signals related to CdS pigment, associated binding medium, and degradation products were taken from multiple locations across the painting and were chosen for analysis. These samples were compared to artificially aged reference paints to investigate the processes of pigment and binder degradation, along with previous restoration efforts. Results showed that SIMS allowed for the identification of degradation products previously unobserved with methods such as Scanning Electron Microscope coupled with Energy Dispersive X-ray (SEM-EDX). Across the four samples, CdS pigment and CdCl
2 were adjoined throughout the various paint layers. The presence of CdSO
4 and CdCO
3 identified the mechanism through which the CdS pigment was degraded and explained the now faded color of the work. SIMS also identified CdC
2O
4 as evidence of the binding medium’s degradation, which was a potential source of the fragility of the upper paint layers present in the painting.
1.3. Metals and Alloys for Biomedical Applications
ToF-SIMS is commonly used to study biological samples, from microbes to drug analysis
[15][16]. Biomedical surface preparation for implants, where residue can be harmful if not removed, has benefitted from SIMS’s ability to characterize surface interfaces where interfacing of implant and bone is desired.
Göttlicher et al. investigated low-temperature oxidation behaviors induced by plasma for orthopedic Ti-40Nb alloys
[17]. Low-temperature oxidation was induced by plasma to study the growth of the surface oxide. ToF-SIMS depth profiles showed that the impurities SiO
2, FeO, CrO, and Pt decreased in intensity with surface depth. Suggestions were made to prevent impurity transference, such as adding additional magnetic confinement of the plasma. Ti ions were also observed to have a faster migration than Nb, leading to concentration gradients after exposure to the plasma. Eriksson et al. focus on devices post-implantation
[18]. Six implants, each with various surface treatments, were placed within the tibia of a rat for 7 days. They were then removed to compare the effect of porosity on hydroxyapatite formation, called mineralization, using ToF-SIMS imaging. Positive spectra were obtained, and the profiles of select ions were imaged. Ca
2+ and CaOH, characteristic peaks of hydroxyapatite, were detected across all six surfaces after one week. From the results, porosity was shown to have a clear influence over mineralization. It was also concluded that for the one-week period, ToF-SIMS was more reliable for predicting biocompatibility than other markers such as bone-to-metal-contact.
In another study, Xu et al. considered osteoconduction of alkaline-treated Ti surfaces of two-month implants to investigate the chemical composition of new bone formed on the treated surface
[19]. ToF-SIMS analysis indicated changes in the bone/Ti interface and uniform distribution of Ca and P deficiency. Ca was shown to have a higher deposition rate on the treated surface, with the SIMS imaging providing further insight into bone mineralization. The analysis also indicated lower concentrations of PO
4 and OH, constituents of hydroxyapatite, near the material interface. This deficiency implies an intermediate mineral phase or hydroxyapatite with large numbers of imperfections.
2. Magnetic SIMS
Due to the continually operating ion beam, magnetic SIMS offers depth profiling capabilities with extremely high depth resolution. This, coupled with high SI transmission and a high mass resolving power, makes magnetic sector SIMS an excellent option when extremely precise elemental and isotopic analysis is required. Semiconductor analysis, alloy-element distribution, and geologic dating are all excellent applications for magnetic-sector SIMS due to their features of precise examination of often small quantities of analyte within a given substance.
2.1. Semiconductor Materials
Magnetic-sector SIMS is an extremely useful tool for characterizing semiconductor materials. Depth profiles and imaging offer data into diffusion barriers or dopants, and elemental distribution can track the evolution of changing microstructural elements and elemental additives. Gu et al. have shown how SIMS may be used to study dielectrics such as HfSixOy, which is one of the most promising high-k materials. High-k materials are a class that is under study in an attempt to reduce semiconductor device dimensions
[20]. One requirement for this type of dielectric is that the constituent elements cannot be allowed to diffuse into adjacent regions in the device during processing. SIMS analysis provides results that were stated to be unobtainable by any other method used previously. Sufficient depth resolution was obtained to define the substrate from the HfSiO layer, as well as the identification of an apparent interfacial layer that was previously thought to be SiO
2 but was shown to contain Hf as well, with no indication of Hf diffusion into the substrate observed.
Quantification of data using SIMS is possible, as is shown by Lee et al. in studying Cu(In,Ga)Se
2 (CIGS) thin films
[21]. CIGS films are popular due to their high absorption coefficients and band gap properties. Analysis of these absorbing layers, therefore, allows for insight into increasing the efficiency of solar cells that contain CIGS. Magnetic-sector SIMS depth profiling of copper, indium, gallium, and selenium was performed and showed a selenium-rich, copper-poor surface region in the CIGS film. Relative sensitivity factors were calculated using integrated intensities gathered from SIMS data. Atomic compositions calculated were shown to be within 1% of other quantification methods, such as inductively coupled plasma atomic emission spectroscopy. This method was found to have great advantages in quantification, and magnetic SIMS was demonstrated for the potential application of magnetic-SIMS for the quantification of these multilayer thin films.
