Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valli Kamala Laxmi Ramya Chittoory | -- | 1473 | 2024-02-08 17:18:14 | | | |
| 2 | Camila Xu | Meta information modification | 1473 | 2024-02-09 01:57:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chittoory, V.K.L.R.; Filipsika, M.; Bartoš, R.; Králová, M.; Dzik, P. Physicochemical Properties of Tungsten Trioxide Photoanodes. Encyclopedia. Available online: https://encyclopedia.pub/entry/54944 (accessed on 08 February 2026).
Chittoory VKLR, Filipsika M, Bartoš R, Králová M, Dzik P. Physicochemical Properties of Tungsten Trioxide Photoanodes. Encyclopedia. Available at: https://encyclopedia.pub/entry/54944. Accessed February 08, 2026.
Chittoory, Valli Kamala Laxmi Ramya, Marketa Filipsika, Radim Bartoš, Marcela Králová, Petr Dzik. "Physicochemical Properties of Tungsten Trioxide Photoanodes" Encyclopedia, https://encyclopedia.pub/entry/54944 (accessed February 08, 2026).
Chittoory, V.K.L.R., Filipsika, M., Bartoš, R., Králová, M., & Dzik, P. (2024, February 08). Physicochemical Properties of Tungsten Trioxide Photoanodes. In Encyclopedia. https://encyclopedia.pub/entry/54944
Chittoory, Valli Kamala Laxmi Ramya, et al. "Physicochemical Properties of Tungsten Trioxide Photoanodes." Encyclopedia. Web. 08 February, 2024.
Copy Citation
Advanced Oxidation Processes (AOPs) are widely regarded as the most effective method for rapidly degrading and oxidizing organic pollutants in water treatment, with chemical methods demonstrating high efficiency, especially for addressing organic wastewater.
electrophotocatalysis
tungsten trioxide
photoanodes
brick-and-mortar
1. Introduction
The urgent requirement to confront the pervasive pollution of groundwater, surface water, and drinking water by toxic and long-lasting organic chemicals stemming from industrial and domestic sources demands an increased emphasis on the development of wastewater treatment technologies [1][2]. Advanced wastewater treatment typically involves the use of physicochemical and combined physical and biological methods, which are categorized into three main groups: physical, chemical, and biological treatments [3][4]. Commonly used physical methods for water treatment include sedimentation, adsorption, and membrane separation [5]. Biological treatments can be an effective solution for removing organic matter from water, as conventional physical processes have limitations in this area. However, even though there have been advancements in membrane bioreactors, they have not yet been successful in removing micropollutants from wastewater. This is because the membrane components of these technologies become clogged with particles from previous processes, which hinders the separation process [6]. Advanced Oxidation Processes (AOPs) are widely regarded as the most effective method for rapidly degrading and oxidizing organic pollutants in water treatment, with chemical methods demonstrating high efficiency, especially for addressing organic wastewater [7].
Advanced oxidation processes possess several advantages, such as a high mineralization efficiency, rapid reaction rates, and minimal secondary pollution [8]. Organic products are highly susceptible to oxidation by hydroxyl radicals, which are generated by holes and other reactive oxygen species (ROS) that exist between electrons and molecular oxygen [9]. AOPs rely on efficient ROS generation and reduced mass transfer resistance to determine their oxidation capacity. Unlike other AOPs, electrochemical oxidation breaks down pollutants without producing secondary products at a relatively low cost. The anodic reaction is influenced by the organic compounds’ affinity for the oxidant and results in the formation of active oxygen, which can lead to either the complete degradation or simplification of pollutants [7][8][10]. Photochemical AOPs offer a promising solution for both water treatment and energy production due to their capacity to break down persistent compounds using sunlight. Nevertheless, their relatively low efficiency in harnessing light energy and the rapid reunion of electron–hole pairs at the photocatalyst’s surface present substantial obstacles to their practical implementation [11]. By integrating multiple advanced oxidation processes (AOPs), the oxidation efficiency of treating organic wastewater can be enhanced. This method aims to overcome the drawbacks and high operational costs of individual treatment technologies, by leveraging synergistic effects to improve the efficiency of organic degradation. PEC facilitates the separation of electron–hole pairs, thereby promoting the mineralization of organic pollutants in wastewater [8][9][12]. The conductivity of urban and industrial wastewater is adequate due to the presence of electrolytes [13]. The majority of PEC research has typically utilized synthetic wastewater composed of ultrapure water and electrolytes [12][14][15][16][17]. The process-specific parameters, system design, and water quality all play a role in determining the formation of radicals [4][18][19]. The efficiency of contaminant destruction in surface-based advanced oxidation processes (AOPs) is influenced by several factors, including radical scavenging, radical transfer, and hydrodynamics [8]. Optimal performance of a photocatalyst depends on effective morphology control, as it impacts the photocatalytic properties, specific surface area, quantum efficiency, and porosity [7]. Oxidation of these organic pollutants at the surface of photocatalyst is of the utmost importance [20]. The photocatalytic approaches have been the latest developments for the degradation of organic pollutants, based on semiconductor materials as catalysts in conjunction with the advanced oxidation processes (AOPs) under the irradiation of solar light [21].
