
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aida A. Abd El-Wahed | -- | 1562 | 2024-02-08 05:33:25 | | | |
| 2 | Lindsay Dong | Meta information modification | 1562 | 2024-02-08 08:56:38 | | |
Video Upload Options
Royal jelly (RJ), a natural bee product rich in bioactive components, as an alternative strategy for managing metabolic diseases. RJ exhibits diverse therapeutic properties, including antimicrobial, estrogen-like, anti-inflammatory, hypotensive, anticancer, and antioxidant effects.
1. Introduction
Royal jelly (RJ) is a “rich source of nutrients” that nurse bees produce and feed to worker larvae and queen bees. RJ supplementation is beneficial for a variety of disorders, including diabetes [1][2], gastrointestinal diseases [3][4], and cardiovascular diseases [5][6]. The active ingredients of RJ, including its proteins, carbohydrates, and fats, as well as its minerals, amino acids, vitamins, enzymes, hormones, and polyphenols, are what provide its biological properties [7].
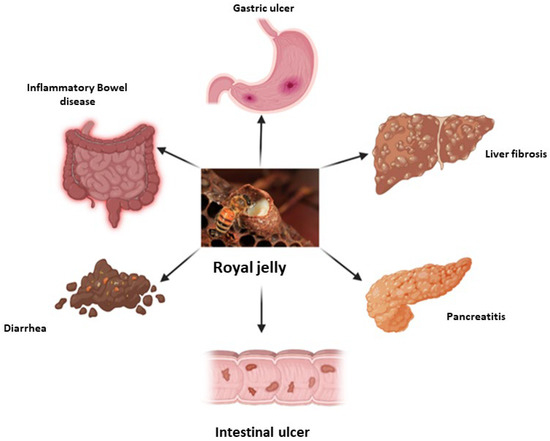
2. Royal Jelly in Gastrointestinal Diseases
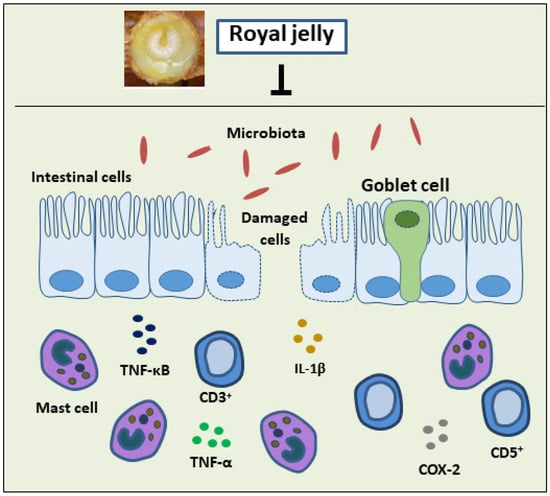
2.1. Inflammatory Bowel Diseases
2.2. Lactose Intolerance
2.3. Chronic Diarrhea and Constipation
2.4. Gastrointestinal Ulcer Disease
2.5. Liver Disease
2.5.1. Preclinical Studies
2.5.2. Clinical Studies
References
- Asgari, M.; Asle-Rousta, M.; Sofiabadi, M. Effect of Royal Jelly on Blood Glucose and Lipids in Streptozotocin Induced Type 1 Diabetic Rats. Arak Med. Univ. J. 2017, 20, 48–56.
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of Royal Jelly Supplementation on Body Weight and Dietary Intake in Type 2 Diabetic Females. Health Promot. Perspect. 2012, 2, 231–235.
- Duran, Y.; Karaboğa, İ.; Polat, F.R.; Polat, E.; Erboğa, Z.F.; Ovalı, M.A.; Öztopuz, R.Ö.; Çelikkol, A.; Yılmaz, A. Royal Jelly Attenuates Gastric Mucosal Injury in a Rat Ethanol-Induced Gastric Injury Model. Mol. Biol. Rep. 2020, 47, 8867–8879.
- Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Wakuda, H.; Yamada, S.; Shinozuka, K. Effect of Royal Jelly on Mouse Isolated Ileum and Gastrointestinal Motility. J. Med. Food 2019, 22, 789–796.
- Ohba, K.; Miyata, Y.; Shinzato, T.; Funakoshi, S.; Maeda, K.; Matsuo, T.; Mitsunari, K.; Mochizuki, Y.; Nishino, T.; Sakai, H. Effect of Oral Intake of Royal Jelly on Endothelium Function in Hemodialysis Patients: Study Protocol for Multicenter, Double-Blind, Randomized Control Trial. Trials 2021, 22, 950–957.
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal Jelly Is an Effective and Relatively Safe Alternative Approach to Blood Lipid Modulation: A Meta-Analysis. J. Funct. Foods 2018, 41, 202–209.
- Bagameri, L.; Baci, G.-M.; Dezmirean, D.S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics 2022, 14, 1142.
- Ananthakrishnan, A.N.; Xavier, R.J. Gastrointestinal Diseases. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 16–26.
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3.
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979–1983.
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033.
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201.
- Proestos, C. Superfoods: Recent Data on Their Role in the Prevention of Diseases. Curr. Res. Nutr. Food Sci. 2018, 6, 576–593.
- Mostafa, R.E.; El-Marasy, S.A.; Abdel Jaleel, G.A.; Bakeer, R.M. Protective Effect of Royal Jelly against Diclofenac-Induced Hepato-Renal Damage and Gastrointestinal Ulcerations in Rats. Heliyon 2020, 6, e03330.
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382.