2.2. Small Additive Transport and Incorporation in Materials
Given the ever-increasing complexity of alloy compositions, analysis of the behavior of small percentages of alloying elements is paramount. Castro et al. apply magnetic-sector SIMS imaging to an Al–Li alloy to observe small weight percent additions of Cu, Mn, Zn, and Mg, which are commonly added to obtain certain desirable properties and are usually added into the microstructure as fine nanoscale precipitates. Other techniques, such as EDX, have insufficient spatial resolution or sensitivity for such tasks
[22]. Magnetic sector SIMS was employed for this purpose to observe the microstructural distribution of these low-percent additives. Images of
7Li,
55Mn,
56Fe, and
63Cu were acquired for analysis, where Li was shown to segregate at grain boundaries in a phase identified as the phase (Al
2CuLi, T1) based on the work by Xu et al.
[23]. Magnesium was also detected despite a relatively low concentration of 0.2%. It was concluded that the alloy was not homogenous, comprising precipitate phases with Mg partitioning and Zn incorporation into an interface phase. Overall, the resolution of the magnetic sector instrument helped to identify these nanodomains where other techniques, such as EDX and nano-XRF, could not
[24][25].
III-Nitride (i.e., GaN, AlGaN) is of interest in high-power electronic and optoelectronic devices, where quantification of impurity species allows for information relating to dopant and impurity control. Gu et al. analyzed such materials with magnetic-sector SIMS, relying on its high dynamic depth resolution and high transmission, obtaining relative sensitivity factors for impurity species
[26]. These factors were calculated for various impurities in AlGaN, which revealed that the relative sensitivity factors for Mg and Si appeared to remain relatively stable when normalized to N-containing matrix ions. This normalization was stated to provide a valuable quantitative tool for analyzing such materials due to the constant concentration of matrix ions in AlGaN.
Titanite, a titanium silicate mineral, can be grown with trace metallic inclusions for use as standards or as experimental starting points for other analytical techniques. Mazdab et al. use the SHRIMP instrument to study trace element incorporation in natural titanite
[27]. Sc, Cr, Ni, Y, Zr, Nb, Hf, Ta, Th, and U can all be identified as trace elements of approximately 50 ppm in natural titanite as well as when doped in grown titanite crystals. Grown titanite was shown to also contain Na and smaller concentrations of B from the flux used in manufacturing. SHRIMP analysis confirmed that the trace elements were successfully incorporated into structural sites.
2.3. Geologic Formations and Minerals
One large application of magnetic SIMS, particularly the Sensitive High-Resolution Ion Microprobe (SHRIMP) instrument, is the geosciences, being well suited for analysis of geological formations, using ionic ratios to date minerals and rock formations. Many studies, including one by Zhuchenko et al., focus on the analysis and dating of zircon, a mineral that holds information from ancient geological eras
[28]. Zhuchenko employs a SHRIMP instrument to date zircons by analyzing the ratio of U-Pb ions to gain insight into ancient geological activity. The analyzed zircons were taken from mafic granulite, and the zircon’s U–Pb ratio dating revealed the rough timeline of the beginning of magmatism in the region of interest (Ukraine), as well as another, newer recrystallization period that corresponded to a separate metamorphic era. Zirconium dating was also applied to better understand the synchroneity of geological activities across what is now Asia and North America
[29]. The analysis revealed the age of the zircons collected from the area of interest, the upper Xieshuihe formation, in south China, fell within the error margins for SHRIMP analysis of zircons originating from separate geological formations in northern Idaho and Utah, USA, as well as in Yukon Canada. This analysis suggested that the two differing locations were subjected to similar conditions around similar times, dating the geological behavior due to the formation conditions and, thus, isotopic distributions of the analyzed zircons.
Shatkov et al. used a similar method to analyze zircons in the uranium-bearing Transbaikalia geological structure in Russia to determine the formation of the Tulukuev caldera
[30]. U-Pb SHRIMP analysis revealed lower counts of U and Th than other regions of the caldera, mostly the core and close edges, than the further, cooler regions. SHRIMP analysis also showed that locations of radioactive uranium isotopes were restricted to certain regions as well, which was evidence of U being moved and separated throughout the structure, hinting at the formation of the structure. Shi et al. employed SHRIMP to analyze Hf isotopes alongside U–Pb analysis. Samples were taken from various types of rock from the North China Craton. SHRIMP zircon ages for each type documented granitoid formation and allowed for an understanding of the date of underlying formative metamorphic events, including evidence of vertical crust growth based on isotopic Hf ratios with Lu varying from region to region within the area of interest.
