Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Sanches Silva | -- | 2625 | 2024-02-08 01:49:11 | | | |
| 2 | Catherine Yang | Meta information modification | 2625 | 2024-02-08 01:54:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tábuas, B.; Cruz Barros, S.; Diogo, C.; Cavaleiro, C.; Sanches Silva, A. Pyrrolizidine Alkaloids. Encyclopedia. Available online: https://encyclopedia.pub/entry/54883 (accessed on 10 March 2026).
Tábuas B, Cruz Barros S, Diogo C, Cavaleiro C, Sanches Silva A. Pyrrolizidine Alkaloids. Encyclopedia. Available at: https://encyclopedia.pub/entry/54883. Accessed March 10, 2026.
Tábuas, Bruna, Sílvia Cruz Barros, Catarina Diogo, Carlos Cavaleiro, Ana Sanches Silva. "Pyrrolizidine Alkaloids" Encyclopedia, https://encyclopedia.pub/entry/54883 (accessed March 10, 2026).
Tábuas, B., Cruz Barros, S., Diogo, C., Cavaleiro, C., & Sanches Silva, A. (2024, February 08). Pyrrolizidine Alkaloids. In Encyclopedia. https://encyclopedia.pub/entry/54883
Tábuas, Bruna, et al. "Pyrrolizidine Alkaloids." Encyclopedia. Web. 08 February, 2024.
Copy Citation
Pyrrolizidine alkaloids (PAs) are a group of natural compounds that are present in several plant families, among which the most predominant are Boraginaceae, Asteraceae, and Fabaceae, and are produced as a defense response against herbivores. PAs are known for their toxicity and for posing an elevated risk to human and animal health when consumed in large quantities over long periods of time.
pyrrolizidine alkaloids
toxicology
metabolism
chromatography
1. Chemistry of PAs
PAs are organic compounds characterized by the heterocyclic nucleus of pyrrolizidine, hydroxymethylated at position 1 (necine), and generally esterified with aliphatic acids, which are generically referred as necic acids [1] (Figure 1).
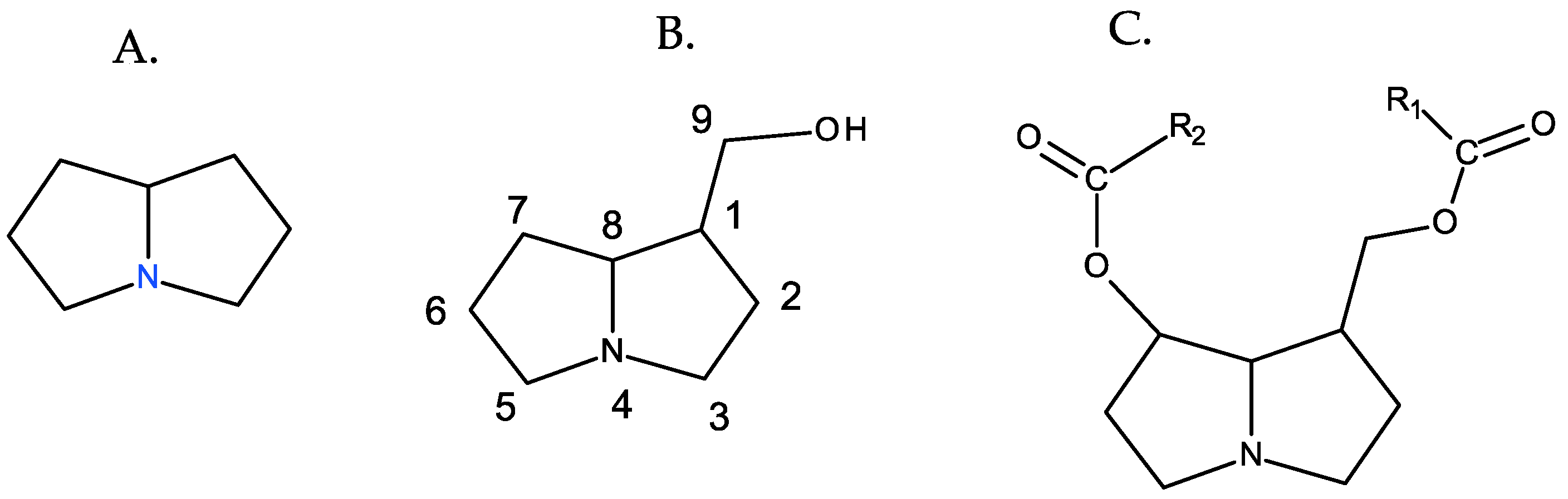
Figure 1. Fundamental structures of PAs. From left to right: (A) pyrrolizidine, (B) 1-hydroxymethylpyrrolizidine (necine), and (C) necine diester.
