
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aspasia Manta | -- | 2003 | 2024-02-09 09:17:10 | | | |
| 2 | Catherine Yang | Meta information modification | 2003 | 2024-02-19 09:09:47 | | |
Video Upload Options
Type 2 diabetes mellitus is a major health problem worldwide with a steadily increasing prevalence reaching epidemic proportions. The major concern is the increased morbidity and mortality due to diabetic complications. Traditional but also nontraditional risk factors have been proposed to explain the pathogenesis of type 2 diabetes mellitus and its complications. Hyperglycemia has been considered an important risk factor, and the strict glycemic control can have a positive impact on microangiopathy but not macroangiopathy and its related morbidity and mortality. Thus, the therapeutic algorithm has shifted focus from a glucose-centered approach to a strategy that now emphasizes target-organ protection. Sodium-glucose transporter 2 inhibitors is an extremely important class of antidiabetic medications that, in addition to their glucose lowering effect, also exhibit cardio- and renoprotective effects. Various established and novel biomarkers have been described, reflecting kidney and cardiovascular function.
1. Introduction
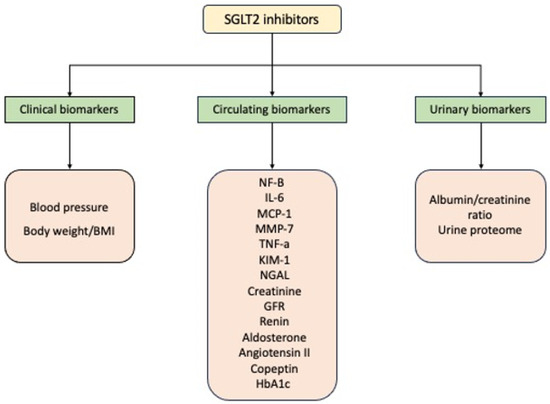
2. Renal Biomarkers and SGLT2i Treatment
2.1. Renal Clinical Biomarkers and SGLT2i Treatment
2.2. Renal Circulating Biomarkers and SGLT2i Treatment
2.3. Renal Urinary Biomarkers in Treatment with SGLT2i
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107.
- Ling, W.; Huang, Y.; Huang, Y.-M.; Fan, R.-R.; Sui, Y.; Zhao, H.-L. Global Trend of Diabetes Mortality Attributed to Vascular Complications, 2000–2016. Cardiovasc. Diabetol. 2020, 19, 182.
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442.
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172.
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of Cardiovascular Disease in Type 2 Diabetes: A Systematic Literature Review of Scientific Evidence from across the World in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83.
- Brunetti, L.; Kalabalik, J. Management of Type-2 Diabetes Mellitus in Adults: Focus on Individualizing Non-Insulin Therapies. Pharm. Ther. 2012, 37, 687–696.
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20.
- Araki, E.; Tanaka, A.; Inagaki, N.; Ito, H.; Ueki, K.; Murohara, T.; Imai, K.; Sata, M.; Sugiyama, T.; Ishii, H.; et al. Diagnosis, Prevention, and Treatment of Cardiovascular Diseases in People with Type 2 Diabetes and Prediabetes―A Consensus Statement Jointly from the Japanese Circulation Society and the Japan Diabetes Society. Circ. J. 2020, 85, 82–125.
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323.
- Sheahan, K.H.; Wahlberg, E.A.; Gilbert, M.P. An Overview of GLP-1 Agonists and Recent Cardiovascular Outcomes Trials. Postgrad. Med. J. 2020, 96, 156–161.
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z.I. Prescribing SGLT2 Inhibitors in Patients with CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476.
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966.
- Ni, L.; Yuan, C.; Chen, G.; Zhang, C.; Wu, X. SGLT2i: Beyond the Glucose-Lowering Effect. Cardiovasc. Diabetol. 2020, 19, 98.
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128.
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of Dapagliflozin on Major Adverse Kidney and Cardiovascular Events in Patients with Diabetic and Non-Diabetic Chronic Kidney Disease: A Prespecified Analysis from the DAPA-CKD Trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31.
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Docherty, K.F.; Jhund, P.S.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; DeMets, D.L.; Sabatine, M.S.; Bengtsson, O.; et al. Effects of Dapagliflozin in DAPA-HF According to Background Heart Failure Therapy. Eur. Heart J. 2020, 41, 2379–2392.
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients with Heart Failure with and Without Diabetes. JAMA 2020, 323, 1353.