SIMS and Fourier Transform Infrared (FTIR) analysis has been used to measure hydrogen abundance within both experimentally annealed natural mantle materials
[31]. The abundance was employed for calibration to be used in measuring H
2O concentrations in a variety of minerals. The relationship between anhydrous materials and silicate melts was selected to aid in understanding the distribution of H between various phases of the mantle and the processes that influence H distribution. FTIR has had previous success in measuring low abundances of hydrogen in small samples. SIMS offered considerable advantages for quantitative analysis of hydrogen equilibrated between phases, including insensitivity to crystal orientation, low detection limit, and high spatial resolution. FTIR and SIMS were compared for their capacity to analyze anhydrous materials through annealing experiments using synthesized crystals with varying H
2O concentrations in the testing environment. Results showed that SIMS had better lateral and depth resolution than FTIR, with FTIR averaged absorption over the full thickness of the sample. Using both techniques, it was possible to compare the minimum atom count required for each technique, with SIMS requiring three orders of magnitude fewer atoms than FTIR and thus being three orders of magnitude more sensitive for measuring hydrogen abundance. SIMS and FTIR could be employed as complimentary techniques. SIMS allows for in situ analysis of small single spots, and FTIR provides information on substitution mechanisms.
This notion of SIMS’s superiority over FTIR, but having the potential to be complementary, was shared when volatile species in volcanic glasses were measured by Hauri et al.
[32]. SIMS was utilized to measure the isotopic abundances and compositions of volatile elements in standard glasses and compared to FTIR measurements of the same materials for use in studying volcanic degassing of volatile species. The comparison showed that SIMS offered easier sample preparation, sufficient detection limits, and sufficient spatial resolution to collect data from secondary phases, which hindered other vacuum extraction techniques
[33]. Its excellent resolution also facilitated the acquisition of isotopic data for H, C, and S, which aided in providing constraints to magma evolutions, degassing, and contamination processes.
3. Large Geometry SIMS
An important role for SIMS is the characterization of particles. Large geometry SIMS achieves high transmission at high mass resolving power and is commonly utilized to study environmental samples, such as meteorites, and as nuclear safeguards by measuring actinide particle compositions.
3.1. Extraterrestrial Materials
Large geometry SIMS provides an optimal platform for cosmochemistry due to the capacity for in-situ trace element analysis of complex minerals. In applying large geometry SIMS to the study of extraterrestrial materials, Merle et al. employed large geometry SIMS to study lunar basalt and mafic plutonic rocks
[34]. Their objective was to further understand the early moon’s crust-mantle differentiation by obtaining radiogenic isotopic compositions due to the constraints they place upon the composition of the mantle source they formed from. The accurate measuring of the selected Pb ions for dating, as well as the excellent spatial resolution achieved by the instrument, allowed for precise comparison of Pb isotopic ratios in samples. Dating results showed clear evidence of magmatic activity on the moon from 3100 to 300 million years ago, and it was not continuous as was previously suggested. Pack et al. used large geometry SIMS to investigate the silicon content of iron meteorites, which are predominantly made of iron and nickel alloys, as well as minor inclusions of other compounds of FeS, FeNi
3C, and silicates
[35]. The meteorites chosen are of a type with largely unfractionalized trace elements
[36]. This analysis was performed due to the thermal history of the meteorites being derived from the metal structure and phases present within the bulk. Thus, if a conventional theory and model of formation are correct, then silicates and metals must have entered an equilibrium phase. Silicate partitioning into the metal phase can be calculated and compared to the stability and activity coefficients of Si in Ni and Fe alloys. This comparison allowed for silicate concentrations to be calculated and compared with the Si contents measured in the meteorites.
28SI and
54Fe were utilized to represent Si and Fe content, and sensitivity factors for
28Si/
54Fe were determined across 15 meteorite samples. No silicate inclusions were observed, with Si content low and steady across all samples within a narrow range. Ni-rich regions were identified as probable intergrowths of metallic phases, and it was suggested that the observed low Si presence was due to metal and silicates achieving solid state equilibrium below 1270 K.
Soens et al. utilized a similar experimental apparatus to analyze a refractory phase-bearing micrometeorite to understand its origin
[37]. It was found to be rich in silicates as well as Ca-Al inclusions and Mg oxides. Oxygen concentrations were found to be consistent with the other meteorites of its type. Based on these inclusions of MgO, Ca, and Al, as well as the measured oxygen isotope concentrations, it was suggested that its origin might be linked to main belt or Jupiter family comets, based on similarities of previous results.
3.2. Particle Heterogeneity/Homogeneity
Jovanovic et al. summarize three different large geometry instruments analyzing two uranium dioxide pellets with similar bulk isotope compositions but different spatial uranium isotope distributions
[38]. Each instrument analyzed a set of pellets and obtained and reported results in terms of isotopic ratios and distributions. They demonstrated the capacity for measurement reproducibility for each instrument produced similar results across the two samples. The first particle, it was concluded, was a combination of low-enriched uranium and depleted uranium. SIMS was the only technique among several used to detect and characterize the 235U/238U ratio of the second particle due to that particle’s smaller domains, and it was found to be similar in composition to the first. Large geometry SIMS was able to perform high precision and high spatial resolution characterization for the two particles and provide direct spatial visualization and structural information on isotope distribution.
