Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya D. Klabukov | -- | 2671 | 2024-02-11 11:34:08 | | | |
| 2 | Rita Xu | Meta information modification | 2671 | 2024-02-18 06:22:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Klabukov, I. Gene-Activated Materials in Regenerative Dentistry and Periodontology. Encyclopedia. Available online: https://encyclopedia.pub/entry/54984 (accessed on 07 February 2026).
Klabukov I. Gene-Activated Materials in Regenerative Dentistry and Periodontology. Encyclopedia. Available at: https://encyclopedia.pub/entry/54984. Accessed February 07, 2026.
Klabukov, Ilya. "Gene-Activated Materials in Regenerative Dentistry and Periodontology" Encyclopedia, https://encyclopedia.pub/entry/54984 (accessed February 07, 2026).
Klabukov, I. (2024, February 11). Gene-Activated Materials in Regenerative Dentistry and Periodontology. In Encyclopedia. https://encyclopedia.pub/entry/54984
Klabukov, Ilya. "Gene-Activated Materials in Regenerative Dentistry and Periodontology." Encyclopedia. Web. 11 February, 2024.
Copy Citation
Treatment of a wide variety of defects in the oral and maxillofacial regions requires the use of innovative approaches to achieve best outcomes. One of the promising directions is the use of gene-activated materials (GAMs) that represent a combination of tissue engineering and gene therapy.
dentistry
gene activation
gene therapy
periodontology
regenerative medicine
bioengineering
tissue engineering
1. Introduction
Application of regenerative medicine and tissue engineering methods has great potential for improvement of oral and dental tissue regeneration and can be called regenerative dentistry [1]. Regenerative techniques have been studied for the treatment of periodontal disease, jaw bone and gingiva defects, age-related disorders, etc., with the aim of restoring tissue properties, functions, and structure. Since tissue regeneration in the oral and maxillofacial regions involves multifaceted and complex processes, the enhancement of tissue-engineering tools with gene therapy promises to improve outcomes. The concept of gene-activated materials (GAMs) represents the combination of tissue engineering and gene therapy [2]. The GAM approach implies that biocompatible materials will be enriched with vectors carrying therapeutic genes and will result in transfection of recipient’s cells after implantation [3][4].
Previous studies have shown that the use of various growth factors can increase clinical attachment level, reduce gingival recession, and enhance linear bone gain in patients with periodontal osseous defects, and stimulate proliferation of gingival fibroblasts, osteoblasts, periodontal ligament cells, etc. [5][6][7][8]. However, the benefits of growth factors face a problem of rapid protein degradation requiring the use of supraphysiological doses [9]. Transfection of cells with genes encoding therapeutic proteins helps to overcome these disadvantages and facilitates gradual prolonged protein secretion by recipients’ cells in the defect site converting them into bioreactors in situ [10].
Composition of plasmid-based GAMs implies the presence of the following essential elements: naked plasmid or plasmid/delivery system complex, encoded therapeutic protein, and biocompatible material, enriched with plasmid. The way of attaching plasmid to the material is also of importance since it may influence kinetics of plasmid release. Variability inside GAM elements allows achievement of different effects. Thus, various encoded proteins exert different therapeutic functions. Materials used for GAM creation may also possess different properties depending on chemical structure and biomechanical parameters that may influence tissue regeneration and clinical outcome [11][12][13]. The exact GAM composition may be chosen depending on the clinical task to be solved.
The use of GAM has already shown its potential in pre-clinical studies for the treatment of bone and skin defects [14][15]. In the field of regenerative dentistry, GAMs were successfully used in vivo for teeth replantation, treatment of alveolar bone defects after tooth extraction, and even showed their safety and efficacy for jaw bone grafting in clinical trial [16][17][18]. Osseointegration of titanium implants with the use of the GAM approach was also investigated in vivo [19].
2. Gene-Activated Materials’ Mechanism of Action
GAMs’ mechanism of action is based on the combination of the properties of the material to form a provisional template for new tissue formation in the area of the defect and the properties of a gene-carrying vector to transfect recipient’s cells and stimulate local secretion of encoded protein. Exact mechanisms of action are deeply dependent on pathology being addressed, type of biocompatible material used, encoded protein, type of delivery system, and approach to attaching plasmid (or a plasmid/delivery system complex) to the material.
