Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SeungRock Lee | -- | 2360 | 2024-02-12 16:27:24 | | | |
| 2 | Jessie Wu | + 5 word(s) | 2365 | 2024-02-18 01:53:06 | | | | |
| 3 | Jessie Wu | + 8 word(s) | 2373 | 2024-02-18 01:56:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Trinh, V.H.; Nguyen Huu, T.; Sah, D.K.; Choi, J.M.; Yoon, H.J.; Park, S.C.; Jung, Y.S.; Lee, S. Oxidative Inhibition of PTEN by Reactive Oxygen Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/55002 (accessed on 07 February 2026).
Trinh VH, Nguyen Huu T, Sah DK, Choi JM, Yoon HJ, Park SC, et al. Oxidative Inhibition of PTEN by Reactive Oxygen Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/55002. Accessed February 07, 2026.
Trinh, Vu Hoang, Thang Nguyen Huu, Dhiraj Kumar Sah, Jin Myung Choi, Hyun Joong Yoon, Sang Chul Park, Yu Seok Jung, Seung-Rock Lee. "Oxidative Inhibition of PTEN by Reactive Oxygen Species" Encyclopedia, https://encyclopedia.pub/entry/55002 (accessed February 07, 2026).
Trinh, V.H., Nguyen Huu, T., Sah, D.K., Choi, J.M., Yoon, H.J., Park, S.C., Jung, Y.S., & Lee, S. (2024, February 12). Oxidative Inhibition of PTEN by Reactive Oxygen Species. In Encyclopedia. https://encyclopedia.pub/entry/55002
Trinh, Vu Hoang, et al. "Oxidative Inhibition of PTEN by Reactive Oxygen Species." Encyclopedia. Web. 12 February, 2024.
Copy Citation
Phosphatase and tensin homolog (PTEN) is a tumor suppressor due to its ability to regulate cell survival, growth, and proliferation by downregulating the phosphoinositide 3-kinases/protein kinase B (PI3K/AKT) signaling pathway. In addition, PTEN plays an essential role in other physiological events associated with cell growth demands, such as ischemia-reperfusion, nerve injury, and immune responsiveness.
PTEN
redox regulation
oxidative inhibition
ROS
cell signaling
1. Cardiovascular Remodeling
Studies indicate the involvement of the serine/threonine kinase AKT as a mediator in the process of ischemic preconditioning, a short transient period of sustenance during ischemia-reperfusion injury [1][2][3][4]. In ischemic preconditioning, AKT signaling is upregulated and prevents cardiomyocytes from undergoing apoptosis [3][4][5][6]. The phosphoinositide 3-kinases/protein kinase B (PI3K/AKT/mTOR) pathway plays a significant role in protecting against ischemia-reperfusion injury, particularly in the context of ischemic preconditioning in cardiac tissue. Accordingly, reversible PTEN downregulation has been suggested as a viable therapeutic approach to mitigate ischemia-reperfusion-related cardiac damage [7]. A study revealed that PTEN plays a pivotal role in the post-myocardial infarction remodeling process: Partial PTEN inactivation, by regulating the AKT signaling pathway, can increase interleukin IL-10 and consequently decrease tumor necrosis factor TNFα and matrix metalloproteinase MMP2 expression in the heart. However, the authors were not able to determine the exact source of generated IL-10, apart from immune cells. It probably comes from endothelial cells and fibroblasts [8]. Several research studies demonstrate that IL-10 can eventually attenuate apoptosis and facilitate cardiac remodeling after myocardial infarction [9][10][11][12]. Hence, PTEN inhibition could be an effective approach for improving cardiac conservation after ischemia [13][14].
During acute myocardial infarction, the heart suffers from oxidative stress with increased reactive oxygen species (ROS) levels [15]. In the acute and chronic cellular response to this event, NOX2 is overexpressed in human cardiomyocytes, which may not interfere with the activity of macrophages [16][17][18]. Since PTEN oxidation is likely to occur near the site of ROS formation and both PIP3 and the NOX complex are located in the plasma membrane, H2O2 generated from NOXs is the primary candidate for inhibiting the PI3K/AKT pathway via PTEN oxidation. There is substantial supporting evidence indicating that elevated PIP3 signaling contributes to the activation of the NOX complex in both phagocytic and non-phagocytic cells. The increase in PIP3 levels is proposed to be a key factor in initiating the activation of the NOX complex [19][20]. This may create a circular impact, where ROS generated from NOXs can inhibit PTEN and enhance the PI3K/AKT pathway, which, in turn, promotes NOX activity.
Cai and Semenza were the first to describe the modulation of PTEN during ischemia-reperfusion injury. During the first 15 min of ischemia, PTEN undergoes dephosphorylation and proteasomal degradation. However, the kinetics reveal that not all PTEN activity is impaired during this initial phase and AKT phosphorylation increases without any significant changes. This indicates that the dephosphorylation and degradation of PTEN do not greatly hinder its function. However, in the subsequent initial phase of reperfusion, there is a notable increase in oxidized PTEN and, consequently, phosphorylated AKT. Their findings clarify that the surge in AKT phosphorylation during this short reperfusion period is caused by the oxidative inhibition of the remaining PTEN [21]. Simultaneously, elevated levels of ROS have been observed in both injured cardiomyocytes and intact hearts during ischemia-reperfusion events [21][22]. Therefore, the oxidation of PTEN during the initial reperfusion period is related to the concurrent rise in ROS levels. (Figure 1).