Varga et al. would perform a similar analysis using large geometry SIMS to verify the analysis of inhomogeneous samples containing uranium particles of various enrichments
[39]. The measured uranium standards were U
3O
8 with given U
234/U
238 and U
235/U
238 ratios and were of various sizes ranging from sub-micrometers to a few hundred micrometers. Around 2200 uranium particles were found in automatic screening. More precise measurements found around 30 particles that were able to be separated into two different populations based on their
235U enrichment values of 1.01% and 3.06%, respectively. It was noted that the two different reference samples demonstrated differences between these populations. One had two statistically significant
235U populations present, one at 0.97% and the other at 1.01%, while the other reference sample was found to be more homogenous. Overall, a similar conclusion was drawn, as in Jovanovic et al.’s study, that large geometry SIMS could be used to validate and confirm uranium ratios, providing an excellent tool for nuclear safeguarding.
It has quickly expanded in biological and medical research domains and has been applied in diverse fields, including material sciences, cosmochemistry, and geosciences.
4. NanoSIMS
NanoSIMS has high spatial resolution and high collection efficiency, which allows for specialization in elemental and isotopic analysis. NanoSIMS, therefore, is an ideal technique for use in fields from analyzing biological samples, such as single cells, clays, and sediments, geochemistry, cosmochemistry, and material science, where it is commonly applied for both analyzing nanoscale elemental segregation for light element and isotopic analysis.
4.1. Study of Elemental Segregation in Metals and Alloys Using NanoSIMS
Elemental segregation at grain boundaries is a common cause of altered behavioral properties such as embrittlement
[40]. A study of Inconel 718 by Talukder et al. focuses on small additions of B, P, and C, which have been shown to result in drastic changes in mechanical behavior
[41]. While the alloy is very desirable for its mechanical properties, its preferred method of repair is welding. B and P additions improve the life of the alloy but have been shown to have detrimental effects on weldability, while C helps to mitigate this negative effect of the B and P additions. NanoSIMS is used to observe the presence of B, P, and C segregations to the grain boundaries of the alloy, as the alloy suffers micro-fissuring during welding in the heat-affected zone near the welding site. Carbon’s effect in mitigating the negative welding ability was investigated. Two alloy compositions—one with 0.006 wt.% of added C content and one with 0.033 wt.% C content—were selected for study. Selected ions for analysis included
12C
−,
16O
−,
11B,
16O
−,
31P
−, and
58Ni
−.
Images to accompany the elemental analysis were acquired. The images and elemental analysis show that the Ni content was identical in both measured alloys. B segregation was observed similarly for both alloy samples, although it was significantly reduced for the sample with the increased carbon presence. Alternatively, no P or C segregation could be detected for the grain boundary, but the author stated that it was possibly due to the segregation of these elements being below the detection limit for the given experimental conditions. The B to Ni ratio across the grain boundary shows a dramatic increase in the B segregation in the sample with higher carbon content. Therefore, it is hypothesized that carbon influences the segregation of B across a larger area away from the material’s grain boundaries than was originally thought. Rosa et al. also investigated B inclusion but focused on its segregation at austenite grain boundaries in low-carbon steel
[42]. NanoSIMS was selected based on the expected concentrations of B at grain boundaries because other techniques, such as atom probe tomography (APT), had insufficient detection limits (a few ppm for NanoSIMS vs. tens of ppm for APT)
[43][44]. The segregation of B at the grain boundaries as a function of temperature was determined using NanoSIMS, and it revealed boron distribution in the microstructure consisting of martensite. Although various boundaries were observed, B was only found at observed γGBs and not at formed packets, blocks, and laths. This NanoSIMS data contributed to the construction of modeling boron segregation kinetics. The modeling and experimental observations revealed that the B inclusions were very mobile at high temperatures, with fast quenching being insufficient to restrain segregation behavior during the quenching process. It was also observed that the ratio between boron segregation at γGBs and boron in solution in grains decreases with increasing temperature.
A multi-phase steel was examined using NanoSIMS to study the microstructural distribution of carbon
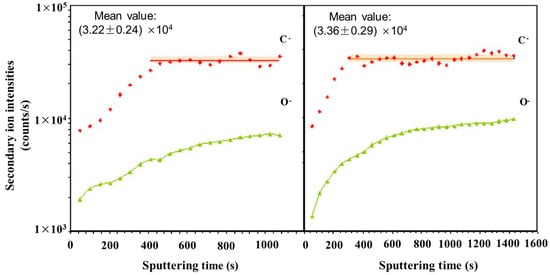
[45]. NanoSIMS was selected due to its up to 50 nm lateral resolution and high sensitivity, allowing for small changes in carbon content to be observed. The techniques used previously, such as Transmission Electron Microscopy using parallel electron energy loss spectroscopy (TEM-PEELS), are very complex, and thus NanoSIMS provided an alternative method utilizing its excellent lateral resolution and high sensitivity. The carbon concentrations ranged from 0.2 wt.% to 0.8 wt.%. With a 100 nm probe, the sensitivity of the technique is sufficient to detect small variations of carbon within the same phase.