- Li, S.; Tao, L.; Yu, X.; Zheng, H.; Wu, J.H.F. Royal Jelly Proteins and Their Derived Peptides: Preparation, Properties, and Biological Activities. J. Agric. Food Chem. 2021, 69, 14415–14427.
- Jairath, V.; Feagan, B.G. Global Burden of Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3.
- Karaca, T.; Uz, Y.H.; Demirtas, S.; Karaboga, I.; Can, G. Protective Effect of Royal Jelly in 2,4,6 Trinitrobenzene Sulfonic Acid-Induced Colitis in Rats. Iran. J. Basic Med. Sci. 2015, 18, 370–379.
- Karaca, T.; Bayiroglu, F.; Yoruk, M.; Kaya, M.S.; Uslu, S.; Comba, B.; Mis, L. Effect of Royal Jelly on Experimental Colitis Induced by Acetic Acid and Alteration of Mast Cell Distribution in the Colon of Rats. Eur. J. Histochem. 2010, 54, 158–161.
- Karaca, T.; Şimşek, N.; Uslu, S.; Kalkan, Y.; Can, I.; Kara, A.; Yörük, M. The Effect of Royal Jelly on CD3+, CD5+, CD45+ T-Cell and CD68+ Cell Distribution in the Colon of Rats with Acetic Acid-Induced Colitis. Allergol. Immunopathol. 2012, 40, 357–361.
- Chi, X.; Liu, Z.; Wang, H.; Wang, Y.; Xu, B.; Wei, W. Regulation of a New Type of Selenium-Rich Royal Jelly on Gut Microbiota Profile in Mice. Biol. Trace Elem. Res. 2022, 200, 1763–1775.
- Guo, J.; Ma, B.; Wang, Z.; Chen, Y.; Tian, W.; Dong, Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients 2022, 14, 2069.
- Li, J.; Zhang, W.; Wang, C.; Yu, Q.; Dai, R.; Pei, X. Lactococcus Lactis Expressing Food-Grade β-Galactosidase Alleviates Lactose Intolerance Symptoms in Post-Weaning Balb/c Mice. Appl. Microbiol. Biotechnol. 2012, 96, 1499–1506.
- Kavas, N. Functional Probiotic Yoghurt Production with Royal Jelly Fortification and Determination of Some Properties. Int. J. Gastron. Food Sci. 2022, 28, 100519.
- Oak, S.J.; Jha, R. The Effects of Probiotics in Lactose Intolerance: A Systematic Review–The Effects of Probiotics in Lactose Intolerance: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2018, 8398, 1425977.
- Hassan, A.A.M.; Elenany, Y.E.; Nassrallah, A.; Cheng, W.; Abd El-Maksoud, A.A. Royal Jelly Improves the Physicochemical Properties and Biological Activities of Fermented Milk with Enhanced Probiotic Viability. LWT 2022, 155, 112912.
- Vazhacharickal, J.P. A Review on Health Benefits and Biological Action of Honey, Propolis and Royal Jelly. J. Med. Plants Stud. 2021, 9, 1–13.
- Ali, M.W.; Sabir, A.M.; Gadour, M.O. Gum Arabic in Treatment of Functional Constipation in Children in Sudan. Sudan JMS 2012, 8, 73–76.
- Postali, E.; Peroukidou, P.; Giaouris, E.; Papachristoforou, A. Investigating Possible Synergism in the Antioxidant and Antibacterial Actions of Honey and Propolis from the Greek Island of Samothrace through Their Combined Application. Foods 2022, 11, 2041.
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active Components and Biological Functions of Royal Jelly. J. Funct. Foods 2021, 82, 104514.
- Bílikova, K.; Huang, S.-C.; Lin, I.-P.; Šimuth, J.; Peng, C.-C. Structure and Antimicrobial Activity Relationship of Royalisin, an Antimicrobial Peptide from Royal Jelly of Apis Mellifera. Peptides 2015, 86, 190–196.
- Chantawannakul, P. From Entomophagy to Entomotherapy. Front. Biosci. 2020, 25, 179–200.
- Sofiabadi, M.; Samiee-Rad, F. Royal Jelly Accelerates Healing of Acetate Induced Gastric Ulcers in Male Rats. Gastroenterol. Hepatol. Bed Bench 2020, 13, 18–22.
- Abd El-Naeem, A. Effect of Nicotine on the Structure of Gastric Mucosa of Adult Male Albino Rats and the Possible Protective Effect of Royal Jelly (Light and Scanning Electron Microscopic Study). Egypt. J. Histol. 2021, 45, 404–415.
- Ahmed, W.M.S.; Khalaf, A.A.; Moselhy, W.A.; Safwat, G.M. Royal Jelly Attenuates Azathioprine Induced Toxicity in Rats. Environ. Toxicol. Pharmacol. 2014, 37, 431–437.
- Felemban, A.H.; Alshammari, G.M.; Yagoub, A.E.G.A.; Al-Harbi, L.N.; Alhussain, M.H.; Yahya, M.A. Activation of AMPK Entails the Protective Effect of Royal Jelly against High-Fat-Diet-Induced Hyperglycemia, Hyperlipidemia, and Non-Alcoholic Fatty Liver Disease in Rats. Nutrients 2023, 15, 1471.
- Fang, C.; Pan, J.; Qu, N.; Lei, Y.; Han, J.; Zhang, J.; Han, D. The AMPK Pathway in Fatty Liver Disease. Front. Physiol. 2022, 13, 970292.
- Bahaaldin-Beygi, M.; Kariminik, A.; Arababadi, M.K. Royal Jelly Significantly Alters Inflammasome Pathways in Patients with Chronic Hepatitis B. Indian J. Exp. Biol. 2022, 60, 875–879.