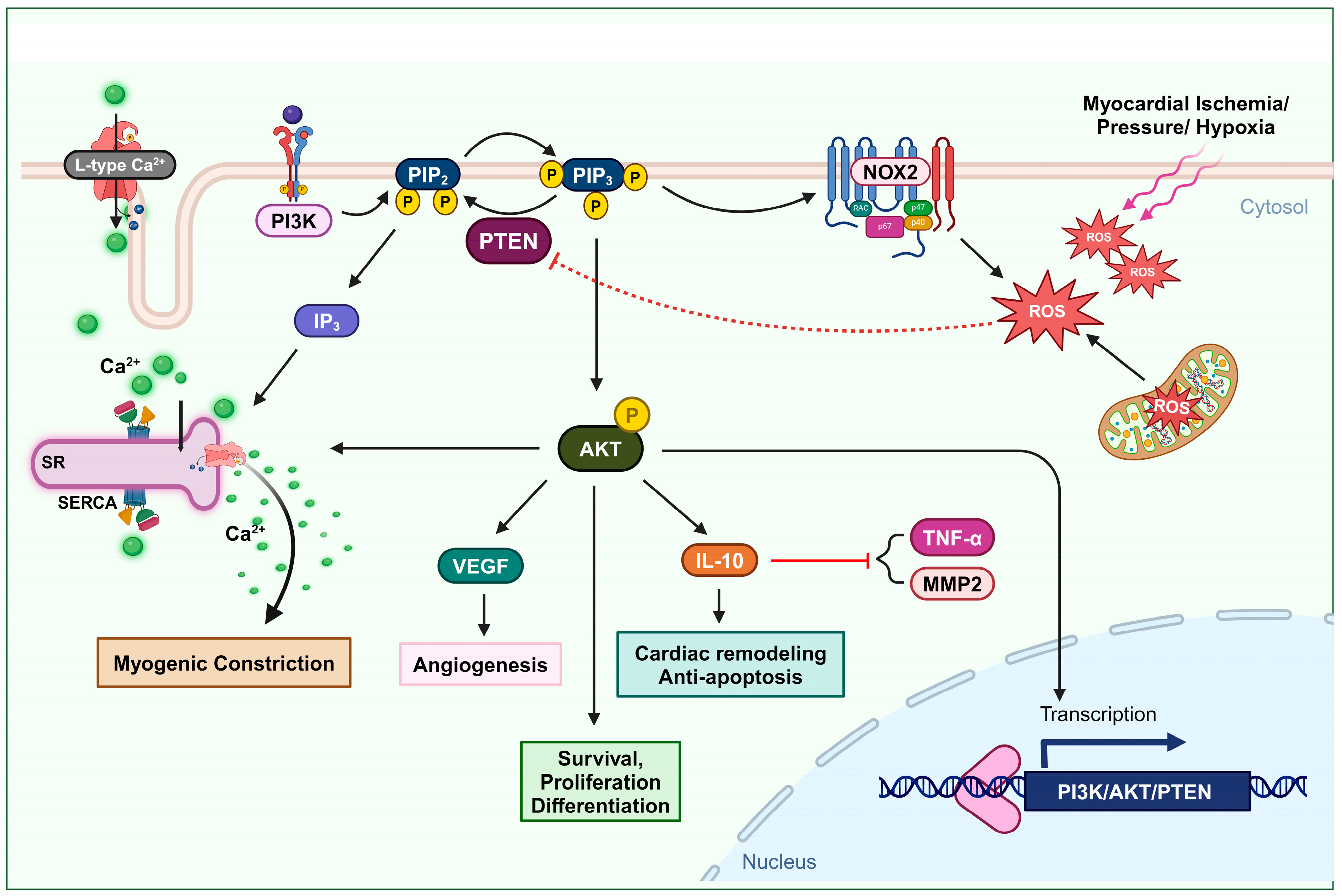
Figure 1. Oxidation of PTEN in cardiovascular remodeling and myogenic constriction. Ischemia or elevated blood pressure conditions induce the production of ROS. These ROS deactivate PTEN, leading to an increase in the AKT signaling pathway. The activation of the AKT pathway enhances cell survival, proliferation, and differentiation. Furthermore, PTEN-mediated AKT activation upregulates IL-10 expression, promoting cardiac remodeling and preventing apoptosis. It also elevates VEGF expression, facilitating angiogenesis. This mechanism also involves L-type calcium channel activity and the formation of IP3, which stimulates Ca2+ secretion, thus increasing intracellular Ca2+ levels and promoting myogenic constriction.
One vital mechanism of injured tissue in cases of blood supply shortage, due to ischemia or infarction events, is angiogenesis. Angiogenesis is defined as the formation of new blood vessels [23]. Vascular endothelial growth factor (VEGF) is associated with promoting angiogenesis. Upregulation of VEGF can be a potential treatment approach to induce axonal outgrowth and following angiogenesis after cerebral ischemia [24], as well as to restore blood flow in ischemic tissues after myocardial infarction [25]. Experimental data reported by Connor et al. indicate that the overexpression of manganese superoxide dismutase (SOD2) increases the production of mitochondrial H2O2, which triggers angiogenic activity. In this process, mitochondrial H2O2 can oxidize PTEN and upregulate the PI3K/AKT signaling axis, subsequently activating VEGF production [26] (Figure 1).
2. Vascular Constriction
Accumulating evidence highlights the significant role of PI3K/AKT-dependent signaling pathways in various fundamental cellular functions within the cardiovascular system. These functions include processes such as the maturation and growth, mechanotransduction, contractility, and proliferation and migration of both cardiac and vascular smooth muscle cells [27][28][29][30][31]. Dysfunction of this signaling pathway plays an essential role in cardiovascular pathophysiological conditions, such as heart failure, atherosclerosis, and hypertension [32][33][34][35]. Wu et al. observed that in the rostral ventrolateral medulla of spontaneously hypertensive rats, ROS originating from NOXs and mitochondrial oxidative stress reduced the catalytic ability of PTEN via oxidation. Consequently, the ensuing activation of the PI3K/AKT signaling pathway may lead to neurogenic hypertension [35].