Transfection of cells may be performed in vitro by culturing cells with non-viral vectors, followed by seeding of cells on the material and implantation into the damaged area [20]. However, the GAM approach implies that the material will be enriched with plasmid or a plasmid/delivery system complex (in contrast to preliminary transfected cells), implanted into the damaged area, followed by the recipient’s cell transfection and secretion of encoded protein (Figure 1). Such an approach helps to elude the costly and labor-intensive stage of in vitro cell culture and does not fall under strict legal requirements for cell culture.
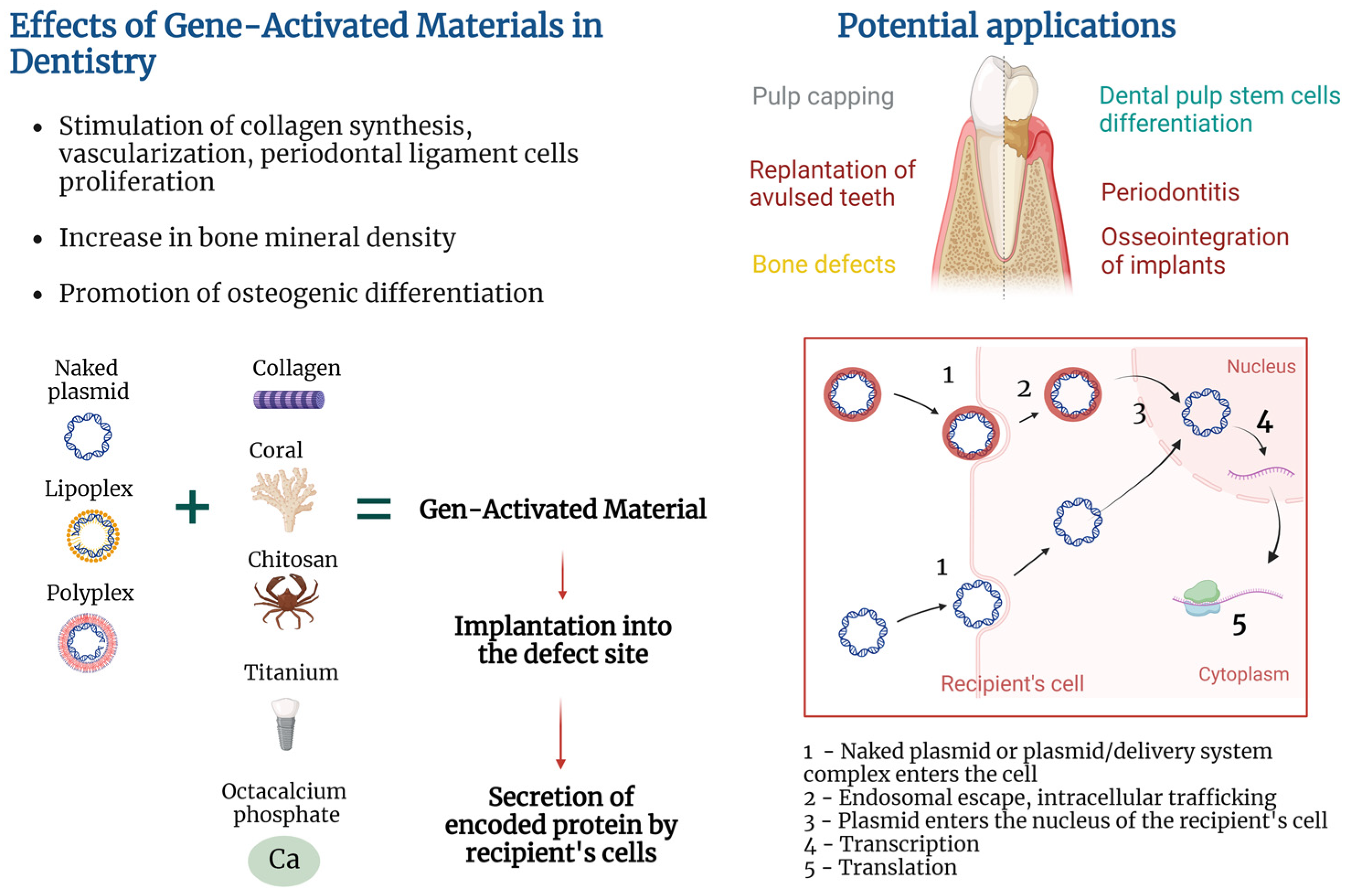
Figure 1. Gene-activated materials’ mechanism of action.
