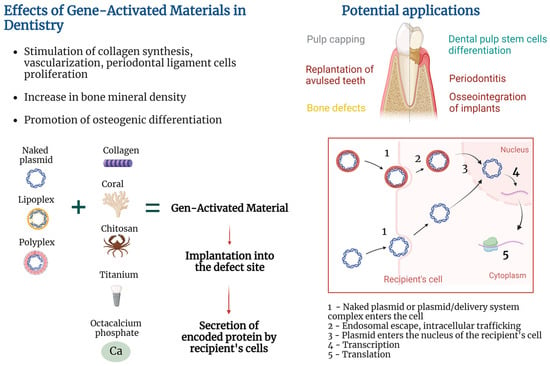
2. Gene-Activated Materials’ Mechanism of Action
GAMs’ mechanism of action is based on the combination of the properties of the material to form a provisional template for new tissue formation in the area of the defect and the properties of a gene-carrying vector to transfect recipient’s cells and stimulate local secretion of encoded protein. Exact mechanisms of action are deeply dependent on pathology being addressed, type of biocompatible material used, encoded protein, type of delivery system, and approach to attaching plasmid (or a plasmid/delivery system complex) to the material.
Transfection of cells may be performed in vitro by culturing cells with non-viral vectors, followed by seeding of cells on the material and implantation into the damaged area
[20][21]. However, the GAM approach implies that the material will be enriched with plasmid or a plasmid/delivery system complex (in contrast to preliminary transfected cells), implanted into the damaged area, followed by the recipient’s cell transfection and secretion of encoded protein (
Figure 1). Such an approach helps to elude the costly and labor-intensive stage of in vitro cell culture and does not fall under strict legal requirements for cell culture.
Figure 1. Gene-activated materials’ mechanism of action.
2.1. Implantation of GAM into a Damaged Area
The technique of GAM positioning at the injury site depends on material and treated pathology. For example, fixation of gene-activated octacalcium phosphate (OCP) within a bone grafting site was achieved via soft tissue suturing in a clinical trial
[18]. In vivo studies also demonstrated various techniques of GAM implantation; for example, covering of the root of avulsed teeth with poly(D,L-lactic-co-glycolic acid) (PLGA) in dogs
[16], placing collagen into an alveolar bone defect followed by muscle suturing and skin incision closure with clips in rats
[21][22], filling of sockets after tooth extraction with gelatin sponges followed by coverage with periodontal dressing in rats
[17], and layer-by-layer coating of a titanium implant followed by implantation in dogs
[19].
2.2. Colonization of the Material with Cells/Release of the Plasmid from the Material
After implantation, the GAM starts to exert dual functions explained by a combination of tissue engineering and gene therapy in the GAM approach. Aside from being tools for incorporation of the plasmid or plasmid/delivery system complex, the paradigm of tissue engineering implies that materials should represent a provisional template for cell migration, adhesion, proliferation, differentiation, and deposition of extracellular matrix at the defect site
[2]. Release of the plasmid or plasmid/delivery system complex from GAM occurs in the course of material degradation or plasmid diffusion depending on the type of material and method of plasmid attachment
[4]. Tailoring physicochemical parameters of the materials, as well as choosing ways of adhering plasmid to the material, allow the achievement of different plasmid release kinetics, which is valuable for temporal control over gene expression
[22][23][23,24].
2.3. Recipient’s Cell Transfection
Transfection of cells occurs via uptake of naked plasmids of the plasmid/delivery system complex. Some works used naked plasmids for cell transfection in vivo and in vitro, suggesting their ability to be internalized and result in increased protein secretion
[24][25], or in reporter gene expression
[4]. At the same time, there is an opinion that entry of naked plasmids into eukaryotic cells may be hampered by the negative charge of the plasmid, so a variety of approaches were used to increase transfection efficiency
[25][26]. For example, cationic polymers facilitate plasmid/delivery system complex uptake via neutralization of DNA negative charge followed by endocytosis and endosomal escape
[26][27].
2.4. Cytoplasmic Transport and Nucleus Entry
After entering the cell, free DNA is complexed with intracellular proteins that facilitate microtubule-mediated cytoplasmic transport of plasmid DNA to the surface of the cell nucleus
[27][28][28,29]. Plasmids may enter the nucleus of dividing cells at the time of nuclear envelope disassembly or cross the nuclear envelope of non-dividing cells via nuclear pore complex
[27][29][28,30].
2.5. Transcription, Translation, Expression Duration
The host’s RNA polymerase II facilitates plasmid DNA transcription and mRNA synthesis. The duration of transgene expression greatly depends on the cell type that was transfected. For example, muscle cells are characterized by long-lasting expression in mice for more than a year, presumably because of the absence of cell division
[30][31]. When GAMs are concerned, duration of expression also depends on plasmid release kinetics from the materials, with a longer release period yielding prolonged expression
[23][24].