In tungsten(VI) oxide (WO3), the lower size of the bandgap starts at 2.6 eV, and photocatalysis can be excited by blue radiation of the visible spectrum, which has an edge for WO3 over titania [22]. There are different mechanisms through which tungsten oxides act. One includes the bandgap value and band edge positions, allowing carrier generation in the healthcare system [23]. The energy of the bandgap can vary depending on the crystalline phase of the photocatalyst [24]. Photocatalysts face a significant challenge due to faster electron–hole recombination time, making it difficult in PEC cell activity [25][26][27]. The photoelectrochemical cell comprises a photoanode and a cathode immersed in an aqueous electrolyte, as shown in Figure 1. The smaller the photocatalytic particles, the greater the total number of charge separations [28][29][30]. Combining band structure engineering, geometric engineering, and heterostructure engineering may enhance the photocatalytic activity. The setup comprises a photoanode and a cathode immersed in an aqueous electrolyte, as shown in Figure 1. In the n-type photoanode, photogenerated holes move to the semiconductor interface to oxidize organic pollutants to carbon dioxide.
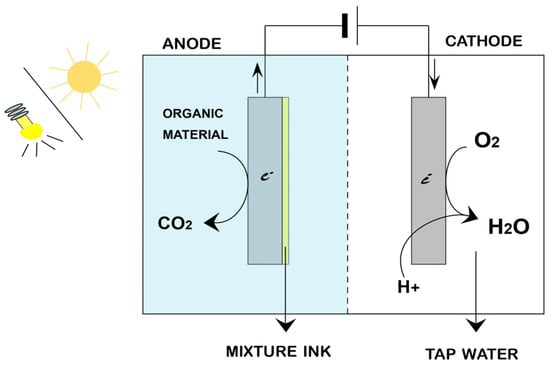
Figure 1. Schematic overview of photoelectrochemical cell.
The nanomaterial preparation can be divided into two basic procedures: top-down and bottom-up. In the top-down procedure, the bulk material is broken down into nanosized particles by different physical, chemical, and mechanical processes. Various synthetic methods can be utilized under this approach such as mechanical milling, laser ablation, and ion sputtering [31]. Mechanical mills of various types include planetary, attrition, vibration, low-energy ball, and high-energy ball mills [32]. Using the bottom-up procedure, larger layers can be prepared from soluble precursors and such processes include the sol–gel method. The precursors utilized in the sol–gel method are heated to a very high temperatures to achieve one of the immobilization conditions, namely, adhesion to the substrate [32].
2. Tungsten(VI) Oxide Layers Prepared by Top-Down Approach
Ball milling is commonly used in industrial mass production for ores, ceramics, and pigments [33]. The milling technique is quite suitable for morphological modifications of nanostructures and It comes with an advantage of low-cost preparation can use it at room temperature [34]. Ball milling is advantageous over laser ablation in terms of large-scale production [35]. Ball milling is a widely used technique for synthesizing amorphous materials [36]. A miller setup was designed to reduce WO3 to metallic tungsten [37][38].