2.1. Implantation of GAM into a Damaged Area
The technique of GAM positioning at the injury site depends on material and treated pathology. For example, fixation of gene-activated octacalcium phosphate (OCP) within a bone grafting site was achieved via soft tissue suturing in a clinical trial [18]. In vivo studies also demonstrated various techniques of GAM implantation; for example, covering of the root of avulsed teeth with poly(D,L-lactic-co-glycolic acid) (PLGA) in dogs [16], placing collagen into an alveolar bone defect followed by muscle suturing and skin incision closure with clips in rats [21], filling of sockets after tooth extraction with gelatin sponges followed by coverage with periodontal dressing in rats [17], and layer-by-layer coating of a titanium implant followed by implantation in dogs [19].
2.2. Colonization of the Material with Cells/Release of the Plasmid from the Material
After implantation, the GAM starts to exert dual functions explained by a combination of tissue engineering and gene therapy in the GAM approach. Aside from being tools for incorporation of the plasmid or plasmid/delivery system complex, the paradigm of tissue engineering implies that materials should represent a provisional template for cell migration, adhesion, proliferation, differentiation, and deposition of extracellular matrix at the defect site [2]. Release of the plasmid or plasmid/delivery system complex from GAM occurs in the course of material degradation or plasmid diffusion depending on the type of material and method of plasmid attachment [4]. Tailoring physicochemical parameters of the materials, as well as choosing ways of adhering plasmid to the material, allow the achievement of different plasmid release kinetics, which is valuable for temporal control over gene expression [22][23].
2.3. Recipient’s Cell Transfection
Transfection of cells occurs via uptake of naked plasmids of the plasmid/delivery system complex. Some works used naked plasmids for cell transfection in vivo and in vitro, suggesting their ability to be internalized and result in increased protein secretion [24], or in reporter gene expression [4]. At the same time, there is an opinion that entry of naked plasmids into eukaryotic cells may be hampered by the negative charge of the plasmid, so a variety of approaches were used to increase transfection efficiency [25]. For example, cationic polymers facilitate plasmid/delivery system complex uptake via neutralization of DNA negative charge followed by endocytosis and endosomal escape [26].
2.4. Cytoplasmic Transport and Nucleus Entry
After entering the cell, free DNA is complexed with intracellular proteins that facilitate microtubule-mediated cytoplasmic transport of plasmid DNA to the surface of the cell nucleus [27][28]. Plasmids may enter the nucleus of dividing cells at the time of nuclear envelope disassembly or cross the nuclear envelope of non-dividing cells via nuclear pore complex [27][29].
2.5. Transcription, Translation, Expression Duration
The host’s RNA polymerase II facilitates plasmid DNA transcription and mRNA synthesis. The duration of transgene expression greatly depends on the cell type that was transfected. For example, muscle cells are characterized by long-lasting expression in mice for more than a year, presumably because of the absence of cell division [30]. When GAMs are concerned, duration of expression also depends on plasmid release kinetics from the materials, with a longer release period yielding prolonged expression [23].
2.6. Protein Secretion
Transfected cells start to secrete plasmid-encoded proteins which may be directed to the achievement of various goals. For example, chitosan/collagen matrix enriched with plasmid encoding platelet-derived growth factor (pPDGF) promoted proliferation of human periodontal ligament cells (hPDLCs) and formation of a periodontal connective tissue-like structure in vitro [4]. At the same time, Yang et al. reported slower proliferation of dental pulp stem cells (DPSCs) cultured on chitosan/collagen matrix enriched with plasmids carrying the bone morphogenetic protein-7 (BMP) gene and hypothesized that this may be due to the reverse correlation between the DPSCs’ odontoblastic differentiation caused by BMP-7 secretion and proliferation [31].
3. Heterogeneous Design of Gene-Activated Materials
In the field of regenerative dentistry, there is heterogeneity in the design of plasmid-based GAMs. It is explained by the diversity in the following fundamental structural elements of the GAMs: (1) type of material used, (2) type of encoded protein, (3) type of delivery system, (4) method of attaching plasmid or plasmid/delivery system complex to the material. The exact GAM design may be chosen considering the type of treated pathology and individual patient characteristics.