Maintaining a consistent cerebral blood flow distribution through myogenic tone development is vital for neurons, which lack glucose storage and rely solely on a continuous blood supply of glucose and oxygen for normal metabolic function and under conditions of increased demands [36]. The role of PI3K in mediating the impact of physical forces, such as pressure, shearing, and stretching, on vascular smooth muscle cells and various other cell types, is well recognized [37]. Gebremedhin et al. found that elevated intraluminal pressure in cerebral arteries leads to an increase in ROS generation, leading to the oxidative inactivation of PTEN. This, in turn, results in the upregulation of PI3K/AKT activity and the release of IP3. The activation of AKT can induce the inhibition of arterial calcium-activated potassium channels, membrane depolarization, and L-type calcium channels. In addition, the formation of inositol (3,4,5)-triphosphate (IP3) stimulates the sarcoplasmic reticulum to release Ca2+, resulting in an increase in intracellular Ca2+ levels and the initiation of pressure-dependent myogenic constriction in cerebral arteries [36] (Figure 1).
3. Neuro-Regeneration and Neuro-Survival
PTEN activity has been shown to substantially limit cell survival in the challenging context of cerebral ischemia [14][16][17][18][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]. Numerous studies have demonstrated that inhibiting PTEN to activate the PI3K/AKT pathway provides protection to the brain during stroke [39][40][41][42][43][44]. The reduction in the PI3K/AKT/GSK-3β/mTOR signaling pathway by neuronal PTEN impairs axon growth and nerve regeneration in both the peripheral and central nervous systems, post-neuronal injuries, and ischemic conditions. Strong evidence consistently supports PTEN’s inhibitory role in critical neurological processes in pathological contexts [45][46][47][48][49][50][51]. Enhancing the activity of the PI3K/AKT pathway has been shown to increase axon growth [52]. Therefore, it is clear that PTEN, an intrinsic inhibitor of the PI3K pathway, plays a significant role in regulating the growth of central axons. PTEN’s activity also impedes nerve regeneration following neuronal injury, which is crucial for neural function recovery [49]. Hence, deliberately inhibiting PTEN activity emerges as a strategically advantageous approach with pronounced benefits for facilitating neuronal regeneration following injury. Empirical evidence shows that deleting PTEN in the spinal cord or optic nerve significantly enhances nerve regeneration after injury [53]. Targeted application of local pharmacological agents to suppress PTEN or the precise utilization of siRNA-based techniques to specifically downregulate PTEN expression at injury sites serves as a potent and effective strategy for accelerating the intricate axon outgrowth process and expediting the overall neuronal recovery [54]. Even in genetic diseases, such as spinal muscular atrophy, managing protein synthesis in motor neurons via PTEN depletion could be a therapeutic strategy [55][56]. Experimental data demonstrate that ROS signaling plays an essential role in promoting the self-renewal, proliferation, and differentiation of neural stem cells and neural progenitor cells via a regulatory mechanism in which the oxidation of PTEN by ROS upregulates the PI3K/AKT signaling pathway [57].