In most cases, the necine base, in addition to the hydroxymethyl at C-1, is also hydroxylated at C-7. This hydroxyl can also be esterified, and depending on whether esterification occurs at one or both hydroxyl groups (positions C-7 and C-9), PAs can be monoesters or diesters and, in the latter case, open-chain esters or cyclic esters [1] (Figure 2).
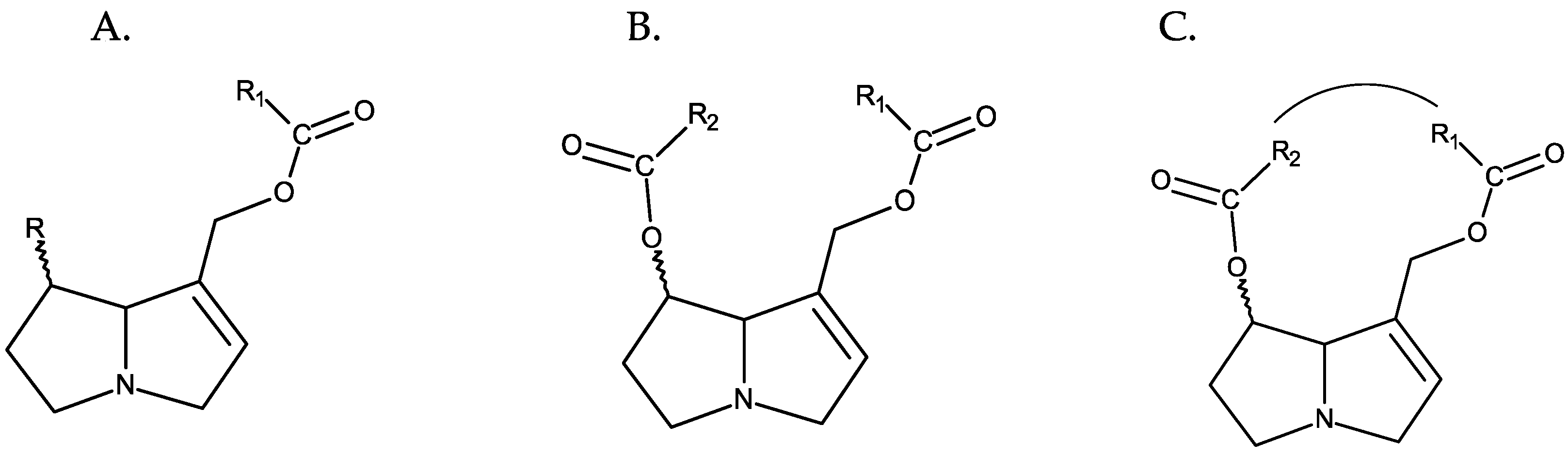
Figure 2. Different types of esterification of PAs. From left to right: (A) monoester, (B) open-chain diester, and (C) cyclic diester.
Depending on the structure of the necine base, PAs can be divided into four main types: retronecine, heliotridine, otonecine, and platynecine (Figure 3). Retronecine, heliotridine, and otonecine types include PAs derived from 1,2-unsaturated pyrrolizidine (unsaturated bases), while those of the platynecine type are derived from the saturated base. From a structural point of view, the otonecine type is the most peculiar, as it is derived from a monocyclic base, has a carbonyl group at C-8 and a methylated nitrogen. Retronecine- and heliotridine-type PAs are diastereoisomers at C-7 [2]. Besides these main types of necine bases, there are other types; however, they are of minor significance.