Figure 3 shows the carbon content of a bainite/martensite sample vs. sputtering time. Carbon was shown to have been enriched in the martensite regions due to bainite transformation. High carbon content was also shown in areas perpendicular to ferrite laths.
Figure 3. Evolution of carbon and oxygen secondary ion intensities versus sputtering time during two analyses in a sample with 0.97 at.% C. Adapted from Ref.
[45].
When compared with other microanalysis techniques, NanoSIMS had the ability to study the same area as in SEM and, if offered, visualization of carbon repartition within the microstructure of the steel. It was also concluded that because the detection limit was so low, 0.0063 wt.% for carbon in iron, the characterization of non-stable phases, such as bainite, which contained low concentrations of carbon, was possible. Nano SIMS allowed for a more complete understanding of bainitic transformation where the lack of quality data was at least partly assigned to the lack of detectability for techniques such as SEM and the higher detection limit of techniques, such as TEM-PEELS (1000 ppm).
4.2. Hydrogen Isotopes in Metals and Alloys
NanoSIMS offers the ability to analyze hydrogen isotopes such as deuterium and tritium, and it has an advantage when studying behaviors such as hydrogen embrittlement and blistering. Greg McMahon investigated the role of hydrogens in the deterioration of materials, specifically structural materials, through hydrogen-assisted cracking
[46]. APT was also previously utilized as a possible option due to its good atomic resolution, but it has a much smaller sample volume (100,000 nm
3 vs. 1 × 10
9 nm
3 S) and higher detection limits when compared to NanoSIMS or SIMS
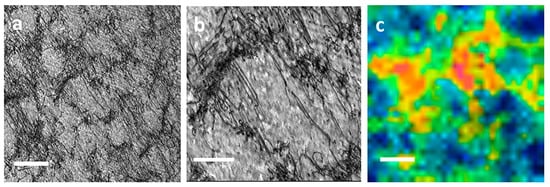
[43][46]. The latter is generally ten times better in detection sensitivity. The same is true with scanning probe methods such as scanning electrochemical microscope (SECM) analysis in which quantification is possible but whose resolution is on the order of hundreds of microns. NanoSIMS imaging was considered a prime technique to bridge these two extremes of operating parameters, providing better resolution than, for example, tritium audiography and better sample volume and detection sensitivity than APT. McMahon shows the distribution of hydrogen in the form of deuterium around primary and tertiary crack fatigue crack tips in two stainless steels. Deuterium, with its low natural abundance, was chosen for analysis. The ratio of deuterium to oxygen was determined due to oxygen’s presence as a matrix signal, with images acquired approximately 4–5 μm ahead of the crack tips. A cellular structure was found and is present in
Figure 4, where dislocations create the structure’s bounds (
Figure 4b). Bringing
Figure 4a,b to even further magnifications allows for a hue saturation intensity image for deuterium and oxygen. These images revealed localized regions with clusters of enriched D/O ratio values and suggest that deuterium is trapped at sites of dislocations sinks. It was described that the distribution of these deuterium hot spots in localized regions might point to hydrogen influencing the steel’s deformation process and help narrow down which of the specific hydrogen-assisted cracking theorems are responsible for crack growth. This result was stated to agree with recent modeling efforts by Dadfarnia et al. and offers a way to validate models of hydrogen transport by dislocations using NanoSIMS
[47].
Figure 4. Dislocation structure at the crack tip. (
a) TEM bright field image approximately 4–5 μm from crack. Scale bar is 500 nm; (
b) Same region at higher magnification showing dislocations clusters observed in a. Scale bar is 200 nm; (
c) 2H/16O ratio displayed as HSI image in the crack wake region 4–5 μm from the crack. The scale bar is 500 nm. Reproduced with permission from Ref.
[46]. Copyright 2018 npj.
Tarzimoghadam et al. investigated the hydrogen distribution and desorption behavior of a Ni-Nb alloy using NanoSIMS. The effect of the needle δ phase on hydrogen embrittlement was studied by mapping the hydrogen distribution within the Ni–Nb alloy. Applications of this alloy include use in hydrogen-containing atmospheres, which required investigation into the hydrogen distribution and embrittlement behavior of the alloy, to which NanoSIMS’s excellent resolution makes it a logical choice for analysis. Previous studies revealed that the δ phase affects the alloy’s sensitivity to hydrogen embrittlement; thus, the relationship between this phase and hydrogen trapping is of great interest. NanoSIMS analysis enabled the deuterium distribution within the microstructure to be detected and mapped. The results confirmed higher deuterium content in the Ni–Nb solid solution than in the δ phase.