3. Tungsten(VI) Oxide Layers by Bottom-Up Approach
Different tungsten precursors were used in the literature for formation of tungsten(VI) oxide films via the sol–gel method. Suitable structure-directing agents and binders help in tailoring the morphology of WO3 thin films. The peroxotungstic acid (PTA) derivatives are widely reported for casting tungsten(VI) oxide films in the sol–gel route. De Moura, D.S. et al. reported on a system to synthesize WO3 powders via PTA and polyvinyl alcohol as precursor [39]. Fang, Y. et al. reported WO3 films derived from the sol–gel synthesis of acetylated peroxotungstic acid (APTA), and polyethylene glycol layers deposited by spin coating [40]. Işık et al. reported the influence of tungsten(VI) oxide microstructures on the electrochromic properties obtained from tungsten peroxo complexes via the sol–gel method for the application of tungsten coatings [41]. The authors of article [36] published a study on the formation of thin oxide layers of modified tungsten chloride by the sol–gel method. Zhang et al. reported on the formation of thermally stable mesoporous tungsten oxide films by the sol–gel method using a surfactant [42].
4. Fabrication of WO3 Layers by Brick-and-Mortar Approach
The brick-and-mortar approach serves as a connection matrix, combining the two aforementioned methodologies, as shown in Figure 2. The authors of [43] used an innovative brick-and-mortar approach to prepare WO3 nanoparticles and the photoanodes showed high photocurrents of 3.04 mA cm−2.
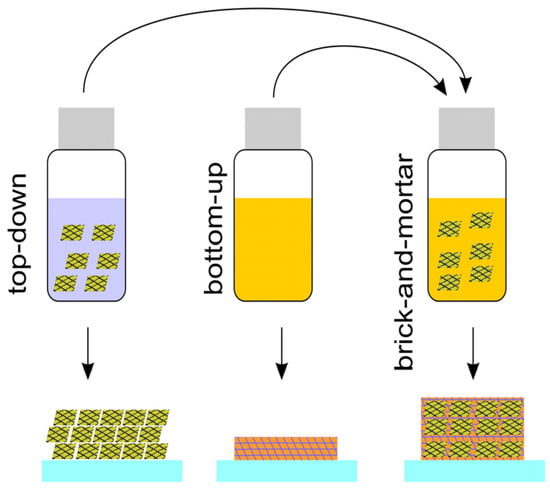
Figure 2. Illustration of top-down, bottom-up, and brick-and-mortar methods.
5. Wet Coating Techniques–Deposition of Liquid Formulations onto Substrates
Meyer rod coating is utilized in the production of the prepared inks for the larger electrodes to achieve uniform thickness, without the need for any additional processes. Ojeda, M. et al. reported on the fabrication of WO3 thin films using a simple spin-coating route [44]. Sadale and Neumann-Spallart drop-casted WO3 films to study the degradation of azo dyes [45]. Wang et al. [46] studied the deposition of tungsten trioxide films with photocatalytic and electrochromic properties by synthesizing a precursor solution with WO3 powder in hydrogen peroxide. Table 1 summarizes the comparative analysis of wet coating methods used for depositing liquid formulations on substrates.
Table 1. Presents a comparison of the advantages and disadvantages of using wet coating techniques for depositing liquid formulations.
| S. No | Technique | Advantages | Disadvantages |
|---|---|---|---|
| 1. | Screen Printer | Low cost [47][48] | One of the principal drawbacks of this method is the lack of flexibility in modifying the morphology and film thickness [49]. |
| 2. | Inkjet Printer | Inkjet printing offers several advantages, including non-contact, maskless, and combinatorial processing. It also consumes minimal materials and generates minimal waste [50][51]. | Nozzle clogging, wetting behaviour, and film homogeneity [52][53][54][55][56]. |
| 3. | Spin Coater | A primary factor contributing to the popularity of spin coating is its ease of handling and rapid processing [57]. | The utilization of spin-coating for automated fabrication is not feasible and lacks the capability to pattern substrates selectively. Furthermore, it has high material waste consumption [58][59]. |
| 4. | Meyer rod | The technique referred to as bar coating does not involve any additional processes such as pre-patterning of the substrate. Its purpose is to achieve a uniform, homogeneous coating with efficient processing [60]. | The thickness of the laminating layer varies and depends on the range of possible bar diameters [61]. |
References
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176.