After neuronal injury, the injured axons are exposed to a highly oxidative and inflammation-driven environment. Under these conditions, growth cones, which are crucial for axon extension, initially collapse and retract. This process involves the oxidation of actin and produces ROS [58]. In a study, two experimental models were used to investigate the role of ROS generation in neuronal death and the involvement of PTEN in neurodegenerative diseases. Oxygen–glucose deprivation and the neurotoxin 1-methyl-4-phenylpyridinium iodide were applied to neural cells to simulate cerebral ischemia and Parkinson’s disease. However, it was found that ROS generated under these conditions did not cause oxidative inactivation to all cellular PTEN, allowing PTEN to maintain its functional activity. The suggested explanation is that the deactivation of PTEN phosphatase by ROS requires suitable intracellular co-localization with the site where these ROS are actively produced [59].
Experimental data demonstrate that the presence of peroxynitrite can prevent etoposide-induced apoptotic cell death in primary cortical neurons. This effect is primarily due to the oxidation of PTEN and the subsequent upregulation of the PI3K/AKT signaling pathway. Although the anti-apoptotic implication of peroxynitrite is subject to dispute, these data concurrently strengthen the potential of PTEN oxidation in promoting neuroprotection [60] (Figure 2).
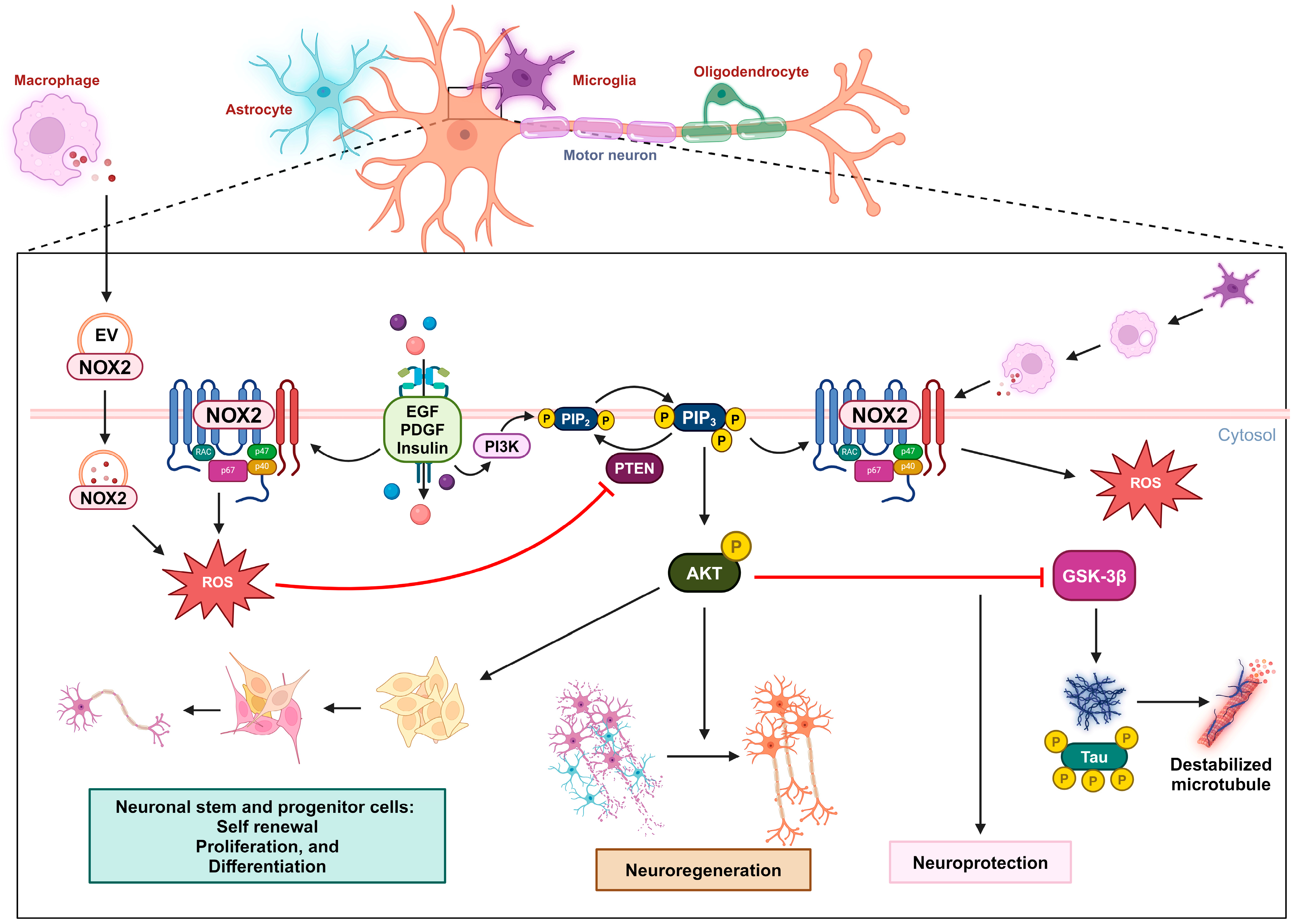
Figure 2. Oxidative inactivation of PTEN in nerve survival and regeneration. During neuronal injury, the NOX2-derived ROS concentration increases due to receptor kinase stimulation or extracellular vesicles released by macrophages. These ROS oxidize PTEN, leading to the activation of the PIP3/AKT signaling pathway, which promotes nerve regeneration. This mechanism can also promote self-renewal, proliferation, and differentiation in neuronal stem and progenitor cells. In the context of Alzheimer’s disease, the activation of the AKT pathway can downregulate GSK3β activity and the subsequent phosphorylation of the tau protein, providing neuroprotection.
3. Immune Responsiveness
Granulopoiesis is an emergency response to acute infection or inflammation, in which neutrophils are rapidly and massively produced and deployed from the bone marrow. Cytokines such as IL-6 and granulocyte colony-stimulating factor (G-CSF) are usually elevated during acute inflammation and may play a role in emergency granulopoiesis by inducing granulocyte differentiation [61][62]. In acute myocardial infarction, the myocardium also releases IL-6 and TNFα, and plasma levels of these cytokines increase after a brief episode of coronary artery blockage [63][64][65]. Kwak et al. demonstrated that an increase in ROS levels in the bone marrow alone is sufficient to trigger granulopoiesis. The elevated ROS concentration is important in promoting the proliferation and differentiation of myeloid progenitor cells via upregulated AKT signal transduction, which occurs due to the oxidative inhibition of PTEN’s phosphatase activity. During emergency granulopoiesis, these ROS are mainly produced by myeloid cells via phagocytic NOX2 activity, which can be induced by the cytokines G-CSF and TNFα. Therefore, the oxidative inactivation of PTEN by NADPH-oxidase-dependent ROS is an essential mechanism for prompting emergency granulopoiesis [66]. PI3K/AKT activity has also been shown to be a robust pivotal factor in the development of ROS-producing macrophages [67] (Figure 3).
