
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raffaele Pellegrino | -- | 2767 | 2024-02-08 08:13:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 2767 | 2024-02-08 08:58:33 | | |
Video Upload Options
Immunotherapy has emerged as a pivotal component in the treatment of various malignancies, encompassing lung, skin, gastrointestinal, and head and neck cancers. The foundation of this therapeutic approach lies in immune checkpoint inhibitors (ICI). While ICIs have demonstrated remarkable efficacy in impeding the neoplastic progression of these tumours, their use may give rise to substantial toxicity, notably in the gastrointestinal domain, where ICI colitis constitutes a significant aspect. The optimal positioning of Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway inhibitors in the therapeutic management of ICI colitis remains unclear. Numerous reports have highlighted notable improvements in ICI colitis through the application of pan-JAK-STAT inhibitors, with tofacitinib, in particular, reporting evident clinical remission of colitis.
1. Introduction
2. Immunotherapy, Generalities, and Toxicity: The Dimensions That Matter
3. Clinical Evidence Concerning JAK-STAT Inhibitors and ICI Colitis
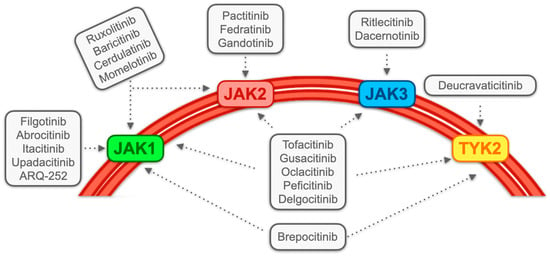
3.1. JAK Inhibitors: Generality and Classification
3.2. Contemporary Management in Accordance with Established Guidelines and Exploration of Giverse Alternatives, with Limited Emphasis on JAK Inhibitors
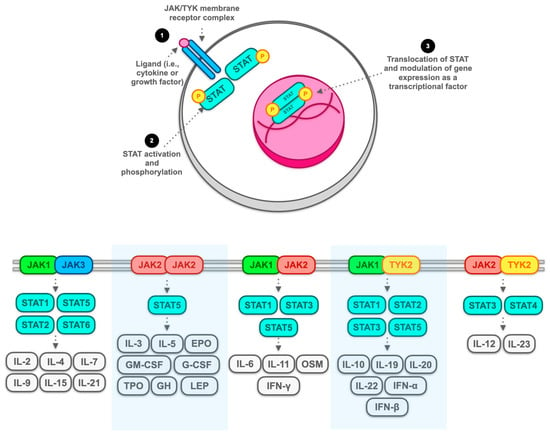
3.3. The Hyperactivation of T Cells as a Potential Therapeutic Target for JAK Inhibitors: General Overview with a Focus on CD8+ Resident Memory T Cells
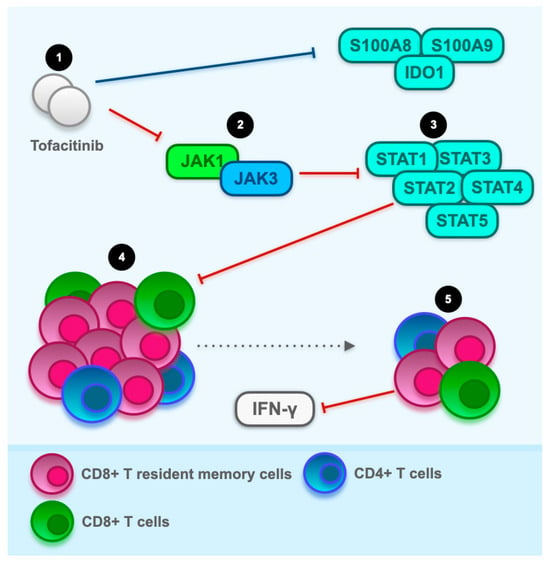
4. Conclusions
References
- Gong, Z.; Wang, Y. Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis: A Clinical Review. JCO Oncol. Prac. 2020, 16, 453–461.
- Singh, B.P.; Marshall, J.L.; He, A.R. Workup and Management of Immune-Mediated Colitis in Patients Treated with Immune Checkpoint Inhibitors. Oncologist 2020, 25, 197–202.
- Macovei Oprescu, A.; Tulin, R.; Slavu, I.; Venter, D.P.; Oprescu, C. Immune Checkpoint Inhibitor-Induced Gastrointestinal Toxicity: The Opinion of a Gastroenterologist. Cureus 2021, 13, e19945.
