| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | zahra asadzadeh | + 1322 word(s) | 1322 | 2020-12-18 07:53:22 | | | |
| 2 | Bruce Ren | -21 word(s) | 1301 | 2020-12-25 02:50:08 | | |
Video Upload Options
Cell death resistance is a key feature of tumor cells. One of the main anticancer therapies is increasing the susceptibility of cells to death. Cancer cells have developed a capability of tumor immune escape. Hence, restoring the immunogenicity of cancer cells can be suggested as an effective approach against cancer. Accumulating evidence proposes that several anticancer agents provoke the release of danger-associated molecular patterns (DAMPs) that are determinants of immunogenicity and stimulate immunogenic cell death (ICD).
1. Introduction
Immunotherapy has been considered as a promising therapeutic modality in oncology that aims to re-activate the immune system which is stopped by the tumor cells and creates a robust antitumor response [1]. Tumor cells, by having resistance to cell death and escaping from immunological surveillance, are durable in cancer patients [2]. So, restoring the susceptibility of cancer cells to death and intensifying immune recognition of poorly immunogenic tumor cells create strategies for therapeutic success [3]. The combination of these strategies, which would take advantage of the probable immunogenic traits of diverse forms of cancer cell death, is an interesting viewpoint [4]. The antitumor immune response can be provided by immunogenic cell death (ICD), a different class of cell death described by release or expression of calreticulin (CRT), adenosine triphosphate (ATP), high mobility group box 1 (HMGB1), heat-shock proteins (HSPs), ANXA1, and stimulation of type I interferon [5][6][7]. Binding of these danger-associated molecular patterns (DAMPs) to their receptors leads to immune cells’ recruitment and induction. Lastly, they lead to recognition, phagocytosis, and activation of T lymphocytes to eradicate cancer cells [8]. Up to now, some single-agent ICD inducers have been introduced, comprising standard chemotherapeutics, targeted anticancer factors, and numerous other treatment options [9][10]. It was documented that the immunogenicity of tumor cells can be promoted by particular treatments (e.g., chemotherapy, radiotherapy, and photodynamic therapy) and leads to antitumor immunity [11][12].
2. Immunogenic Cell Death: New Meaning in Cancer Therapy
Naturally, the immune system can recognize and destroy cancer cells and plays a significant role in the regulation of tumor progression. The immune system is educated in such a way that it does not respond to normal cells, while several mutations in cancer cells result in the expression of tumor-specific antigens that can be identified as non-self and activate the immune system, finally resulting in the elimination of cancer cells. The term “antitumor immunity” defines the innate and adaptive immune responses that regulate tumor. Both innate and adaptive immunity play a role in the identification and fight against tumors, and a successful antitumor immune response is related to the close interaction of several factors of innate and adaptive immune responses [13]. They are composed of antigen-presenting cells, various subsets of T cells, B cells, and NK cells. However, tumors use several ways of immunosuppression to stop the antitumor effect of immune cells. Dysregulation of the balance between the effector and regulatory cell compartments is one of the key strategies for tumors to escape immune eradication [14]. A better understanding of the vital immune cells and the regulatory networks participating in the interaction between tumor cells and the immune system is central for the improvement of therapeutic strategies to strengthen the immune system against cancers.
Numbers of cells die every day as a result of normal tissue turnover that is central for homeostasis maintenance in organisms [15]. Therefore, the existence of several forms of cell death is not unexpected [16][17]. Cell death can be categorized according to its morphological appearance, enzymological criteria, functional features, or immunological properties [18]. Classification based on morphological criteria proposes the existence of three various forms of cell death [19].
Type 1 cell death, or apoptosis, is described by some characteristic morphological changes such as condensation of nuclear material, DNA degradation, cell shrinkage, membrane blebbing, and presence of apoptotic debris [20]. Apoptotic cell death occurs constantly in multicellular organisms and is crucial for normal growth, tissue homeostasis, and many other physiological functions [21]. For supporting the host, physiological apoptosis is quickly recognized by phagocytic cells such as macrophages and dendritic cells (DCs) [22]. Phagocytic clearance of apoptotic bodies is a silent process. These apoptotic cells get removed without inducing immunological responses due to the release of anti-inflammatory signals [23]. So, apoptosis has been introduced as an immunologically quiet form of cell death.