3.1. Materials Used for Creation of GAMs in the Field of Regenerative Dentistry
In tissue engineering, a variety of natural and synthetic materials are used. In order to be used for creation of a GAM, material must not only be biocompatible and support tissue regeneration but also be able to attach a naked plasmid or a plasmid/delivery system complex. To create GAMs in the field of regenerative dentistry, various materials are used depending on the scientific and clinical problem being solved.
Collagen is a biocompatible biodegradable material capable of stimulating migration of human oral region fibroblasts, endothelial, and periodontal ligament cells [32], and healing of bone, cementum, and periodontal ligament [33], and alveolar bone defects [34]. Chitosan is a natural biocompatible, biodegradable polymer with a low immunogenicity possessing bacteriostatic, fungistatic, and anti-inflammatory properties [35][36], capable of promoting the proliferation of human hPDLCs [37].
Hydroxyapatite is frequently used for bone grafting due to the resemblance of its structure and composition to the natural mineral phase of human bone tissue, and osteoconductive and osteoinductive properties [38]. In dentistry, hydroxyapatite is frequently combined with collagen to achieve enhanced mechanical properties [39].
Coral is a natural porous biocompatible material also used for bone tissue engineering due to its osteoconductive and mechanical properties [40]. Coral scaffolds gene-activated with pPDGF-B were shown to stimulate proliferation of hPDLCs and synthesis of type I collagen, suggesting its possible use for periodontal regeneration [41].
Poly(lactic-co-glycolic acid) is a biocompatible and biodegradable copolymer of lactic and glycolic acid. Depending on the composition and manufacturing conditions, PLGA may be tailored to have a certain degradation and drug/plasmid release rates [42][43], mechanical properties, and porosity [44].
Gelatin is a biocompatible biodegradable natural bio polymer characterized by cost-effectiveness and low immunogenicity, as well as various physical and chemical properties depending on the preparation technique [45].
Octacalcium phosphate (OCP) is a mineral precursor of apatite crystals in bone tissue; it possesses osteoconductive and osteoinductive properties and allows adhesion of bioactive molecules [46].
Titanium and its alloys are widely used in dental prosthetics and for the reconstruction of maxillofacial bone defects as well as in orthopedics [47][48]. Despite excellent biocompatibility, surface modifications of titanium implants are studied to improve their osseointegration [47].
3.2. Types of Encoded Proteins Used in GAMs in Regenerative Dentistry
The importance of growth factors in soft and bone tissue regeneration has already been shown in multiple studies. Next, various proteins encoded by plasmids in GAMs used in regenerative dentistry will be briefly overviewed.
Vascular endothelial growth factor (VEGF) not only acts as a mitogen for endothelial cells stimulating angiogenesis but also promotes human periodontal ligament stem cells’ (hPDLSCs) odonto-/osteogenic differentiation in vitro [49][50]. Genetic activation of materials with pVEGF is a promising direction for the stimulation of bone tissue regeneration since sufficient vascularization is necessary for successful osteogenesis [18].
Platelet-derived growth factor is capable of stimulating osteoblast, periodontal ligament cell, and gingival fibroblast proliferation [6][8]. Clinical trials showed that PDGF in combination with beta-tricalcium phosphate resulted in an increased clinical attachment level and reduced gingival recession at 3 months, and greater linear bone gain and defect fill at 6 months compared to scaffold alone [7].
Fibroblast growth factors (FGFs) are a large family of regulatory molecules that have promoting effects on a variety of cells including hPDLCs and hPDLSCs [5][50]. An in vitro study with the use of a mouse periodontal ligament cell line also showed the ability of FGF-2 to stimulate VEGF-A secretion [51]. Notably, recombinant human FGF-2 in combination with modified Widman periodontal surgery increased bone fill in patients with periodontitis in a clinical trial [52].
Bone morphogenetic proteins are signaling molecules with multiple functions and members of the TGF-β superfamily [53]. BMP-2 is used for the treatment of bone lesions since this protein possesses high osteogenic activity via promoting osteogenic differentiation of pre-osteoblastic cells [54][55].
Although the benefits of using growth factors have been shown in many studies, their use has some disadvantages, including a short half-life when applied to the defect site and application of supraphysiological doses [9]. The use of GAMs allows for the prevention of the problem of rapid degradation of recombinant growth factors as well as for gradual secretion of the growth factor by the recipient’s cells at the defect site.