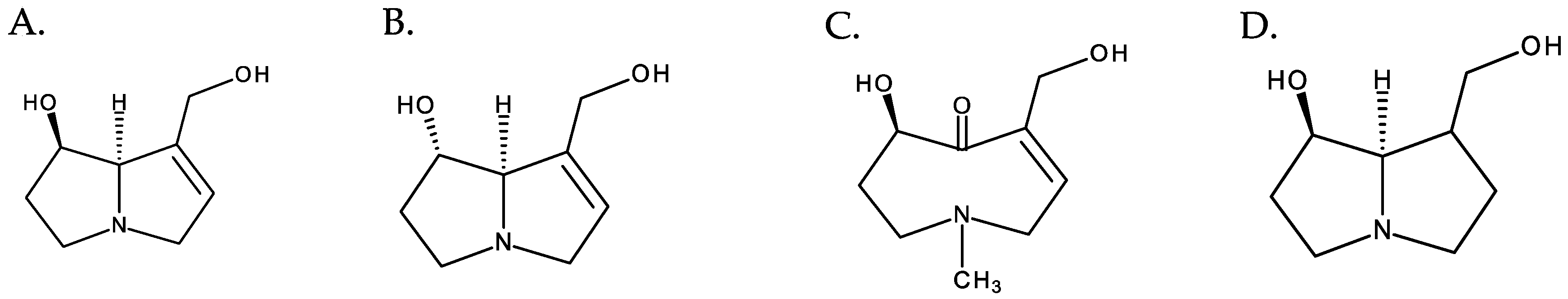
Figure 3. Structures of the most common necine bases. From left to right: (A) retronecine, (B) heliotridine, (C) otonecine, and (D) platynecin.
PAs normally occur in the form of tertiary bases or of their N-oxides, often coexisting in both forms. PANOs are ionic structures resulting from the oxidation of the nitrogen atom of the necine base [3], and they correspond to the most predominant form in plants [4] (Figure 4).
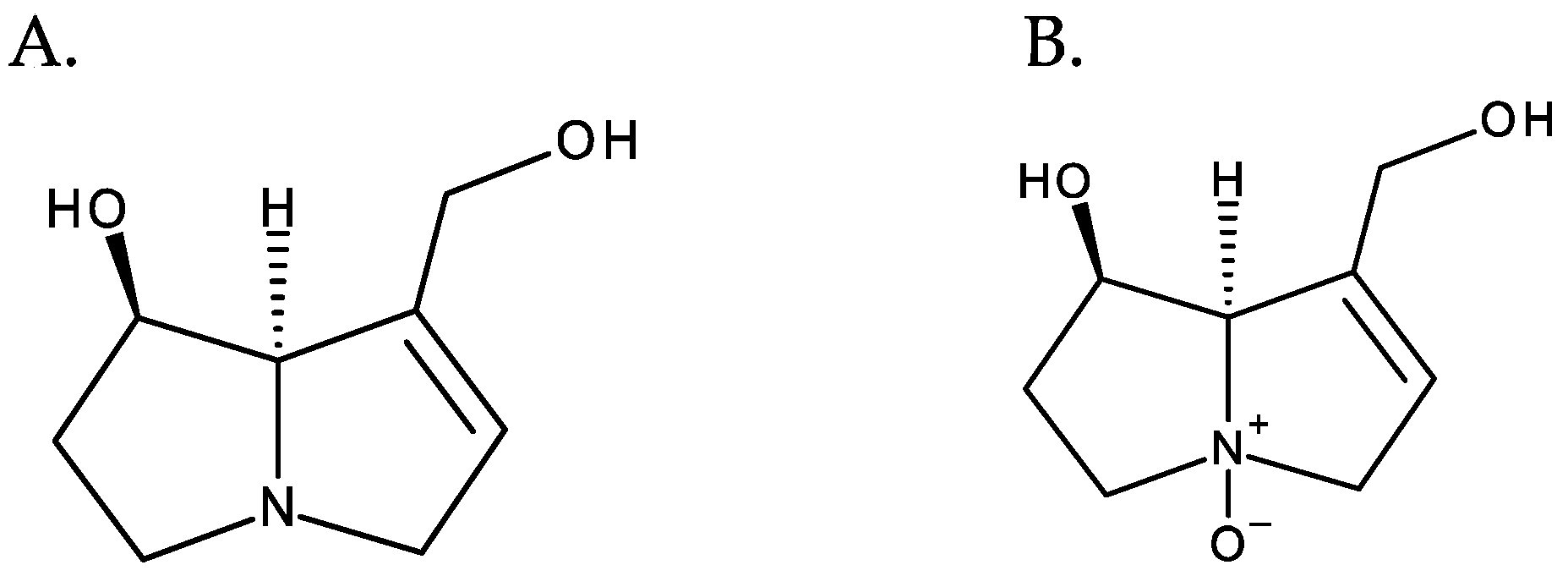
Figure 4. From left to right: (A) retronecine and (B) retronecine N-oxide.