In pressurized water reactors, the cladding around fuel rods is often made with zirconium alloy tubes, chosen for their low neutron capture cross section and good oxidation resistance. Therefore, understanding the hydrogen pickup of these at operating temperatures allows for the safer operation of these reactors. Li et al. described a method of 3D mapping deuterium distribution in oxidized Zircaloy-4
[48]. The high resolution required for the mapping of deuterium made NanoSIMS an excellent technique. Comparison among cross-sectional and depth profile measurements of the alloy showed the 3D distribution of deuterium in the material with the morphology of the deuterium trapping sites suggested. It was shown that the deuterium concentrated in the oxide near the water/oxide interface. A gradual decrease in deuterium concentrations was observed when approaching the oxide/metal interface. This behavior was interrupted by the local trapping sites (i.e., porosity, cracks) that were linked by diffusion paths into the metal bulk.
In another study, NanoSIMS was used to determine hydrogen’s distribution through the Zr oxide growth of Zircaloy-4, and various ratios of a Zr–Nb alloy were obtained
[49]. Subjecting the materials to neutron irradiation was shown to increase the deuterium diffusion coefficient, the deuterium concentration trapped within the oxide, as well as the pickup fraction. Results, similar to Li et al., showed a concentration of decreasing deuterium from the oxide/water interface towards the deeper oxide layers with strong upticks in deuterium concentration hinting at strong trapping sites within the oxide. Zircaloy samples were found to have a high deuterium trapping ratio in the oxide layer and a high diffusion coefficient in the oxides. The diffusion coefficient in Nb containing samples’ oxides was much lower, a repeated result for similar conditions and materials from previous studies. NanoSIMS has been successfully applied for the hydrogen-induced cracking behavior of a Ni-based alloy
[50]. NanoSIMS imaging produced ion and ratio maps taken from the passive oxide layer. Deuterium enrichments were found along dislocation slip bands as well as the intersections between them. This observation was attributed to hydrogen diffusion through mobile dislocations. Deuterium was also observed in twin boundary enrichments and along a particular phase boundary that is exhibited within the studied alloy.
5. Correlative Imaging Using SIMS
SIMS’s unique benefits can allow it to complement a wide variety of analysis techniques. Correlative imaging allows for a diverse range of information to benefit and improve upon the strengths of other techniques beyond what could be performed using isolated methods. The study of irradiated materials and semiconductors is characterized by complex microstructural evolution and is a common application of SIMS correlative imaging techniques.
5.1. Irradiated Materials
Tritium and Li (
6Li and
7Li) transport within neutron-irradiated functional intermetallic coatings, specifically Fe–Al alloys, are common concerns for their use in fission and future nuclear fusion applications
[51]. Yu et al. used a focused ion beam with scanning electron microscopes (FIB-SEM) to prepare lift-outs of intermetallic coatings for analysis by scanning transmission electron microscopy (STEM), atomic force microscopy (AFM), and ToF-SIMS. Excellent isotopic detection of light elements was illustrated using ToF-SIMS. For example, ToF-SIMS’s excellent mass resolution and sensitivity allowed for the analysis of light elemental isotopes such as hydrogen/deuterium and
6Li/
7Li.
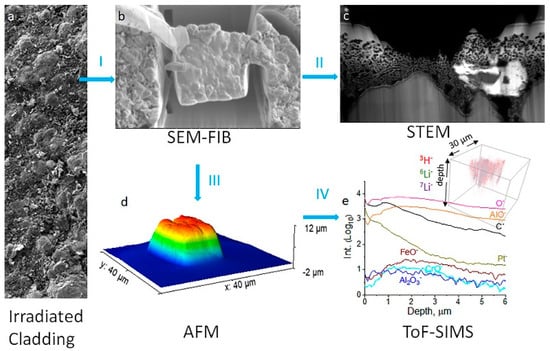
Figure 5 presents a multimodal analysis workflow and attainment of information from each technique, which was selected due to the complementary information over an area when compared to bulk techniques on irradiated samples.
Figure 5. Multimodal chemical imaging of an irradiated tube (
a) using (
b) SEM-FIB to prepare the lift-out, (
c) STEM to determine nanostructures and elemental mapping, (
d) AFM to obtain the lift-out dimensions nondestructively, and (
e) ToF-SIMS to acquire sensitive surface and isotopic, elemental, and molecular 3D mapping. Rainbow color indicates sample topographical height. Blue arrows are used to show workflow. Reproduced with permission from Ref.
[51]. Copyright 2021 Elsevier.