2.6. Protein Secretion
Transfected cells start to secrete plasmid-encoded proteins which may be directed to the achievement of various goals. For example, chitosan/collagen matrix enriched with plasmid encoding platelet-derived growth factor (pPDGF) promoted proliferation of human periodontal ligament cells (hPDLCs) and formation of a periodontal connective tissue-like structure in vitro
[4]. At the same time, Yang et al. reported slower proliferation of dental pulp stem cells (DPSCs) cultured on chitosan/collagen matrix enriched with plasmids carrying the bone morphogenetic protein-7 (BMP) gene and hypothesized that this may be due to the reverse correlation between the DPSCs’ odontoblastic differentiation caused by BMP-7 secretion and proliferation
[31][32].
3. Heterogeneous Design of Gene-Activated Materials
In the field of regenerative dentistry, there is heterogeneity in the design of plasmid-based GAMs. It is explained by the diversity in the following fundamental structural elements of the GAMs: (1) type of material used, (2) type of encoded protein, (3) type of delivery system, (4) method of attaching plasmid or plasmid/delivery system complex to the material. The exact GAM design may be chosen considering the type of treated pathology and individual patient characteristics.
3.1. Materials Used for Creation of GAMs in the Field of Regenerative Dentistry
In tissue engineering, a variety of natural and synthetic materials are used. In order to be used for creation of a GAM, material must not only be biocompatible and support tissue regeneration but also be able to attach a naked plasmid or a plasmid/delivery system complex. To create GAMs in the field of regenerative dentistry, various materials are used depending on the scientific and clinical problem being solved.
Collagen is a biocompatible biodegradable material capable of stimulating migration of human oral region fibroblasts, endothelial, and periodontal ligament cells
[32][33], and healing of bone, cementum, and periodontal ligament
[33][34], and alveolar bone defects
[34][35]. Chitosan is a natural biocompatible, biodegradable polymer with a low immunogenicity possessing bacteriostatic, fungistatic, and anti-inflammatory properties
[35][36][36,37], capable of promoting the proliferation of human hPDLCs
[37][38].
Hydroxyapatite is frequently used for bone grafting due to the resemblance of its structure and composition to the natural mineral phase of human bone tissue, and osteoconductive and osteoinductive properties
[38][39]. In dentistry, hydroxyapatite is frequently combined with collagen to achieve enhanced mechanical properties
[39][40].
Coral is a natural porous biocompatible material also used for bone tissue engineering due to its osteoconductive and mechanical properties
[40][41]. Coral scaffolds gene-activated with pPDGF-B were shown to stimulate proliferation of hPDLCs and synthesis of type I collagen, suggesting its possible use for periodontal regeneration
[41][42].
Poly(lactic-co-glycolic acid) is a biocompatible and biodegradable copolymer of lactic and glycolic acid. Depending on the composition and manufacturing conditions, PLGA may be tailored to have a certain degradation and drug/plasmid release rates
[42][43][43,44], mechanical properties, and porosity
[44][45].
Gelatin is a biocompatible biodegradable natural bio polymer characterized by cost-effectiveness and low immunogenicity, as well as various physical and chemical properties depending on the preparation technique
[45][46].
Octacalcium phosphate (OCP) is a mineral precursor of apatite crystals in bone tissue; it possesses osteoconductive and osteoinductive properties and allows adhesion of bioactive molecules
[46][47].
Titanium and its alloys are widely used in dental prosthetics and for the reconstruction of maxillofacial bone defects as well as in orthopedics
[47][48][48,49]. Despite excellent biocompatibility, surface modifications of titanium implants are studied to improve their osseointegration
[47][48].
3.2. Types of Encoded Proteins Used in GAMs in Regenerative Dentistry
The importance of growth factors in soft and bone tissue regeneration has already been shown in multiple studies. Next, various proteins encoded by plasmids in GAMs used in regenerative dentistry will be briefly overviewed.
Vascular endothelial growth factor (VEGF) not only acts as a mitogen for endothelial cells stimulating angiogenesis but also promotes human periodontal ligament stem cells’ (hPDLSCs) odonto-/osteogenic differentiation in vitro
[49][50][50,51]. Genetic activation of materials with pVEGF is a promising direction for the stimulation of bone tissue regeneration since sufficient vascularization is necessary for successful osteogenesis
[18].
Platelet-derived growth factor is capable of stimulating osteoblast, periodontal ligament cell, and gingival fibroblast proliferation
[6][8][6,8]. Clinical trials showed that PDGF in combination with beta-tricalcium phosphate resulted in an increased clinical attachment level and reduced gingival recession at 3 months, and greater linear bone gain and defect fill at 6 months compared to scaffold alone
[7].
Fibroblast growth factors (FGFs) are a large family of regulatory molecules that have promoting effects on a variety of cells including hPDLCs and hPDLSCs
[5][50][5,51]. An in vitro study with the use of a mouse periodontal ligament cell line also showed the ability of FGF-2 to stimulate VEGF-A secretion
[51][52]. Notably, recombinant human FGF-2 in combination with modified Widman periodontal surgery increased bone fill in patients with periodontitis in a clinical trial
[52][53].