3.3. Types of Non-Viral Delivery Systems Used in GAMs in the Field of Regenerative Dentistry
Ensuring the efficient transfer of genetic material into the cell is one of the main challenges in gene therapy. To enhance plasmid transfection efficiency, a variety of non-viral delivery agents are used. Non-viral delivery systems may be represented by biodegradable and non-biodegradable polymers, lipids, peptides, inorganic materials (e.g., gold and magnetic nanoparticles), hybrid systems [56], calcium phosphate [57], exosomes [58], etc. Researchers will briefly describe non-viral delivery systems that are used as part of GAMs in the field of regenerative dentistry.
Polyethyleneimine (PEI) is a positively charged cationic polymer that facilitates plasmid entry through negatively charged cell membranes via condensation of negatively charged plasmid DNA [59]. One of the most common cationic polymers is represented by PEI, with 25 kDa branched PEI being considered one of the most efficient types [17]. However, the non-biodegradable nature of PEI may result in a cytotoxic effect as seen in human periodontal ligament fibroblasts and gingival fibroblasts [21]. Some authors suggested that cytotoxicity is higher in PEI with high molecular weight compared to low molecular weight [60]. Importantly, Plonka et al. showed that even though PEI reduced viability of human periodontal ligament fibroblasts and gingival fibroblasts, transfection of PEI/pPDGF polyplexes nivelated detrimental impact of PEI suggesting the stimulating effect of transfection with growth factor-encoding plasmid [21].
Chitosan is a natural cationic biodegradable polymer capable of interacting with negatively charged plasmid DNA via electrostatic forces [61]. Low cytotoxicity was also reported for the chitosan-based delivery system [62]. In the study by Peng et al. (2009), transfection of hPDLCs with pDNA/chitosan resulted in higher cell viability compared to naked plasmid alone [4]. At the same time, many studies combined chitosan with other delivery systems or subjected it to various modifications to improve its transfection efficiency [63].
Cationic liposomes are widely used gene delivery systems. Cationic liposomes act by binding positively charged head groups of lipids with negatively charged phosphate groups of plasmid DNA and by surrounding DNA molecules [64].
Delivery of naked plasmid DNA is also considered as a promising direction and has some advantages, among which is the absence of delivery systems with potential cytotoxicity. Supercoiling of plasmid DNA also aids more efficient transfection [65].
3.4. Methods of Binding Plasmids or Plasmid/Delivery System Complexes to the Materials
Various methods of attaching the plasmid or plasmid/delivery system complex to the material are used and are of importance since this parameter may affect the kinetics of plasmid release and duration of transgene expression [23]. Methods of attaching plasmids or plasmid/delivery system complexes to materials may be divided into chemical and physical methods. Chemical methods rely on the creation of bonds that facilitate plasmid attachment to the material. For example, calcium-containing materials may bind DNA via electrostatic interactions due to the presence of phosphate groups on the DNA backbone.
Physical methods do not imply creation of bonds between plasmid and material and include emulsion electrospinning that allows for the incorporation of the buffer with the target plasmid into the volume of polymeric microfibers for subsequent release in the course of material degradation [16][66]. Importantly, several authors noted that incorporation of the plasmid into the material may cause DNA damage due to interaction with solvents during manufacture [2][23]. One of the solutions to this issue is coaxial (core-shell) electrospinning that allows for the obtaining of core-shell fibers, the inner compartment of which may be filled with a plasmid-containing buffer avoiding plasmid interaction with damaging solvents [23].
From a technical point of view, the attachment of a naked plasmid or a plasmid/delivery system complex to the material may be achieved by different methods, some of which were described by Peng et al. [4]. Among such methods are dropping plasmid-containing suspension on the material or soaking of the material in the suspension followed by some time of incubation [17][67], injection into the volume of the material [68], or coating of the material with other plasmid-containing materials [41]. The layer-by-layer technique allows for the coating of the materials with multiple oppositely charged plasmid-containing layers [19].
References
- Tran, S.D.; Bakkar, M.O.; Sumita, Y.; Kishimoto, N. Regenerative dentistry in periodontics. Saudi Dent. J. 2019, 31, 301.
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polymer 2014, 46, 476–482.
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369.