- König, D.; Läubli, H. Mechanisms of Immune-Related Complications in Cancer Patients Treated with Immune Checkpoint Inhibitors. Pharmacology 2021, 106, 123–136.
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355.
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune Checkpoint Inhibitor-Induced Colitis: A Comprehensive Review. World J. Clin. Cases 2019, 7, 405–418.
- Abu-Sbeih, H.; Ali, F.S.; Luo, W.; Qiao, W.; Raju, G.S.; Wang, Y. Importance of Endoscopic and Histological Evaluation in the Management of Immune Checkpoint Inhibitor-Induced Colitis. J. Immunother. Cancer 2018, 6, 95.
- Hashash, J.G.; Francis, F.F.; Farraye, F.A. Diagnosis and Management of Immune Checkpoint Inhibitor Colitis. Gastroenterol. Hepatol. 2021, 17, 358–366.
- Shivaji, U.N.; Jeffery, L.; Gui, X.; Smith, S.C.L.; Ahmad, O.F.; Akbar, A.; Ghosh, S.; Iacucci, M. Immune Checkpoint Inhibitor-Associated Gastrointestinal and Hepatic Adverse Events and Their Management. Therap. Adv. Gastroenterol. 2019, 12, 1756284819884196.
- Abu-Sbeih, H.; Wang, Y. Management Considerations for Immune Checkpoint Inhibitor-Induced Enterocolitis Based on Management of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 662–668.
- Portenkirchner, C.; Kienle, P.; Horisberger, K. Checkpoint Inhibitor-Induced Colitis-A Clinical Overview of Incidence, Prognostic Implications and Extension of Current Treatment Options. Pharmaceuticals 2021, 14, 367.
- Smyth, M.J.; Teng, M.W. 2018 Nobel Prize in Physiology or Medicine. Clin. Transl. Immunol. 2018, 7, e1041.
- Ljunggren, H.; Jonsson, R.; Höglund, P. Seminal Immunologic Discoveries with Direct Clinical Implications: The 2018 Nobel Prize in Physiology or Medicine Honours Discoveries in Cancer Immunotherapy. Scand. J. Immunol. 2018, 88, e12731.
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. Adv. Exp. Med. Biol. 2020, 1248, 7–32.
- Linsley, P.S.; Greene, J.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) Bind with Similar Avidities but Distinct Kinetics to CD28 and CTLA-4 Receptors. Immunity 1994, 1, 793–801.
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 Is a Second Receptor for the B Cell Activation Antigen B7. J. Exp. Med. 1991, 174, 561–569.
- He, M.; Chai, Y.; Qi, J.; Zhang, C.W.H.; Tong, Z.; Shi, Y.; Yan, J.; Tan, S.; Gao, G.F. Remarkably Similar CTLA-4 Binding Properties of Therapeutic Ipilimumab and Tremelimumab Antibodies. Oncotarget 2017, 8, 67129–67139.
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561.
- Finger, L.R.; Pu, J.; Wasserman, R.; Vibhakar, R.; Louie, E.; Hardy, R.R.; Burrows, P.D.; Billips, L.G. The Human PD-1 Gene: Complete cDNA, Genomic Organization, and Developmentally Regulated Expression in B Cell Progenitors. Gene 1997, 197, 177–187.
- Mallett, G.; Laurence, A.; Amarnath, S. Programmed Cell Death-1 Receptor (PD-1)-Mediated Regulation of Innate Lymphoid Cells. Int. J. Mol. Sci. 2019, 20, 2836.
- Ghosh, C.; Luong, G.; Sun, Y. A Snapshot of the PD-1/PD-L1 Pathway. J. Cancer 2021, 12, 2735–2746.
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742.
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39.
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of Response, Resistance, and Toxicity to Immune Checkpoint Blockade. Cell 2021, 184, 5309–5337.
- Khan, Z.; Di Nucci, F.; Kwan, A.; Hammer, C.; Mariathasan, S.; Rouilly, V.; Carroll, J.; Fontes, M.; Ley Acosta, S.; Guardino, E.; et al. Polygenic Risk for Skin Autoimmunity Impacts Immune Checkpoint Blockade in Bladder Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 12288–12294.