SIMS allowed for a complex investigation of possible lithium mobility within the sample via both spectral analysis and depth profiling. SIMS spectral analysis identified hydrogen, deuterium, and tritium presence, as well as
6Li and
7Li. It was also observed that tritium is deposited on the cladding coating and has a large possibility of being a product during irradiation due to tritium levels being far larger than the natural abundance. Depth profile measurements for Li suggested that it is associated with alumina oxide layers, and tritium signals were much less intense in the middling depths of the cladding. Andersen et al. studied hydrogen inclusion using magnetic sector SIMS
[52]. Mg
2Ni/Mg
2NiH
4 thin films were analyzed with high-resolution imaging and depth profiling to characterize such materials in fields from batteries to high-strength alloys using magnetic sector SIMS
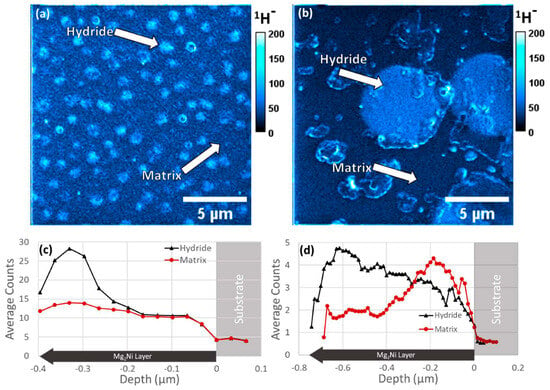
[53][54]. Both images and depth profiles of a Mg
2Ni film can be found in
Figure 6. In this regard, SIMS imaging bridged the gap between TEM and X-ray diffraction (XRD), allowing for the 3D chemical measurement of hydrogen with a resolution of tens of nanometers and a field of view (FOV) of tens of microns.
Figure 6. SIMS images with (
a) equiaxed microstructure showing the summed signal over three slices and (
b) columnar microstructure showing the summed signal over 14 slices. (
c,
d) SIMS localized depth profiles from regions inside and outside of the surface visible hydride areas for (
a) and (
b), respectively, were reproduced with permission from Ref.
[52]. Copyright 2023 Elsevier.
SIMS and EBSD were combined to study polycrystalline nickel and to investigate hydrogen distribution around grain boundaries to see the effect on the grain boundaries
[55]. EBSD inverse pole figure mapping was combined with hydrogen concentration profile mapping from SIMS. This multimodal imaging strategy showed two different types of hydrogen distribution behavior in nickel. The first is categorized by fast hydrogen diffusivity and showed a sharp gap for hydrogen concentration profiles across random grain boundaries. The second category is across special grain boundaries, characterized by low hydrogen diffusivity.
5.2. Semiconductors Using
Semiconductors are a type of material structure that can benefit from multimodal SIMS strategies, as small optimizations in composition can lead to drastic changes in observed properties. Usiobo et al. applied Helium-ion Microscopy (HIM) coupled with SIMS to study mixed organic and mixed halide perovskite semiconductors
[56]. These perovskite semiconductors are used for solar cell devices, and continued efforts have been made to reduce the instability in certain environments through doping. Alkali cation pairs such as K-Cs, K-Rb, and Rb-Cs were analyzed, allowing for both elemental and morphological imaging at the nanometer scale. Correlative imaging permits the characterization of chemical content, distribution of grains, and secondary phases. The fusion of imaging techniques allowed for combinatory structural images and chemical maps. Results showed that Rb accumulates at the semiconductor’s grain boundaries while still having a presence within perovskite grains regardless of the cation pairing chosen.
Kumar et al. employed SEM, TEM, and NanoSIMS to study Si-metal interfaces of screen-printed solar cells, which is a primary source for the cell’s loss of efficiency. NanoSIMS was selected for inclusion as dopant levels within the cells were reported to be below the 0.1 wt.% detection limit of conventional analytical techniques such as EDX. NanoSIMS-enabled dopant distributions were imaged, and SEM allowed for analysis of phases present within the sample. It was discovered that phosphorus-emitting structures, identified by NanoSIMS, and SiNx passivation layers were destroyed if the cells were overfired, which was validated in the correlated SEM analysis. These results point towards diffusion of the dopant species, lowering the overall cell efficiency due to these microstructural losses.
The doping of SI nanocrystals has long been hindered by the separation of theoretical calculations, where thermodynamic equilibrium conditions are usually utilized, and experimental conditions, where nanocrystal incorporation is common. Perego et al. used a multimodal technique to study P-doped Si nanocrystals embedded in SiO
2 to allow for the understanding of kinetics while not being directly tied to equilibrium conditions
[57]. Energy-filtered transmission electron microscopy (EF-TEM) cross-sectional images were obtained, along with ToF-SIMS depth profiling, Rutherford Backscattering Spectrometry (RBS), X-ray photoelectron spectroscopy (XPS), and nuclear reaction analysis (NRA). XPS allowed observation of the diffused P trapped in the nanocrystals and incorporated either in the nanostructure’s core or in an interface region. XPS’s 1000–2000 ppm was insufficient to detect P levels in the surrounding SiO
2. ToF-SIMS depth profiling was coupled with TEM cross-sectional images to compare nanocrystal size distribution before and after annealing. The data were compared to diffusion models to confirm diffusion behavior. It was estimated that the P content in the matrix was a fraction of that contained within the embedded nanocrystals. SIMS analysis at various annealing temperatures provided information into the dynamics of the trapping behavior. Further results revealed that high P concentration in Si nanocrystals embedded in SiO
2 corresponded to a thermodynamically favored system configuration, with six times the solubility in the bulk material. P trapping behavior in the embedded nanocrystals was shown to be limited by diffusion, lacking additional diffusion barriers. It was, therefore, proven possible for high levels of impurities to be introduced into the inner layers of Si nanocrystals with dopant properties finely tunable with changing annealing conditions. This approach could be particularly appealing in conjunction with monolayer doping processes to control dopants introduced in nanostructured systems.