- Gautam, P.; Popat, A.; Lokhandwala, S. Advances & Trends in Advance Oxidation Processes and Their Applications. In Advanced Industrial Wastewater Treatment and Reclamation of Water: Comparative Study of Water Pollution Index during Pre-industrial, Industrial Period and Prospect of Wastewater Treatment for Water Resource Conservation; Roy, S., Garg, A., Garg, S., Tran, T.A., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzwerland, 2022; pp. 45–69. ISBN 9783030838119.
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced Oxidation Processes in the Removal of Organic Substances from Produced Water: Potential, Configurations, and Research Needs. Chem. Eng. J. 2021, 414, 128668.
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99.
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170.
- Atalay, S.; Ersöz, G. Chapter 7—Hybrid Application of Advanced Oxidation Processes to Dyes’ Removal. In Green Chemistry and Water Remediation: Research and Applications; Sharma, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 209–238.
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104.
- Miklos, D.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131.
- Feijoo, S.; Yu, X.; Kamali, M.; Appels, L.; Dewil, R. Generation of Oxidative Radicals by Advanced Oxidation Processes (AOPs) in Wastewater Treatment: A Mechanistic, Environmental and Economic Review. Rev. Environ. Sci. Technol. 2023, 22, 205–248.
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261.
- Swaminathan, M.; Muruganandham, M.; Sillanpää, M. Advanced Oxidation Processes for Wastewater Treatment. Int. J. Photoenergy 2013, 2013, 683682.
- Matilainen, A.; Sillanpää, M. Removal of Natural Organic Matter from Drinking Water by Advanced Oxidation Processes. Chemosphere 2010, 80, 351–365.
- Stefan, M.I. (Ed.) Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2018; ISBN 9781780407197.
- Zhang, X.; Yu, W.; Guo, Y.; Li, S.; Chen, Y.; Wang, H.; Bian, Z. Recent Advances in Photoelectrocatalytic Advanced Oxidation Processes: From Mechanism Understanding to Catalyst Design and Actual Applications. Chem. Eng. J. 2023, 455, 140801.
- Zawadzki, P. Visible Light–Driven Advanced Oxidation Processes to Remove Emerging Contaminants from Water and Wastewater: A Review. Water Air Soil Pollut. 2022, 233, 374.
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641.
- Muruganandham, M.; Suri, R.; Jafari, S.; Sillanpää, M.; Lee, G.; Wu, J.J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014, 821674.
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of Water and Wastewater by Advanced Oxidation Processes. Sci. Total Environ. 2019, 696, 133961.
- Chaplin, B.P. Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203.
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A Review on Nano-TiO2 Sol–Gel Type Syntheses and Its Applications. J. Mater. Sci. 2011, 46, 3669–3686.
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.-X.; Jiang, B.; Xie, S.K.; Habibi, D.; Zhang, L.C. Strong Enhancement on Dye Photocatalytic Degradation by Ball-Milled TiO2: A Study of Cationic and Anionic Dyes. J. Mater. Sci. Technol. 2017, 33, 856–863.
- Strieth-Kalthoff, F.; James, M.J.; Teders, M.; Pitzer, L.; Glorius, F. Energy Transfer Catalysis Mediated by Visible Light: Principles, Applications, Directions. Chem. Soc. Rev. 2018, 47, 7190–7202.
- Sampaio, P.G.V.; González, M.O.A.; de Oliveira Ferreira, P.; da Cunha Jácome Vidal, P.; Pereira, J.P.P.; Ferreira, H.R.; Oprime, P.C. Overview of Printing and Coating Techniques in the Production of Organic Photovoltaic Cells. Int. J. Energy Res. 2020, 44, 9912–9931.
- Veprek, S.; Veprek-Heijman, M.G.J.; Karvankova, P.; Prochazka, J. Different Approaches to Superhard Coatings and Nanocomposites. Thin Solid Films 2005, 476, 1–29.