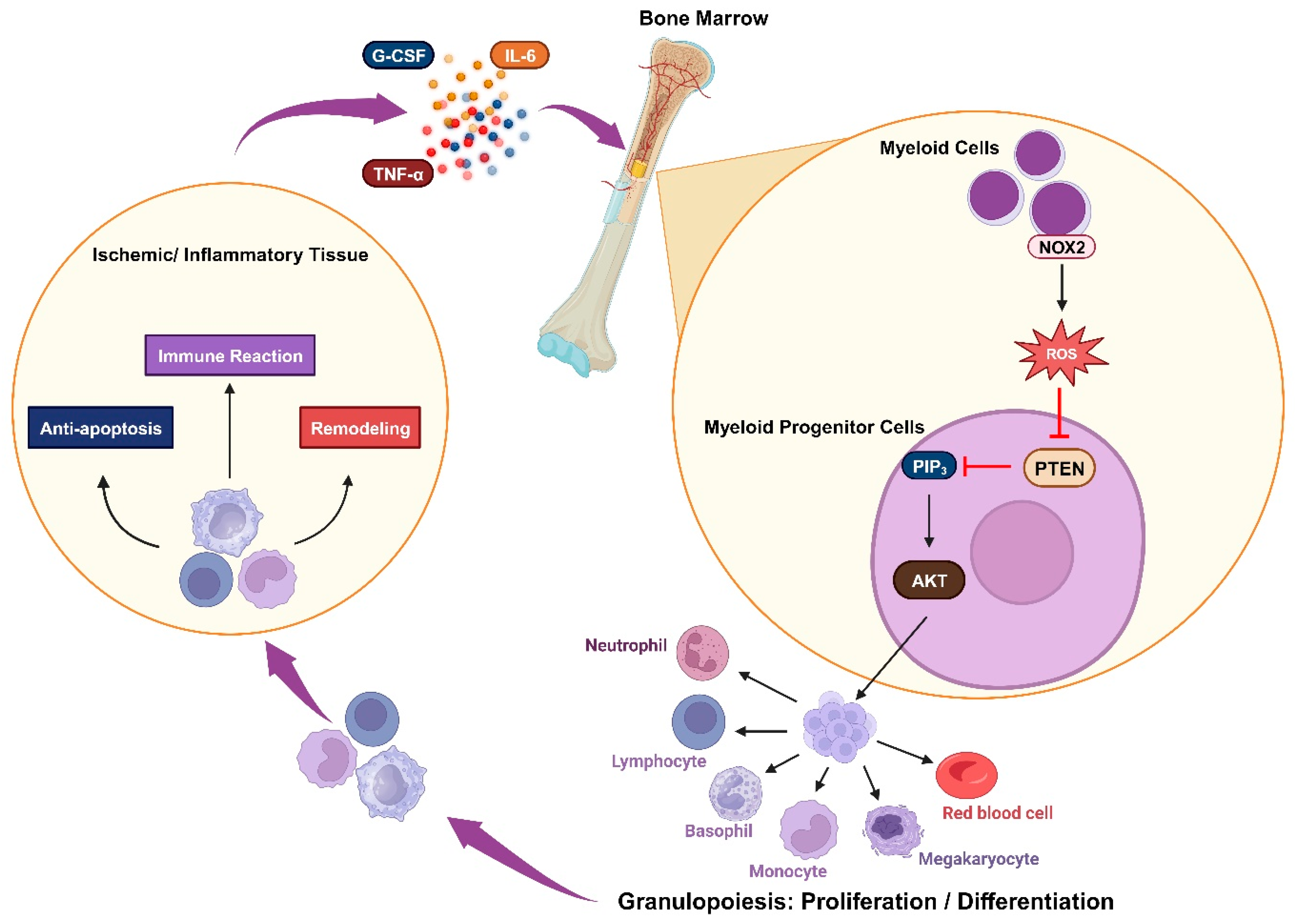
Figure 3. Oxidative inactivation of PTEN in immune responsiveness: Ischemia or inflammation can lead to elevated plasma cytokines, which stimulate myeloid cells to produce NOX2-derived ROS. These ROS mediate the AKT signaling pathway by inhibiting PTEN and trigger granulopoiesis, promoting the proliferation and differentiation of immune cells. These cells engage in immune reactions while also contributing to anti-apoptosis and remodeling processes.
4. Insulin-Related Metabolism
Insulin resistance, which is characterized by a reduced sensitivity to insulin in regulating blood glucose levels, is the primary pathological feature of type 2 diabetes mellitus. The role of ROS in insulin sensitivity is complex, with a dual effect: promoting insulin sensitivity in the early stages of disease, and contributing to insulin resistance as hyperglycemia progresses. The transient and controlled ROS production by NOXs in response to insulin is likely to be beneficial, while the chronic ROS generation by mitochondria during the context of prolonged nutrient overload in the later stages of the disease might be detrimental to insulin responsiveness [68][69]. Insulin stimulation can lead to this temporary increase in ROS levels by activating NOX and subsequently triggering insulin-mediated AKT activation. PIP3 and NOXs are located in the cell’s plasma membrane, suggesting that upon insulin stimulation, PTEN is oxidatively inactivated in close proximity to NOXs, and recruited PI3K can elevate PIP3 levels [70]. PIP3, in turn, triggers the PDK/AKT pathway, which subsequently phosphorylates various targets such as AS160, performing the anabolic effects of insulin stimulation [71][72]. The activated AKT pathway can enhance glucose absorption in adipocytes by facilitating the translocation of glucose transporter GLUT4 to the plasma membrane, as well as elevating GLUT1 expression. This aligns with the proposition that AKT signaling potentially participates in mediating insulin-stimulated responses [73]. Hence, as a negative regulator of the AKT pathway, the knockout of PTEN was experimentally shown to incrementally affect the level of GLUT4 expression in skeletal muscle and white adipose tissue, which consequently increases glucose uptake [74][75]. Additionally, in some studies, inhibiting PTEN’s PIP3-phosphatase activity has been proposed as a potential therapeutic approach for type 2 diabetes [76][77][78]. Loh et al. demonstrated that a slight increase in physiological ROS levels in muscle cells can induce PTEN oxidation and eventually enhance insulin-induced glucose uptake via the PI3K/AKT pathway [69]. Therefore, the redox regulation of PTEN holds promise as a method for managing type 2 diabetes mellitus (Figure 4).
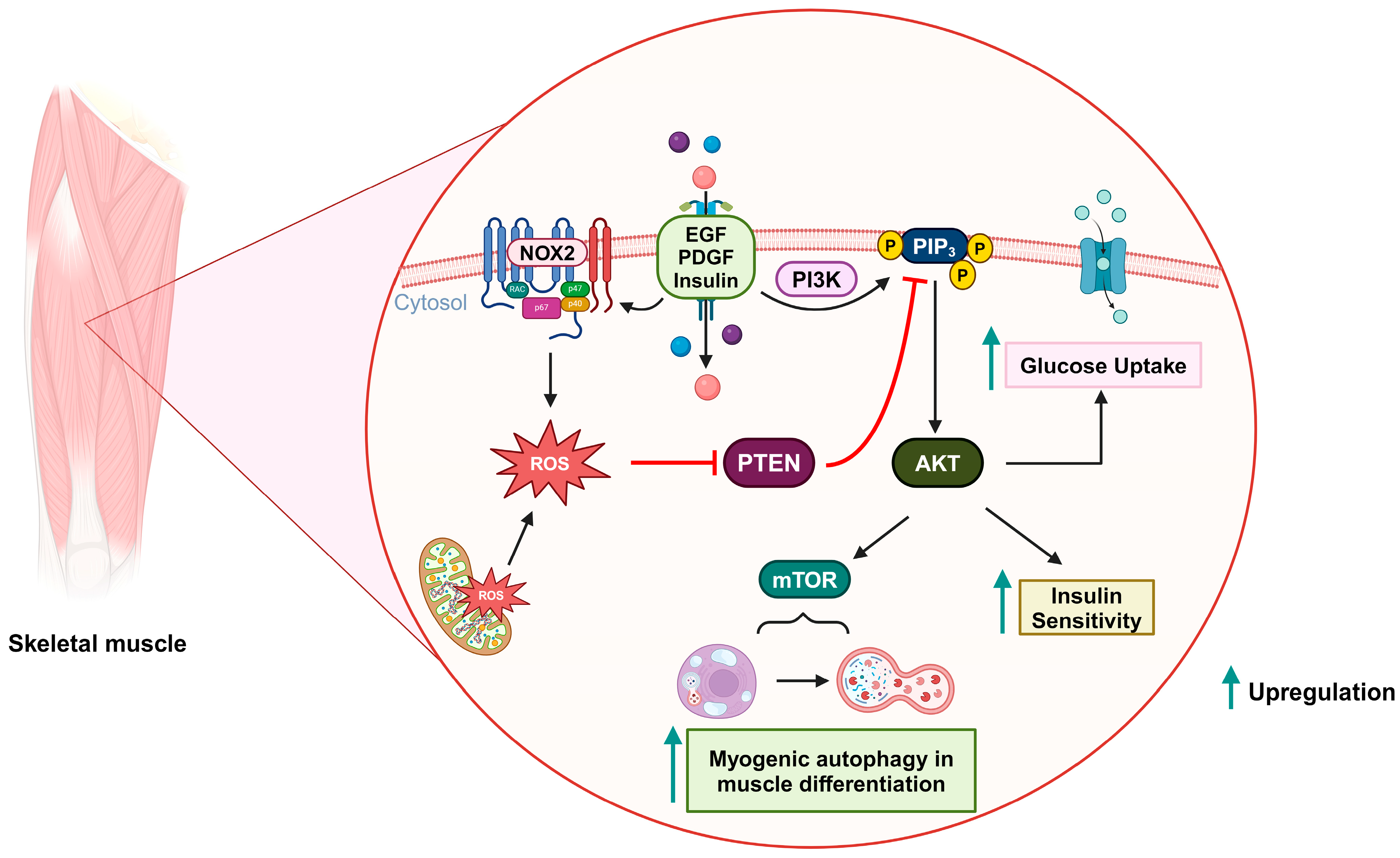
Figure 4. Oxidative inactivation of PTEN in insulin-related metabolism and muscle differentiation. Stimulation of growth factor receptors induces NOX2 activity and the production of ROS, which can oxidize PTEN and upregulate the PI3K/AKT signaling pathway. As a result, glucose uptake and insulin sensitivity are increased. During muscle differentiation, mitochondria-derived ROS can also oxidize PTEN and promote mTOR-induced myogenic autophagy.