- Cherney, D.Z.I.; Charbonnel, B.; Cosentino, F.; Dagogo-Jack, S.; McGuire, D.K.; Pratley, R.; Shih, W.J.; Frederich, R.; Maldonado, M.; Pong, A.; et al. Effects of Ertugliflozin on Kidney Composite Outcomes, Renal Function and Albuminuria in Patients with Type 2 Diabetes Mellitus: An Analysis from the Randomised VERTIS CV Trial. Diabetologia 2021, 64, 1256–1267.
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary. J. Am. Coll. Cardiol. 2019, 74, 1376–1414.
- Jones, B.J.; Tan, T.; Bloom, S.R. Minireview: Glucagon in Stress and Energy Homeostasis. Endocrinology 2012, 153, 1049–1054.
- Cheeseman, C. Solute Carrier Family 2, Member 9 and Uric Acid Homeostasis. Curr. Opin. Nephrol. Hypertens. 2009, 18, 428–432.
- Santos Cavaiola, T.; Pettus, J. Cardiovascular Effects of Sodium Glucose Cotransporter 2 Inhibitors. Diabetes Metab. Syndr. Obes. 2018, 11, 133–148.
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.; Woerle, H.-J.; von Eynatten, M.; Broedl, U.C. The Effect of Empagliflozin on Arterial Stiffness and Heart Rate Variability in Subjects with Uncomplicated Type 1 Diabetes Mellitus. Cardiovasc. Diabetol. 2014, 13, 28.
- Heerspink, H.J.L.; Desai, M.; Jardine, M.; Balis, D.; Meininger, G.; Perkovic, V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J. Am. Soc. Nephrol. 2017, 28, 368–375.
- van Bommel, E.J.M.; Muskiet, M.H.A.; Tonneijck, L.; Kramer, M.H.H.; Nieuwdorp, M.; van Raalte, D.H. SGLT2 Inhibition in the Diabetic Kidney—From Mechanisms to Clinical Outcome. Clin. J. Am. Soc. Nephrol. 2017, 12, 700–710.
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; de Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The Role of Vascular Biomarkers for Primary and Secondary Prevention. A Position Paper from the European Society of Cardiology Working Group on Peripheral Circulation. Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532.
- Wang, T.J. Assessing the Role of Circulating, Genetic, and Imaging Biomarkers in Cardiovascular Risk Prediction. Circulation 2011, 123, 551–565.
- Reed, J. Impact of Sodium–Glucose Cotransporter 2 Inhibitors on Blood Pressure. Vasc. Health Risk Manag. 2016, 12, 393–405.
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood Pressure Lowering in Type 2 Diabetes. JAMA 2015, 313, 603.
- Lastra, G.; Syed, S.; Kurukulasuriya, L.R.; Manrique, C.; Sowers, J.R. Type 2 Diabetes Mellitus and Hypertension. Endocrinol. Metab. Clin. North. Am. 2014, 43, 103–122.
- Mazidi, M.; Rezaie, P.; Gao, H.; Kengne, A.P. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials with 22 528 Patients. J. Am. Heart Assoc. 2017, 6, e004007.
- Wilding, J.; Bailey, C.; Rigney, U.; Blak, B.; Kok, M.; Emmas, C. Dapagliflozin Therapy for Type 2 Diabetes in Primary Care: Changes in HbA1c, Weight and Blood Pressure over 2 Years Follow-Up. Prim. Care Diabetes 2017, 11, 437–444.
- Perseghin, G.; Solini, A. The EMPA-REG Outcome Study: Critical Appraisal and Potential Clinical Implications. Cardiovasc. Diabetol. 2016, 15, 85.
- Idzerda, N.M.A.; Stefansson, B.V.; Pena, M.J.; Sjostrom, D.C.; Wheeler, D.C.; Heerspink, H.J.L. Prediction of the Effect of Dapagliflozin on Kidney and Heart Failure Outcomes Based on Short-Term Changes in Multiple Risk Markers. Nephrol. Dial. Transplant. 2020, 35, 1570–1576.
- Okamoto, A.; Yokokawa, H.; Sanada, H.; Naito, T. Changes in Levels of Biomarkers Associated with Adipocyte Function and Insulin and Glucagon Kinetics During Treatment with Dapagliflozin Among Obese Type 2 Diabetes Mellitus Patients. Drugs R. D 2016, 16, 255–261.