Type 2 cell death, or autophagy, is an intracellular degradation process that plays a central role in the protection of cells and organisms from stressors. Autophagy is described by the removal of materials marked for destruction into double-membrane vesicles called autophagosomes. Autophagosomes fuse with endosomes and then they deliver their content to the hydrolytic interior of lysosomes for degradation. Autophagic degradation is considered as a vital source of amino acids, nucleotides, and fatty acids and provides energy for cells. Autophagy plays a part in physiological processes and its activity is fundamental for adjustment to starvation, cell development, aberrant structures degradation, cell survival, homeostasis, and regulation of cell death [24].
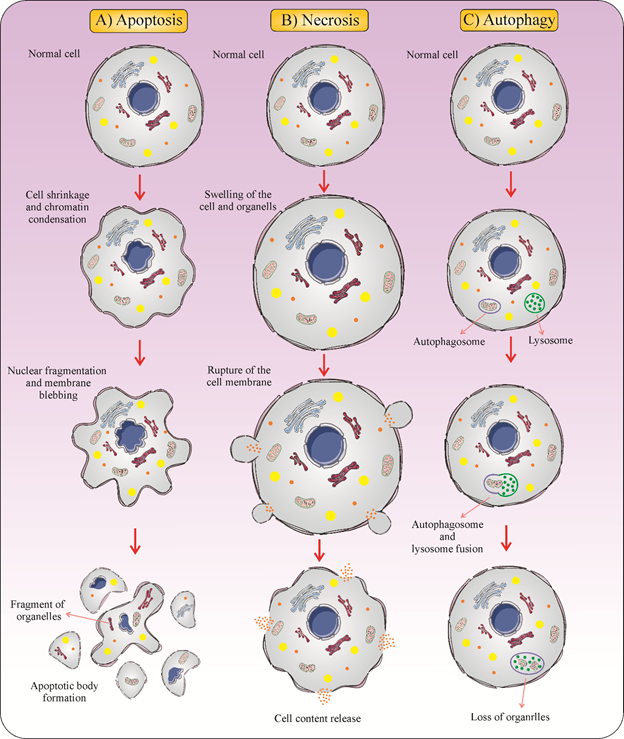
Type 3 cell death, or necrosis, is generally characterized as a form of cell death with no indications of apoptosis or of autophagy. The morphological features of necrosis are cytoplasmic swelling, the plasma membrane breakdown, and loss of intracellular contents [25]. Dissimilar to apoptosis, the biochemical characteristics of necrosis are unknown [26]. Because of the abrupt release of pro-inflammatory modulators, necrosis is commonly believed to be immunologically unsafe [27][28]. Necrotic cell death leads to the release of pro-inflammatory mediators [29] (Figure 1).
Figure 1. Three different forms of cell death. (A) Apoptosis is a form of cell death that some molecular mechanisms in a cell lead to its death. It is generally characterized by distinct morphological features including cell shrinkage, blebbing, and nuclear fragmentation. (B) Necrosis is a type of cell death that is morphologically described by an increasing translucent cytoplasm, swelling of organelles, and increased cell volume. (C) Autophagy is the major intracellular degradation process by which cytoplasm ingredients are translocated to and degraded in the lysosome.
Poorly immunogenic cancer cells avoid destruction by the immune response. Cancer cells express antigens but these prevent separating them from tolerized self-antigens. Normally mutation rates are low in such cancers and exhibit poor de novo antigens [30]. Glioblastoma [31], ovarian cancer [32], and some other types of malignancies lack stimulatory tumor neoantigens and endorse an immunosuppressive tumor milieu through generating anti-inflammatory cytokines [33]. Some cancer therapies induce immunologically silent apoptotic cell death and can attenuate the immune system, facilitating cancer recurrence. Unlike apoptosis, a regulated cell death process in which the plasma membrane does not rupture, necrosis is inevitably associated with the release of DAMPs and intracellular organelles. Pathophysiologically, this stimulates an immune response because new surfaces become available to both the innate and the adaptive immune system during a process defined as necroinflammation [34]. Instead, apoptosis was considered to be non-immunogenic or even tolerogenic. Currently, a set of anticancer therapies has developed that causes a kind of apoptosis named ICD, informing the immune system to the existence of dying cancer cells [35]. ICD describes a functionally distinct type of cell death that results in a T cell-mediated immune response, particularly for dead cell-derived antigens. ICD can be stimulated by different mechanisms and leads to translocation or release of DAMPs from the dying cells that activate the immune response. In contrast to ICD, tolerogenic cell death (TCD) including most types of apoptosis is a non-inflammatory mechanism of cell death that is described by membrane blebbing and loss of DAMP secretion. TCD is unable to stimulate an immune response associated with cell death [36].