5. Myogenic Autophagy in Muscle Differentiation
Autophagy is a crucial intracellular recycling process that eliminates old and dysfunctional cellular proteins and organelles. This process involves the formation of autophagosomes, which envelop parts of the cell’s cytoplasm that contain unnecessary components. As a result, autophagy functions as a dynamic mechanism for maintaining cellular health and resource efficiency [79][80]. Kim et al. demonstrated that the PI3K/AKT/mTOR signaling pathway is upregulated by mitochondrial ROS-derived H2O2, which subsequently implicates myogenesis-specific autophagy during muscle differentiation. In this scenario, PTEN is inactivated via oxidation [81] (Figure 4).
References
- Murphy, E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ. Res. 2004, 94, 7–16.
- Jonassen, A.K.; Sack, M.N.; Mjøs, O.D.; Yellon, D.M. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ. Res. 2001, 89, 1191–1198.
- Matsui, T.; Li, L.; del Monte, F.; Fukui, Y.; Franke, T.F.; Hajjar, R.J.; Rosenzweig, A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 1999, 100, 2373–2379.
- Uchiyama, T.; Engelman, R.M.; Maulik, N.; Das, D.K. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 2004, 109, 3042–3049.
- Tong, H.; Chen, W.; Steenbergen, C.; Murphy, E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ. Res. 2000, 87, 309–315.
- Mocanu, M.M.; Bell, R.M.; Yellon, D.M. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J. Mol. Cell. Cardiol. 2002, 34, 661–668.
- Mocanu, M.; Yellon, D. PTEN, the Achilles’ heel of myocardial ischaemia/reperfusion injury? Br. J. Pharmacol. 2007, 150, 833–838.
- Parajuli, N.; Yuan, Y.; Zheng, X.; Bedja, D.; Cai, Z.P. Phosphatase PTEN is critically involved in post-myocardial infarction remodeling through the Akt/interleukin-10 signaling pathway. Basic Res. Cardiol. 2012, 107, 248.
- Burchfield, J.S.; Iwasaki, M.; Koyanagi, M.; Urbich, C.; Rosenthal, N.; Zeiher, A.M.; Dimmeler, S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 2008, 103, 203–211.
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, e9–e18.
- Stumpf, C.; Seybold, K.; Petzi, S.; Wasmeier, G.; Raaz, D.; Yilmaz, A.; Anger, T.; Daniel, W.G.; Garlichs, C.D. Interleukin-10 improves left ventricular function in rats with heart failure subsequent to myocardial infarction. Eur. J. Heart Fail. 2008, 10, 733–739.
- Yang, Z.; Zingarelli, B.; Szabó, C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000, 101, 1019–1026.
- Keyes, K.T.; Xu, J.; Long, B.; Zhang, C.; Hu, Z.; Ye, Y. Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H1198–H1208.
- Ruan, H.; Li, J.; Ren, S.; Gao, J.; Li, G.; Kim, R.; Wu, H.; Wang, Y. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2009, 46, 193–200.
- Sun, Y. Oxidative stress and cardiac repair/remodeling following infarction. Am. J. Med. Sci. 2007, 334, 197–205.
- Fukui, T.; Yoshiyama, M.; Hanatani, A.; Omura, T.; Yoshikawa, J.; Abe, Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem. Biophys. Res. Commun. 2001, 281, 1200–1206.
- Krijnen, P.; Meischl, C.; Hack, C.; Meijer, C.; Visser, C.; Roos, D.; Niessen, H. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J. Clin. Pathol. 2003, 56, 194–199.