- Peng, L.; Cheng, X.; Zhuo, R.; Lan, J.; Wang, Y.; Shi, B.; Li, S. Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J. Biomed. Mater. Res. Part A 2009, 90, 564–576.
- An, S.; Huang, X.; Gao, Y.; Ling, J.; Huang, Y.; Xiao, Y. FGF-2 induces the proliferation of human periodontal ligament cells and modulates their osteoblastic phenotype by affecting Runx2 expression in the presence and absence of osteogenic inducers. Int. J. Mol. Med. 2015, 36, 705–711.
- Marcopoulou, C.E.; Vavouraki, H.N.; Dereka, X.E.; Vrotsos, I.A. Proliferative effect of growth factors TGF-beta1, PDGF-BB and rhBMP-2 on human gingival fibroblasts and periodontal ligament cells. J. Int. Acad. Periodontol. 2003, 5, 63–70.
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J. Periodontol. 2005, 76, 2205–2215.
- Wu, Y.; Zhang, Y.; Yin, Q.; Xia, H.; Wang, J. Platelet-derived growth factor promotes osteoblast proliferation by activating G-protein-coupled receptor kinase interactor-1. Mol. Med. Rep. 2014, 10, 1349–1354.
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth factor engineering strategies for regenerative medicine applications. Front. Bioeng. Biotechnol. 2020, 7, 469.
- Laird, N.Z.; Acri, T.M.; Tingle, K.; Salem, A.K. Gene-and RNAi-activated scaffolds for bone tissue engineering: Current progress and future directions. Adv. Drug Deliv. Rev. 2021, 174, 613–627.
- Tatani, I.; Panagopoulos, A.; Diamantakos, I.; Sakellaropoulos, G.; Pantelakis, S.; Megas, P. Comparison of two metaphyseal-fitting (short) femoral stems in primary total hip arthroplasty: Study protocol for a prospective randomized clinical trial with additional biomechanical testing and finite element analysis. Trials 2019, 20, 359.
- Xie, Y.; Wang, X.; Jian, Q.; Fan, X.; Yu, Y.; Gu, D.; Wu, W. Three dimensional finite element analysis used to study the influence of the stress and strain of the operative and adjacent segments through different foraminnoplasty technique in the PELD: Study protocol clinical trial (SPIRIT Compliant). Medicine 2020, 99, e19670.
- Waechter, J.; Madruga, M.D.M.; Carmo Filho, L.C.D.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between tapered and cylindrical implants in the posterior regions of the mandible: A prospective, randomized, split-mouth clinical trial focusing on implant stability changes during early healing. Clin. Implant. Dent. Relat. Res. 2017, 19, 733–741.
- Huang, Y.C.; Simmons, C.; Kaigler, D.; Rice, K.G.; Mooney, D.J. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4). Gene Ther. 2005, 12, 418–426.
- Hwang, J.; Kiick, K.L.; Sullivan, M.O. VEGF-Encoding, Gene-Activated Collagen-Based Matrices Promote Blood Vessel Formation and Improved Wound Repair. ACS Appl. Mater. Interfaces 2023, 15, 16434–16447.
- Jiang, L.; Ding, Z.; Xia, S.; Liu, Y.; Lei, S.; Zhong, M.; Chen, X. Poly lactic-co-glycolic acid scaffold loaded with plasmid DNA encoding fibroblast growth factor-2 promotes periodontal ligament regeneration of replanted teeth. J. Periodontal Res. 2020, 55, 488–495.
- Jin, H.; Liu, Z.; Li, W.; Jiang, Z.; Li, Y.; Zhang, B. Polyethylenimine-alginate nanocomposites based bone morphogenetic protein 2 gene-activated matrix for alveolar bone regeneration. RSC Adv. 2019, 9, 26598–26608.
- Bozo, I.Y.; Drobyshev, A.Y.; Redko, N.A.; Komlev, V.S.; Isaev, A.A.; Deev, R.V. Bringing a gene-activated bone substitute into clinical practice: From bench to bedside. Front. Bioeng. Biotechnol. 2021, 32, 599300.
- He, F.M.; Shan, H.Q.; Shen, J.W.; Jiang, Q.H. Bone Formation at Porous Titanium Implants Coated with Multiple Layers of Recombinant Human Bone Morphogenetic Protein-2 cDNA Plasmid in the Posterior Mandible in Dogs. Int. J. Oral Maxillofac. Implant. 2013, 28, 1648–1654.