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin Reduces Inflammation and Fibrosis Biomarkers: A Potential Mechanism of Action for Beneficial Effects of SGLT2 Inhibitors in Diabetic Kidney Disease. Diabetologia 2019, 62, 1154–1166.
- Sen, T.; Li, J.; Neuen, B.L.; Neal, B.; Arnott, C.; Parikh, C.R.; Coca, S.G.; Perkovic, V.; Mahaffey, K.W.; Yavin, Y.; et al. Effects of the SGLT2 Inhibitor Canagliflozin on Plasma Biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS Trial. Diabetologia 2021, 64, 2147–2158.
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 Inhibitor Dapagliflozin on Glomerular and Tubular Injury Markers. Diabetes Obes. Metab. 2018, 20, 1988–1993.
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z.I. Markers of Kidney Injury, Inflammation, and Fibrosis Associated with Ertugliflozin in Patients with CKD and Diabetes. Kidney Int. Rep. 2021, 6, 2095–2104.
- Darawshi, S.; Yaseen, H.; Gorelik, Y.; Faor, C.; Szalat, A.; Abassi, Z.; Heyman, S.N.; Khamaisi, M. Biomarker Evidence for Distal Tubular Damage but Cortical Sparing in Hospitalized Diabetic Patients with Acute Kidney Injury (AKI) While on SGLT2 Inhibitors. Ren. Fail. 2020, 42, 836–844.
- Levey, A.S. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461.
- Choosongsang, P.; Soonthornpun, S. Overestimation of Glomerular Filtration Rate Calculated from Creatinine as Compared with Cystatin C in Patients with Type 2 Diabetes Receiving Sodium-glucose Cotransportor 2 Inhibitors. Diabet. Med. 2022, 39, e14659.
- Miyoshi, H.; Kameda, H.; Yamashita, K.; Nakamura, A.; Kurihara, Y. Protective Effect of Sodium–Glucose Cotransporter 2 Inhibitors in Patients with Rapid Renal Function Decline, Stage G3 or G4 Chronic Kidney Disease and Type 2 Diabetes. J. Diabetes Investig. 2019, 10, 1510–1517.
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446.
- Scholtes, R.A.; Muskiet, M.H.A.; van Baar, M.J.B.; Hesp, A.C.; Greasley, P.J.; Hammarstedt, A.; Karlsson, C.; Hallow, K.M.; Danser, A.H.J.; Heerspink, H.J.L.; et al. The Adaptive Renal Response for Volume Homeostasis During 2 Weeks of Dapagliflozin Treatment in People with Type 2 Diabetes and Preserved Renal Function on a Sodium-Controlled Diet. Kidney Int. Rep. 2022, 7, 1084–1092.
- Harding, A.L.; Bediaga, N.; Galligan, A.; Colman, P.G.; Fourlanos, S.; Wentworth, J.M. Factors That Predict Glycaemic Response to Sodium-glucose Linked Transporter (SGLT) Inhibitors. Intern. Med. J. 2021, 51, 515–519.
- Osonoi, T.; Gouda, M.; Kubo, M.; Arakawa, K.; Hashimoto, T.; Abe, M. Effect of Canagliflozin on Urinary Albumin Excretion in Japanese Patients with Type 2 Diabetes Mellitus and Microalbuminuria: A Pilot Study. Diabetes Technol. Ther. 2018, 20, 681–688.
- Kobayashi, K.; Toyoda, M.; Kaneyama, N.; Hatori, N.; Furuki, T.; Sakai, H.; Takihata, M.; Umezono, T.; Ito, S.; Suzuki, D.; et al. Relation between Blood Pressure Management and Renal Effects of Sodium-Glucose Cotransporter 2 Inhibitors in Diabetic Patients with Chronic Kidney Disease. J. Diabetes Res. 2019, 2019, 9415313.
- Kobayashi, K.; Toyoda, M.; Hatori, N.; Furuki, T.; Sakai, H.; Umezono, T.; Ito, S.; Suzuki, D.; Takeda, H.; Minagawa, F.; et al. Blood Pressure after Treatment with Sodium–Glucose Cotransporter 2 Inhibitors Influences Renal Composite Outcome: Analysis Using Propensity Score-matched Models. J. Diabetes Investig. 2021, 12, 74–81.
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early Detection of Diabetic Kidney Disease by Urinary Proteomics and Subsequent Intervention with Spironolactone to Delay Progression (PRIORITY): A Prospective Observational Study and Embedded Randomised Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312.