- Sirker, A.; Murdoch, C.E.; Protti, A.; Sawyer, G.J.; Santos, C.X.; Martin, D.; Zhang, X.; Brewer, A.C.; Zhang, M.; Shah, A.M. Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J. Mol. Cell. Cardiol. 2016, 98, 11–17.
- Nguyen Huu, T.; Park, J.; Zhang, Y.; Park, I.; Yoon, H.J.; Woo, H.A.; Lee, S.R. Redox Regulation of PTEN by Peroxiredoxins. Antioxidants 2021, 10, 302.
- Leslie, N.R.; Bennett, D.; Lindsay, Y.E.; Stewart, H.; Gray, A.; Downes, C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003, 22, 5501–5510.
- Cai, Z.; Semenza, G.L. PTEN activity is modulated during ischemia and reperfusion: Involvement in the induction and decay of preconditioning. Circ. Res. 2005, 97, 1351–1359.
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxidative Med. Cell. Longev. 2021, 2021, 6614009.
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633.
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; Del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769.
- Zaitone, S.A.; Abo-Gresha, N.M. Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: Role of iNOS and VEGF. Eur. J. Pharmacol. 2012, 691, 134–142.
- Connor, K.M.; Subbaram, S.; Regan, K.J.; Nelson, K.K.; Mazurkiewicz, J.E.; Bartholomew, P.J.; Aplin, A.E.; Tai, Y.-T.; Aguirre-Ghiso, J.; Flores, S.C. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J. Biol. Chem. 2005, 280, 16916–16924.
- Latronico, M.V.; Costinean, S.; Lavitrano, M.L.; Peschle, C.; Condorelli, G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann. N. Y. Acad. Sci. 2004, 1015, 250–260.
- Saward Peter Zahradka, L. Angiotensin II activates phosphatidylinositol 3-kinase in vascular smooth muscle cells. Circ. Res. 1997, 81, 249–257.
- Sugden, P.H. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ. Res. 2003, 93, 1179–1192.
- McDowell, S.A.; McCall, E.; Matter, W.F.; Estridge, T.B.; Vlahos, C.J. Phosphoinositide 3-kinase regulates excitation-contraction coupling in neonatal cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H796–H805.
- Goncharova, E.A.; Ammit, A.J.; Irani, C.; Carroll, R.G.; Eszterhas, A.J.; Panettieri, R.A.; Krymskaya, V.P. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 283, L354–L363.
- Perrino, C.; Schroder, J.N.; Lima, B.; Villamizar, N.; Nienaber, J.J.; Milano, C.A.; Naga Prasad, S.V. Dynamic regulation of phosphoinositide 3-kinase-γ activity and β-adrenergic receptor trafficking in end-stage human heart failure. Circulation 2007, 116, 2571–2579.
- Namgaladze, D.; Brüne, B. Phospholipase A2–modified low-density lipoprotein activates the phosphatidylinositol 3-kinase-akt pathway and increases cell survival in monocytic cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2510–2516.
- Northcott, C.A.; Hayflick, J.S.; Watts, S.W. PI3-Kinase upregulation and involvement in spontaneous tone in arteries from DOCA-salt rats: Is p110δ the culprit? Hypertension 2004, 43, 885–890.
- Wu, K.L.; Wu, C.-A.; Wu, C.-W.; Chan, S.H.; Chang, A.Y.; Chan, J.Y. Redox-sensitive oxidation and phosphorylation of PTEN contribute to enhanced activation of PI3K/Akt signaling in rostral ventrolateral medulla and neurogenic hypertension in spontaneously hypertensive rats. Antioxid. Redox Signal. 2013, 18, 36–50.
- Gebremedhin, D.; Terashvili, M.; Wickramasekera, N.; Zhang, D.X.; Rau, N.; Miura, H.; Harder, D.R. Redox signaling via oxidative inactivation of PTEN modulates pressure-dependent myogenic tone in rat middle cerebral arteries. PLoS ONE 2013, 8, e68498.
- Carnevale, D.; Vecchione, C.; Mascio, G.; Esposito, G.; Cifelli, G.; Martinello, K.; Landolfi, A.; Selvetella, G.; Grieco, P.; Damato, A. PI3Kγ inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc. Res. 2012, 93, 200–209.
- Ning, K.; Pei, L.; Liao, M.; Liu, B.; Zhang, Y.; Jiang, W.; Mielke, J.G.; Li, L.; Chen, Y.; El-Hayek, Y.H.; et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J. Neurosci. 2004, 24, 4052–4060.
- Mao, L.; Jia, J.; Zhou, X.; Xiao, Y.; Wang, Y.; Mao, X.; Zhen, X.; Guan, Y.; Alkayed, N.J.; Cheng, J. Delayed administration of a PTEN inhibitor BPV improves functional recovery after experimental stroke. Neuroscience 2013, 231, 272–281.
- Zhao, J.; Qu, Y.; Wu, J.; Cao, M.; Ferriero, D.; Zhang, L.; Mu, D. PTEN inhibition prevents rat cortical neuron injury after hypoxia–ischemia. Neuroscience 2013, 238, 242–251.
- Wu, J.; Li, J.; Hu, H.; Liu, P.; Fang, Y.; Wu, D. Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell. Mol. Neurobiol. 2012, 32, 1187–1197.
- Guo, J.-Y.; Ding, J.; Yuan, F.; Chen, H.; Chen, S.-W.; Tian, H.-L. Dose-dependent protective effect of bisperoxovanadium against acute cerebral ischemia in a rat model of ischemia/reperfusion injury. Int. J. Mol. Sci. 2013, 14, 12013–12022.
- Wu, D.-N.; Pei, D.-S.; Wang, Q.; Zhang, G.-Y. Down-regulation of PTEN by sodium orthovanadate inhibits ASK1 activation via PI3-K/Akt during cerebral ischemia in rat hippocampus. Neurosci. Lett. 2006, 404, 98–102.