- Camacho, S.Y.T.; Rey, A.M.; Hernández-Alonso, M.D.; Llorca, J.; Medina, F.; Contreras, S. Pd/TiO2-WO3 Photocatalysts for Hydrogen Generation from Water-Methanol Mixtures. Appl. Surf. Sci. 2018, 455, 570–580.
- Ouyang, W.; Kuna, E.; Yepez, A.; Balu, A.M.; Romero, A.A.; Colmenares, J.C.; Luque, R. Mechanochemical Synthesis of TiO2 Nanocomposites as Photocatalysts for Benzyl Alcohol Photo-Oxidation. Nanomaterials 2016, 6, 93.
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871.
- Coronado, J.M. A Historical Introduction to Photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; pp. 1–4. ISBN 9781447150602.
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38.
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and Applications of 3D Printing for Novel Materials. Appl. Mater. Today 2017, 7, 120–133.
- Homola, T.; Ďurašová, Z.; Shekargoftar, M.; Souček, P.; Dzik, P. Optimization of TiO2 Mesoporous Photoanodes Prepared by Inkjet Printing and Low-Temperature Plasma Processing. Plasma Chem. Plasma Process. 2020, 40, 1311–1330.
- Vidmar, T.; Topič, M.; Dzik, P.; Opara Krašovec, U. Inkjet Printing of Sol–Gel Derived Tungsten Oxide Inks. Sol. Energy Mater. Sol. Cells 2014, 125, 87–95.
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball Milling as a Mechanochemical Technology for Fabrication of Novel Biochar Nanomaterials. Bioresour. Technol. 2020, 312, 123613.
- Xu, C.; Sudipta, D.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical Synthesis of Advanced Nanomaterials for Catalytic Applications. Chem. Commun. 2015, 51, 6698–6713.
- Laranjo, M.T.; Ricardi, N.C.; Arenas, L.T.; Benvenutti, E.V.; de Oliveira, M.C.; Buchner, S.; Santos, M.J.L.; Costa, T.M.H. Influence of Ball Milling on Textural and Morphological Properties of TiO2 and TiO2/SiO2 Xerogel Powders Applied in Photoanodes for Solar Cells. J. Solid State Electrochem. 2016, 20, 1731–1741.
- Gies, M.; Michel, F.; Lupó, C.; Schlettwein, D.; Becker, M.; Polity, A. Electrochromic Switching of Tungsten Oxide Films Grown by Reactive Ion-Beam Sputter Deposition. J. Mater. Sci. 2021, 56, 615–628.
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-Gel Synthesized Hexagonal Boron Nitride/Titania Nanocomposites with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2019, 465, 154–163.
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts 2020, 10, 464.
- de Moura, D.S.; Pazinato, J.C.O.; Pereira, M.B.; Mertins, O.; Silva, E.R.; Garcia, I.T.S. Poly(Vinyl Alcohol) as a Structuring Agent for Peroxotungstic Acid. J. Mol. Liq. 2018, 269, 92–100.
- Fang, Y.; Sun, X.; Cao, H. Influence of PEG Additive and Annealing Temperature on Structural and Electrochromic Properties of Sol–Gel Derived WO3 Films. J. Sol-Gel Sci. Technol. 2011, 59, 145–152.
- Işık, D.; Ak, M.; Durucan, C. Structural, Electrochemical and Optical Comparisons of Tungsten Oxide Coatings Derived from Tungsten Powder-Based Sols. Thin Solid Films 2009, 518, 104–111.
- Zhang, Y.; Yuan, J.; Le, J.; Song, L.; Hu, X. Structural and Electrochromic Properties of Tungsten Oxide Prepared by Surfactant-Assisted Process. Sol. Energy Mater. Sol. Cells 2009, 93, 1338–1344.
- Nakajima, T.; Hagino, A.; Nakamura, T.; Tsuchiya, T.; Sayama, K. WO3 Nanosponge Photoanodes with High Applied Bias Photon-to-Current Efficiency for Solar Hydrogen and Peroxydisulfate Production. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 17809–17818.