- Liu, C.; Kieltyka, J.; Fleischmann, R.; Gadina, M.; O’Shea, J.J. A Decade of JAK Inhibitors: What Have We Learned and What May Be the Future? Arthritis Rheumatol. 2021, 73, 2166–2178.
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and Future Status of JAK Inhibitors. Lancet 2021, 398, 803–816.
- Damsky, W.; King, B.A. JAK Inhibitors in Dermatology: The Promise of a New Drug Class. J. Am. Acad. Dermatol. 2017, 76, 736–744.
- Kameda, H. JAK Inhibitors∼Overview∼. Immunol. Med. 2023, 46, 108–111.
- Benucci, M.; Damiani, A.; Infantino, M.; Manfredi, M.; Lari, B.; Grossi, V.; Gobbi, F.L.; Sarzi-Puttini, P. Cardiovascular Safety, Cancer and Jak-Inhibitors: Differences to Be Highlighted. Pharmacol. Res. 2022, 183, 106359.
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 879–913.
- Winthrop, K.L.; Cohen, S.B. Oral Surveillance and JAK Inhibitor Safety: The Theory of Relativity. Nat. Rev. Rheumatol. 2022, 18, 301–304.
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664.
- Terrin, M.; Migliorisi, G.; Dal Buono, A.; Gabbiadini, R.; Mastrorocco, E.; Quadarella, A.; Repici, A.; Santoro, A.; Armuzzi, A. Checkpoint Inhibitor-Induced Colitis: From Pathogenesis to Management. Int. J. Mol. Sci. 2023, 24, 11504.
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736.
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17.
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Schreiber, S.; D’Haens, G.; Tanida, S.; Siffledeen, J.; Enejosa, J.; Zhou, W.; Othman, A.A.; et al. Efficacy of Upadacitinib in a Randomized Trial of Patients with Active Ulcerative Colitis. Gastroenterology 2020, 158, 2139–2149.e14.
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as Induction and Maintenance Therapy for Moderately to Severely Active Ulcerative Colitis: Results from Three Phase 3, Multicentre, Double-Blind, Randomised Trials. Lancet 2022, 399, 2113–2128.
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as Induction and Maintenance Therapy for Ulcerative Colitis (SELECTION): A Phase 2b/3 Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2021, 397, 2372–2384.
- Loftus, E.V.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980.
- Sandborn, W.J.; Feagan, B.G.; Loftus, E.V.; Peyrin-Biroulet, L.; Van Assche, G.; D’Haens, G.; Schreiber, S.; Colombel, J.-F.; Lewis, J.D.; Ghosh, S.; et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients with Crohn’s Disease. Gastroenterology 2020, 158, 2123–2138.e8.
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical Remission in Patients with Moderate-to-Severe Crohn’s Disease Treated with Filgotinib (the FITZROY Study): Results from a Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2017, 389, 266–275.
- Santos, S.; Gamelas, V.; Saraiva, R.; Simões, G.; Saiote, J.; Ramos, J. Tofacitinib: An Option for Acute Severe Ulcerative Colitis? GE Port. J. Gastroenterol. 2022, 29, 132–134.
- Steenholdt, C.; Dige Ovesen, P.; Brynskov, J.; Benedict Seidelin, J. Tofacitinib for Acute Severe Ulcerative Colitis: A Systematic Review. J. Crohns Colitis 2023, 17, 1354–1363.
- Gisbert, J.P.; García, M.J.; Chaparro, M. Rescue Therapies for Steroid-Refractory Acute Severe Ulcerative Colitis: A Review. J. Crohns Colitis 2023, 17, 972–994.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106.
- Sørensen, A.S.; Andersen, M.N.; Juul-Madsen, K.; Broksø, A.D.; Skejø, C.; Schmidt, H.; Vorup-Jensen, T.; Kragstrup, T.W. Tumor Necrosis Factor Alpha Neutralization Attenuates Immune Checkpoint Inhibitor-Induced Activation of Intermediate Monocytes in Synovial Fluid Mononuclear Cells from Patients with Inflammatory Arthritis. Arthritis Res. Ther. 2022, 24, 43.
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238.
- Abu-Sbeih, H.; Ali, F.S.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of Vedolizumab Therapy in Patients with Immune Checkpoint Inhibitor-Induced Colitis: A Multi-Center Study. J. Immunother. Cancer 2018, 6, 142.
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor-Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210.