- Zhao, H.; Sapolsky, R.M.; Steinberg, G.K. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol. Neurobiol. 2006, 34, 249–269.
- Christie, K.J.; Zochodne, D. Peripheral axon regrowth: New molecular approaches. Neuroscience 2013, 240, 310–324.
- Garcia-Junco-Clemente, P.; Golshani, P. PTEN: A master regulator of neuronal structure, function, and plasticity. Commun. Integr. Biol. 2014, 7, e28358.
- Knafo, S.; Esteban, J.A. PTEN: Local and Global Modulation of Neuronal Function in Health and Disease. Trends Neurosci. 2017, 40, 83–91.
- Ohtake, Y.; Hayat, U.; Li, S. PTEN inhibition and axon regeneration and neural repair. Neural Regen. Res. 2015, 10, 1363–1368.
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966.
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50.
- Sun, Y.; Zhang, L.; Chen, Y.; Zhan, L.; Gao, Z. Therapeutic Targets for Cerebral Ischemia Based on the Signaling Pathways of the GluN2B C Terminus. Stroke 2015, 46, 2347–2353.
- Soltoff, S.P.; Rabin, S.L.; Cantley, L.C.; Kaplan, D.R. Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. J. Biol. Chem. 1992, 267, 17472–17477.
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010, 13, 1075–1081.
- Christie, K.J.; Webber, C.A.; Martinez, J.A.; Singh, B.; Zochodne, D.W. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neurosci. 2010, 30, 9306–9315.
- Little, D.; Valori, C.F.; Mutsaers, C.A.; Bennett, E.J.; Wyles, M.; Sharrack, B.; Shaw, P.J.; Gillingwater, T.H.; Azzouz, M.; Ning, K. PTEN depletion decreases disease severity and modestly prolongs survival in a mouse model of spinal muscular atrophy. Mol. Ther. 2015, 23, 270–277.
- Ning, K.; Drepper, C.; Valori, C.F.; Ahsan, M.; Wyles, M.; Higginbottom, A.; Herrmann, T.; Shaw, P.; Azzouz, M.; Sendtner, M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum. Mol. Genet. 2010, 19, 3159–3168.
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71.
- Giridharan, S.S.; Caplan, S. MICAL-family proteins: Complex regulators of the actin cytoskeleton. Antioxid. Redox Signal. 2014, 20, 2059–2073.
- Zhu, Y.; Hoell, P.; Ahlemeyer, B.; Sure, U.; Bertalanffy, H.; Krieglstein, J. Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson’s disease. Neurochem. Int. 2007, 50, 507–516.
- Delgado-Esteban, M.; Martin-Zanca, D.; Andres-Martin, L.; Almeida, A.; Bolaños, J.P. Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J. Neurochem. 2007, 102, 194–205.
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314.
- Walker, F.; Zhang, H.-H.; Matthews, V.; Weinstock, J.; Nice, E.C.; Ernst, M.; Rose-John, S.; Burgess, A.W. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF–and GM-CSF–deficient mice. Blood J. Am. Soc. Hematol. 2008, 111, 3978–3985.
- Gwechenberger, M.; Mendoza, L.H.; Youker, K.A.; Frangogiannis, N.G.; Smith, C.W.; Michael, L.H.; Entman, M.L. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 1999, 99, 546–551.
- Kleinbongard, P.; Heusch, G.; Schulz, R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010, 127, 295–314.
- Frangogiannis, N.G. The mechanistic basis of infarct healing. Antioxid. Redox Signal. 2006, 8, 1907–1939.
- Kwak, H.-J.; Liu, P.; Bajrami, B.; Xu, Y.; Park, S.-Y.; Nombela-Arrieta, C.; Mondal, S.; Sun, Y.; Zhu, H.; Chai, L. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity 2015, 42, 159–171.
- Liu, H.; Perlman, H.; Pagliari, L.J.; Pope, R.M. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages: Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 2001, 194, 113–126.
- Tiganis, T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011, 32, 82–89.
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10, 260–272.
- Seo, J.H.; Ahn, Y.; Lee, S.-R.; Yeo, C.Y.; Hur, K.C. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol. Biol. Cell 2005, 16, 348–357.
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic role of PTEN in insulin signaling and resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137.
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes 2013, 62, 1519–1526.
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 1996, 271, 31372–31378.
- Wang, D.F.; Yang, H.J.; Gu, J.Q.; Cao, Y.L.; Meng, X.; Wang, X.L.; Lin, Y.C.; Gao, M. Suppression of phosphatase and tensin homolog protects insulin-resistant cells from apoptosis. Mol. Med. Rep. 2015, 12, 2695–2700.
- Nakashima, N.; Sharma, P.M.; Imamura, T.; Bookstein, R.; Olefsky, J.M. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 2000, 275, 12889–12895.
- Wang, L.; Liu, Y.; Yan Lu, S.; Nguyen, K.-T.T.; Schroer, S.A.; Suzuki, A.; Mak, T.W.; Gaisano, H.; Woo, M. Deletion of Pten in pancreatic β-cells protects against deficient β-cell mass and function in mouse models of type 2 diabetes. Diabetes 2010, 59, 3117–3126.
- Wijesekara, N.; Konrad, D.; Eweida, M.; Jefferies, C.; Liadis, N.; Giacca, A.; Crackower, M.; Suzuki, A.; Mak, T.W.; Kahn, C.R. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol. Cell. Biol. 2005, 25, 1135–1145.
- Rosivatz, E. Inhibiting PTEN. Biochem. Soc. Trans. 2007, 35, 257–259.
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741.
- Harris, H.; Rubinsztein, D.C. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 2011, 8, 108–117.
- Kim, J.H.; Choi, T.G.; Park, S.; Yun, H.R.; Nguyen, N.N.Y.; Jo, Y.H.; Jang, M.; Kim, J.; Kim, J.; Kang, I.; et al. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ. 2018, 25, 1921–1937.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
525
Revisions:
3 times
(View History)
Update Date:
18 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