Bone morphogenetic proteins are signaling molecules with multiple functions and members of the TGF-β superfamily
[53][54]. BMP-2 is used for the treatment of bone lesions since this protein possesses high osteogenic activity via promoting osteogenic differentiation of pre-osteoblastic cells
[54][55][55,56].
Although the benefits of using growth factors have been shown in many studies, their use has some disadvantages, including a short half-life when applied to the defect site and application of supraphysiological doses
[9]. The use of GAMs allows for the prevention of the problem of rapid degradation of recombinant growth factors as well as for gradual secretion of the growth factor by the recipient’s cells at the defect site.
3.3. Types of Non-Viral Delivery Systems Used in GAMs in the Field of Regenerative Dentistry
Ensuring the efficient transfer of genetic material into the cell is one of the main challenges in gene therapy. To enhance plasmid transfection efficiency, a variety of non-viral delivery agents are used. Non-viral delivery systems may be represented by biodegradable and non-biodegradable polymers, lipids, peptides, inorganic materials (e.g., gold and magnetic nanoparticles), hybrid systems
[56][57], calcium phosphate
[57][58], exosomes
[58][59], etc.
ResWe
archers will briefly describe non-viral delivery systems that are used as part of GAMs in the field of regenerative dentistry.
Polyethyleneimine (PEI) is a positively charged cationic polymer that facilitates plasmid entry through negatively charged cell membranes via condensation of negatively charged plasmid DNA
[59][60]. One of the most common cationic polymers is represented by PEI, with 25 kDa branched PEI being considered one of the most efficient types
[17]. However, the non-biodegradable nature of PEI may result in a cytotoxic effect as seen in human periodontal ligament fibroblasts and gingival fibroblasts
[21][22]. Some authors suggested that cytotoxicity is higher in PEI with high molecular weight compared to low molecular weight
[60][61]. Importantly, Plonka et al. showed that even though PEI reduced viability of human periodontal ligament fibroblasts and gingival fibroblasts, transfection of PEI/pPDGF polyplexes nivelated detrimental impact of PEI suggesting the stimulating effect of transfection with growth factor-encoding plasmid
[21][22].
Chitosan is a natural cationic biodegradable polymer capable of interacting with negatively charged plasmid DNA via electrostatic forces
[61][62]. Low cytotoxicity was also reported for the chitosan-based delivery system
[62][63]. In the study by Peng et al. (2009), transfection of hPDLCs with pDNA/chitosan resulted in higher cell viability compared to naked plasmid alone
[4]. At the same time, many studies combined chitosan with other delivery systems or subjected it to various modifications to improve its transfection efficiency
[63][64].
Cationic liposomes are widely used gene delivery systems. Cationic liposomes act by binding positively charged head groups of lipids with negatively charged phosphate groups of plasmid DNA and by surrounding DNA molecules
[64][65].
Delivery of naked plasmid DNA is also considered as a promising direction and has some advantages, among which is the absence of delivery systems with potential cytotoxicity. Supercoiling of plasmid DNA also aids more efficient transfection
[65][66].
3.4. Methods of Binding Plasmids or Plasmid/Delivery System Complexes to the Materials
Various methods of attaching the plasmid or plasmid/delivery system complex to the material are used and are of importance since this parameter may affect the kinetics of plasmid release and duration of transgene expression
[23][24]. Methods of attaching plasmids or plasmid/delivery system complexes to materials may be divided into chemical and physical methods. Chemical methods rely on the creation of bonds that facilitate plasmid attachment to the material. For example, calcium-containing materials may bind DNA via electrostatic interactions due to the presence of phosphate groups on the DNA backbone.
Physical methods do not imply creation of bonds between plasmid and material and include emulsion electrospinning that allows for the incorporation of the buffer with the target plasmid into the volume of polymeric microfibers for subsequent release in the course of material degradation
[16][66][16,67]. Importantly, several authors noted that incorporation of the plasmid into the material may cause DNA damage due to interaction with solvents during manufacture
[2][23][2,24]. One of the solutions to this issue is coaxial (core-shell) electrospinning that allows for the obtaining of core-shell fibers, the inner compartment of which may be filled with a plasmid-containing buffer avoiding plasmid interaction with damaging solvents
[23][24].
From a technical point of view, the attachment of a naked plasmid or a plasmid/delivery system complex to the material may be achieved by different methods, some of which were described by Peng et al.
[4]. Among such methods are dropping plasmid-containing suspension on the material or soaking of the material in the suspension followed by some time of incubation
[17][67][17,68], injection into the volume of the material
[68][69], or coating of the material with other plasmid-containing materials
[41][42]. The layer-by-layer technique allows for the coating of the materials with multiple oppositely charged plasmid-containing layers
[19].