5.3. SIMS Complementary Techniques
SIMS imaging can be applied alongside a wide range of techniques, providing complementary information for a more well-rounded analysis. Otto et al. employed XPS and ToF-SIMS to better understand the passivation layer of Li-metal interfaces
[58]. Employing XPS for its quantitative element and compound-specific information, SIMS was able to boost the low lateral and depth resolutions of XPS as well as increase the sensitivity of Li and the detection of H. Results showed that the Li passivation layer was mainly homogenous with contaminant presence. A bi-layered structure of a hydroxide and carbonate layer was reported atop an oxide-rich region. The multi-analytical approach was required for a comprehensive characterization of the film. XPS’s quantitative compositional data provided the sequencing information, and ToF-SIMS allowed for depth measurements of the layer thickness, distribution, and homogeneity. Amadelli et al. similarly paired XPS and SIMS to study PbO
2 on Ti when electrodeposited
[59]. Results showed from the complementary techniques that the dopant species affect the accumulating behavior of O species at the oxide surfaces. F
− was found to be incorporated into the PbO
2, and the presence of cations, such as Fe
3+, Ni
2+, and Co
2+, was not found in the coatings, even when added to the used solutions. Kellner et al. employed a multivariate analytical approach to analyze V and Cr-containing metal alloys
[60]. An EDX system measured chemical composition, and TEM/STEM measurements allowed for the distributions of elements and compounds when coupled with EDX results. SIMS was able to support and correlate these results by presenting distribution information of its own, providing insight into the presence of V and Cr. Along with TEM, the results described the effects of V and Cr on the corrosion process. Results showed Cr additions gave better corrosion resistance than V, providing a passivation layer. Grovenor et al. applied SIMS, APT, and TEM to study the oxidation mechanisms of Zr fuel cladding alloys
[61]. Previous EDX results had shown the oxide layers of Zr fuel cladding to include an intermediate oxide layer. APT revealed these suboxide layers within the cladding, and TEM validated these measurements. SIMS was employed to demonstrate the penetrating ability of selected oxidizing species through outer oxide layers. SIMS measurements tracked the specific portions of oxide that were active in the oxidation process during corrosion. In addition, results demonstrated that the transition between the corrosion environment was located at the metal/oxide interface, using the porosity of the layers observed with other techniques as routes of access.
6. Nanomaterials
A specific subset of SIMS applications is in the rising field of nanomaterials. The scope of nanoparticles and nanostructured alloys has been increasing over recent decades due to the opportunity to obtain properties not available in contemporary materials’ equivalents. These materials require reliable and effective tools for characterization to optimize novel systems and processes effectively. SIMS sensitivity and excellent resolution allow for it to serve as a linchpin in the effective analysis of nanomaterials.
Priebe et al. used a collection of TEM and SIMS analysis techniques to study Al nanoparticles in a ZrCuAg matrix
[24]. SIMS was selected for analysis to study elemental composition, while TEM provided nanoparticle size measurements. The objective of these complementary techniques was to characterize nanoparticles and to aid in optimizing the nanocomposite’s properties for medicinal applications. The result was a successful attempt at spatially resolving nanoparticles in an inorganic matrix, using SIMS to provide elemental information and TEM to validate SIMS in determining nanoparticle sizes. Tian et al. use SIMS to analyze Mg–Gd–Y–Zr alloys with continuous gradients and nanograin sizes to observe if solute clustering contributed to alloy strengthening
[62]. SIMS imaging and depth profiling were employed with SEM and EDX. Results showed an even distribution of alloying elements, with little clustering at the surface, confirming that a solid solution contributes most to strengthening behaviors rather than precipitation. Interface/surface segregation was also observed by SIMS; however, it did not exceed the component’s maximum solubility. Choi et al. analyzed a nanostructured Ni-based alloy conjoined via dissimilar weld joints with low alloy steel using SIMS, APT, and TEM
[63]. SIMS was employed to construct the chemical map of the alloy constituents at the weld sites, TEM was used to analyze the transition of crystallographic microstructure, and APT was used to determine the chemical composition of specific boundary regions. Their findings suggest the nano-precipitate distribution to be uneven across the bulk, and the weld region to be divided into several areas including an unmixed zone in the Ni-based nano-alloy, a fusion boundary, and a heat affected zone in the steel. This non-homogeneous distribution included interesting observations, such as higher Fe and lower Mn, Ni, and Cr from the low alloy steel compared to the filler metal utilized, with carbide precipitation near the weld fusion boundary.