It was documented that if specific treatments are used, the immunogenicity of cancer cells increases enough to stimulate long-term antitumor immunity [37]. The ICD induction can change dying cancer cells into “vaccines” to induce anticancer immunity by the DCs’ maturation and cytotoxic T lymphocytes’ (CTLs’) activation [38] plus increasing the cytotoxic function of NK cells [35]. Immunogenic features of ICD are mediated largely by intracellular molecules termed DAMPs, which are invisible in live cells, but they exhibit immunostimulatory effects after exposure to dying cells.
References
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current challenges in cancer immunotherapy: Multimodal approaches to improve efficacy and patient response rates. J. Oncol. 2019, 2019, 4508794.
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta Rev. Cancer 2010, 1805, 53–71.
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008, 15, 3.
- Montico, B.; Nigro, A.; Casolaro, V.; Dal Col, J. Immunogenic Apoptosis as a Novel Tool for Anticancer Vaccine Development. Int. J. Mol. Sci. 2018, 19, 594.
- Clarke, C.; Smyth, M.J. Calreticulin exposure increases cancer immunogenicity. Nat. Biotechnol. 2007, 25, 192.
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353.
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164.
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860.
- Krysko, O.; Aaes, T.L.; Bachert, C.; Vandenabeele, P.; Krysko, D. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013, 4, e631.
- Panzarini, E.; Inguscio, V.; Dini, L. Immunogenic cell death: Can it be exploited in PhotoDynamic Therapy for cancer? Biomed Res. Int. 2013, 2013, 482160.
- Krysko, D. Immunogenic cell death and emission of damps: Calreticulin and ATP. J. Nanomed. Biother. Discov. 2012, 2, doi:10.4172/2155-983X.1000e118.
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281.
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284.
- Russo, V.; Protti, M.P. Tumor-derived factors affecting immune cells. Cytokine Growth Factor Rev. 2017, 36, 79–87.
- Curtin, J.F.; Cotter, T.G. Historical perspectives. Essays Biochem. 2003, 39, 1–10.
- Galluzzi, L.; Vitale, I.; Abrams, J.; Alnemri, E.; Baehrecke, E.; Blagosklonny, M.; Dawson, T.; Dawson, V.; El-Deiry, W.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107.
- Brenner, C.; Kroemer, G. Mitochondria--the death Signal integrators. Science 2000, 289, 1150–1151.
- Melino, G. The Sirens’ song. Nature 2001, 412, 23.
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, e955691.
- Garrido, C.; Kroemer, G. Life’s smile, death’s grin: Vital functions of apoptosis-executing proteins. Curr. Opin. Cell Biol. 2004, 16, 639–646.
- de la Rosa, E.J.; de Pablo, F. Cell death in early neural development: Beyond the neurotrophic theory. Trends Neurosci. 2000, 23, 454–458.
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965.
- Napirei, M.; Mannherz, H.G. Molecules involved in recognition and clearance of apoptotic/necrotic cells and cell debris. In Phagocytosis of Dying Cells: From Molecular Mechanisms to Human Diseases; Springer: Berlin/Heidelberg, Germany, 2009; pp. 103–145.
- Yang, Y.; Klionsky, D.J. Autophagy and disease: Unanswered questions. Cell Death Differ. 2020, 27, 858–871.
- Vanlangenakker, N.; Berghe, T.V.; Krysko, D.V.; Festjens, N.; Vandenabeele, P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008, 8, 207–220.
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43.
- Vakkila, J.; Lotze, M.T. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 2004, 4, 641.
- Hemmat, N.; Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 2019, 77, ftz048.
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J. Immunol. 2001, 166, 6847–6854.
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74.
- Razavi, S.M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune evasion strategies of glioblastoma. Front. Surg. 2016, 3, 11.
- Preston, C.C.; Goode, E.L.; Hartmann, L.C.; Kalli, K.R.; Knutson, K.L. Immunity and immune suppression in human ovarian cancer. Immunotherapy 2011, 3, 539–556.
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692.
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707.
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine 2017, 97, 123–132.
- Garg, A.D.; Agostinis, P. immunogenic Cell death in Cancer: From Benchside research to Bedside reality. Front. Immunol. 2016, 7, 110.
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Pedro, B.S.; Manuel, J.; Breckpot, K.; Brough, D.; Chaurio, R.; et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front. Immunol. 2015, 6, 588.
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. Therapeutic cancer vaccines: Past, present, and future. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 119, pp. 421–475.