- Yang, C.; Liu, Y.; Li, C.; Zhang, B. Repair of mandibular defects by bone marrow stromal cells expressing the basic fibroblast growth factor transgene combined with multi-pore mineralized Bio-Oss. Mol. Med. Rep. 2013, 7, 99–104.
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2017, 24, 31–39.
- Shea, L.D.; Smiley, E.; Bonadio, J.; Mooney, D.J. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999, 17, 551–554.
- Xie, Q.; Jia, L.N.; Xu, H.Y.; Hu, X.G.; Wang, W.; Jia, J. Fabrication of core-shell PEI/pBMP2-PLGA electrospun scaffold for gene delivery to periodontal ligament stem cells. Stem Cells Int. 2016, 2016, 5385137.
- Bettan, M.; Emmanuel, F.; Darteil, R.; Caillaud, J.M.; Soubrier, F.; Delaere, P.; Branelec, D.; Mahfoudi, A.; Duverger, N.; Scherman, D. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle. Mol. Ther. 2000, 2, 204–210.
- Tatlow, D.; Tatlow, C.; Tatlow, S.; Tatlow, S. A novel concept for treatment and vaccination against COVID-19 with an inhaled chitosan-coated DNA vaccine encoding a secreted spike protein portion. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1874–1878.
- Sabin, J.; Alatorre-Meda, M.; Miñones, J., Jr.; Domínguez-Arca, V.; Prieto, G. New insights on the mechanism of polyethylenimine transfection and their implications on gene therapy and DNA vaccines. Colloids Surf. B Biointerfaces 2022, 210, 112219.
- Bai, H.; Lester GM, S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616.
- Badding, M.A.; Vaughan, E.E.; Dean, D.A. Transcription factor plasmid binding modulates microtubule interactions and intracellular trafficking during gene transfer. Gene Ther. 2012, 19, 338–346.
- Dean, D.A. Import of plasmid DNA into the nucleus is sequence specific. Exp. Cell Res. 1997, 230, 293–302.
- Muramatsu, T.; Arakawa, S.; Fukazawa, K.; Fujiwara, Y.; Yoshida, T.; Sasaki, R.; Masuda, S.; Park, H.M. In vivo gene electroporation in skeletal muscle with special reference to the duration of gene expression. Int. J. Mol. Med. 2001, 7, 37–79.
- Yang, X.; Han, G.; Pang, X.; Fan, M. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2011, 108, 2519–2526.
- Asparuhova, M.B.; Stähli, A.; Guldener, K.; Sculean, A. A novel volume-stable collagen matrix induces changes in the behavior of primary human oral fibroblasts, periodontal ligament, and endothelial cells. Int. J. Mol. Sci. 2021, 22, 4051.
- Kosen, Y.; Miyaji, H.; Kato, A.; Sugaya, T.; Kawanami, M. Application of collagen hydrogel/sponge scaffold facilitates periodontal wound healing in class II furcation defects in beagle dogs. J. Periodontal Res. 2012, 47, 626–634.
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83.
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for tissue engineering. Nov. Biomater. Regen. Med. 2018, 1077, 475–485.
- Mohyuddin, S.G.; Qamar, A.; Hu, C.Y.; Chen, S.W.; Wen, J.Y.; Liu, X.X.; Ma, X.B.; Yu, Z.C.; Yong, Y.H.; Wu, L.Y.; et al. Effect of chitosan on blood profile, inflammatory cytokines by activating TLR4/NF-κB signaling pathway in intestine of heat stressed mice. Sci. Rep. 2021, 11, 20608.
- Peng, L.; Cheng, X.R.; Wang, J.W.; Xu, D.X.; Wang, G.E. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J. Bioact. Compat. Polym. 2006, 21, 207–220.
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721.
- Ohba, S.; Sumita, Y.; Nakatani, Y.; Noda, S.; Asahina, I. Alveolar bone preservation by a hydroxyapatite/collagen composite material after tooth extraction. Clin. Oral Investig. 2019, 23, 2413–2419.
- Wu, Y.C.; Lee, T.M.; Chiu, K.H.; Shaw, S.Y.; Yang, C.Y. A comparative study of the physical and mechanical properties of three natural corals based on the criteria for bone–tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 1273–1280.