- Ojeda, M.; Gaster, C.; Harris, C. Fabrication and Internal Functionalization of Highly Macroporous WO3 Thin Films. Preprints 2018, 2018100227.
- Sadale, S.B.; Neumann-Spallart, M. Drop-Cast Tungsten Trioxide Semiconducting Films in Photoelectrocatalysis. J. Electroanal. Chem. 2020, 877, 114502.
- Wang, P.; Wang, S.; Wang, H.; Wu, Z.; Wang, L. Recent Progress on Photo-Electrocatalytic Reduction of Carbon Dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371.
- Trojanowicz, M. Impact of Nanotechnology on Design of Advanced Screen-Printed Electrodes for Different Analytical Applications. Trends Anal. Chem. 2016, 84, 22–47.
- Suresh, R.R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of Screen-Printed Electrodes: Opportunities and Challenges. J. Mater. Sci. 2021, 56, 8951–9006.
- Rong, Y.; Ming, Y.; Ji, W.; Li, D.; Mei, A.; Hu, Y.; Han, H. Toward Industrial-Scale Production of Perovskite Solar Cells: Screen Printing, Slot-Die Coating, and Emerging Techniques. J. Phys. Chem. Lett. 2018, 9, 2707–2713.
- Adly, N.; Bachmann, B.; Krause, K.J.; Offenhäusser, A.; Wolfrum, B.; Yakushenko, A. Three-Dimensional Inkjet-Printed Redox Cycling Sensor. RSC Adv. 2017, 7, 5473–5479.
- Bachalo, W.D.; Bachalo, W.D. Measurements of Inkjet Droplet Size, Velocity, and Angle of Trajectory. In Inkjet Printing in Industry: Materials, Technologies, Systems, and Applications; Wiley: Hoboken, NJ, USA, 2022.
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.-S.; Magdassi, S.; Lee, P.S. Inkjet-Printed All Solid-State Electrochromic Devices Based on NiO/WO3 Nanoparticle Complementary Electrodes. Nanoscale 2016, 8, 348–357.
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305.
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213.
- da Costa, C.H.; Costa, C.; Pinheiro, C.B.; da Silva Henriques, I.D.; Laia, C.A.T. Inkjet Printing of Sol-Gel Synthesized Hydrated Tungsten Oxide Nanoparticles for Flexible Electrochromic Devices. ACS Appl. Mater. Interfaces 2012, 4, 1330–1340.
- Hansora, D.; Cherian, D.; Mehrotra, R.; Jang, J.-W.; Lee, J.S. Fully Inkjet-Printed Large-Scale Photoelectrodes. Joule 2023, 7, 884–919.
- Hui, R.; Wang, Z.; Yick, S.; Maric, R.; Ghosh, D. Fabrication of Ceramic Films for Solid Oxide Fuel Cells via Slurry Spin Coating Technique. J. Power Sources 2007, 172, 840–844.
- Deepa, M.; Saxena, T.K.; Singh, D.P.; Sood, K.N.; Agnihotry, S.A. Spin Coated versus Dip Coated Electrochromic Tungsten Oxide Films: Structure, Morphology, Optical and Electrochemical Properties. Electrochim. Acta 2006, 51, 1974–1989.
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Characterization and Gas Sensing Properties of Spin Coated WO3 Thin Films. Z. Phys. Chem. 2020, 234, 1819–1834.
- Go, M.; Alam, A.; Choie, H.K.; Zhong, Z.; Lee, K.H.; Seo, Y.; Hwang, B.; Woo, K.; Kim, T.-W.; Lim, S. Meyer-Rod Coated 2D Single-Crystalline Copper Nanoplate Film with Intensive Pulsed Light for Flexible Electrode. Coat. World 2020, 10, 88.
- Jung, M.; Kim, J.; Koo, H.; Lee, W.; Subramanian, V.; Cho, G. Roll-to-Roll Gravure with Nanomaterials for Printing Smart Packaging. J. Nanosci. Nanotechnol. 2014, 14, 1303–1317.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
647
Revisions:
2 times
(View History)
Update Date:
09 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