- Zhang, Y.; Wang, Y.; Shi, B.; Cheng, X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 2007, 28, 1515–1522.
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454.
- Vey, E.; Rodger, C.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation kinetics of poly (lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution as revealed by infrared and Raman spectroscopies. Polym. Degrad. Stab. 2011, 96, 1882–1889.
- Leung, L.; Chan, C.; Baek, S.; Naguib, H. Comparison of morphology and mechanical properties of PLGA bioscaffolds. Biomed. Mater. 2008, 3, 025006.
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin based scaffolds for tissue engineering-a review. Polym. Res. J. 2015, 9, 15.
- Habibovic, P.; Van der Valk, C.M.; Van Blitterswijk, C.A.; De Groot, K.; Meijer, G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 373–380.
- Silva, R.C.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.; Santos, L.R.; Vasconcelos, N.F.; Machado, G. Titanium dental implants: An overview of applied nanobiotechnology to improve biocompatibility and prevent infections. Materials 2022, 15, 3150.
- Lim, H.K.; Choi, Y.J.; Choi, W.C.; Song, I.S.; Lee, U.L. Reconstruction of maxillofacial bone defects using patient-specific long-lasting titanium implants. Sci. Rep. 2022, 12, 7538.
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358.
- Lee, J.H.; Um, S.; Jang, J.H.; Seo, B.M. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012, 348, 475–484.
- Yanagita, M.; Kojima, Y.; Kubota, M.; Mori, K.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. Cooperative effects of FGF-2 and VEGF-A in periodontal ligament cells. J. Dent. Res. 2014, 93, 89–95.
- Kitamura, M.; Akamatsu, M.; Machigashira, M.; Hara, Y.; Sakagami, R.; Hirofuji, T.; Hamachi, T.; Maeda, K.; Yokota, M.; Kido, J.; et al. FGF-2 stimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J. Dent. Res. 2011, 90, 35–40.
- Chen, D.I.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241.
- Barboza, E.; Caúla, A.; Machado, F. Potential of recombinant human bone morphogenetic protein-2 in bone regeneration. Implant. Dent. 1999, 8, 360–367.
- Ingwersen, L.C.; Frank, M.; Naujokat, H.; Loger, K.; Bader, R.; Jonitz-Heincke, A. BMP-2 long-term stimulation of human pre-osteoblasts induces osteogenic differentiation and promotes transdifferentiation and bone remodeling processes. Int. J. Mol. Sci. 2022, 23, 3077.
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78.
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165.
- Pan, X.; Veroniaina, H.; Su, N.; Sha, K.; Jiang, F.; Wu, Z.; Qi, X. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 2021, 16, 687–703.
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502.
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497.
- Santos-Carballal, B.; Fernández Fernández, E.; Goycoolea, F.M. Chitosan in non-viral gene delivery: Role of structure, characterization methods, and insights in cancer and rare diseases therapies. Polymers 2018, 10, 444.
- Jayakumar, R.; Chennazhi, K.P.; Muzzarelli RA, A.; Tamura, H.; Nair, S.V.; Selvamurugan, N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr. Polym. 2010, 79, 1–8.
- Kim, T.H.; Jiang, H.L.; Jere, D.; Park, I.K.; Cho, M.H.; Nah, J.W.; Choi, Y.J.; Akaike, T.; Cho, C.S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753.
- Al-Dosari, M.S.; Gao, X. Nonviral gene delivery: Principle, limitations, and recent progress. AAPS J. 2009, 11, 671–681.
- Sousa, F.; Prazeres, D.M.; Queiroz, J.A. Improvement of transfection efficiency by using supercoiled plasmid DNA purified with arginine affinity chromatography. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2009, 11, 79–88.
- Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399.
- Zhang, Y.; Cheng, X.; Wang, J.; Wang, Y.; Shi, B.; Huang, C.; Yang, X.; Liu, T. Novel chitosan/collagen scaffold containing transforming growth factor-β1 DNA for periodontal tissue engineering. Biochem. Biophys. Res. Commun. 2006, 344, 362–369.
- Chakka LR, J.; Vislisel, J.; Vidal CD, M.P.; Biz, M.T.; KSalem, A.; Cavalcanti, B.N. Application of BMP-2/FGF-2 gene–activated scaffolds for dental pulp capping. Clin. Oral Investig. 2020, 24, 4427–4437.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
888
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
18 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