Compared with tertiary bases, which are soluble in non-polar organic solvents and in some polar organic solvents, PANOs are soluble in water, methanol, and other polar organic solvents [2]. These characteristics are relevant in the physiological processes of transport and storage of PAs and have significance for the laboratory procedures of extraction and analysis. All known PAs are capable of forming N-oxide derivatives, except those of the otonecine type [5].
Although necine bases are structurally related, necic acids have a wide structural diversity. They are aliphatic, monocarboxylic, or dicarboxylic acids, most with branched carbon chains and hydroxy and alkoxy substituents. Acetic, angelic and tiglic, trachelantic and viridifloric acids (monocarboxylic acids), and senecic and isatinecic acids (dicarboxylic) are examples necic acids (Figure 5). Necic acids with aromatic systems are rarely found [4].
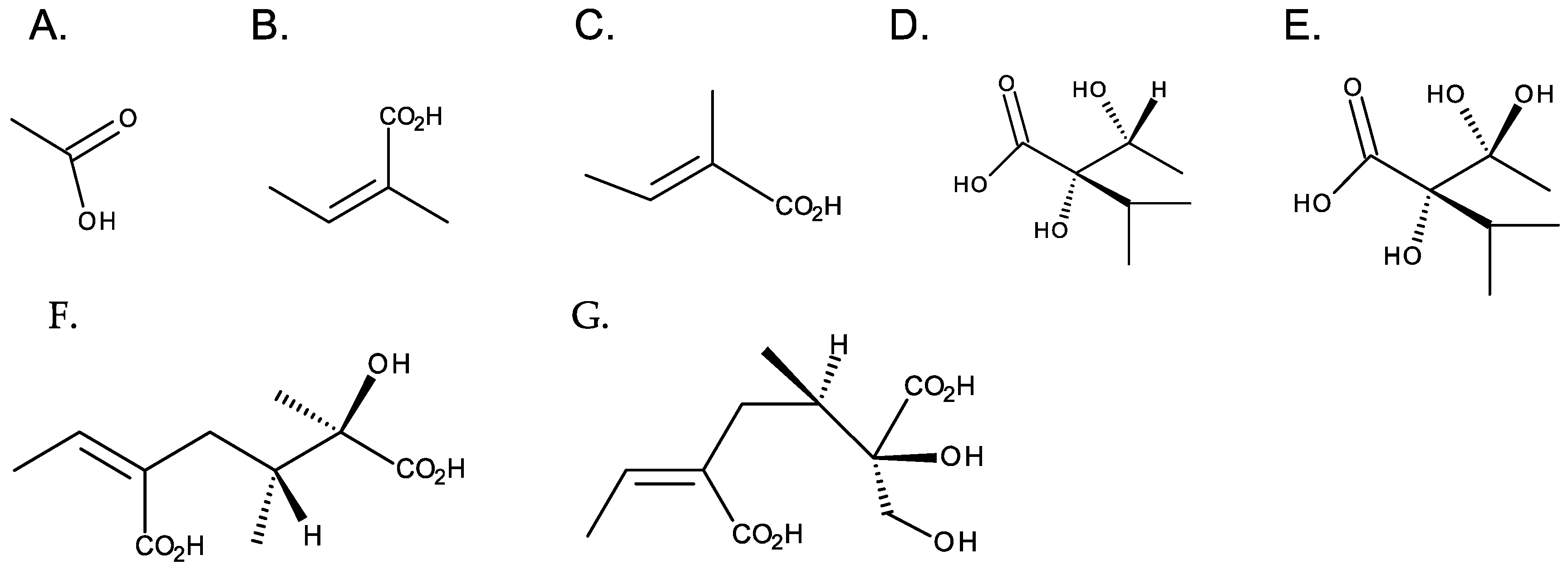
Figure 5. Common necic acids. From left to right: first row—monocarboxylic acids: (A) acetic acid, (B) angelic acid, (C) tiglicic acid, (D) trachelantic acid, and (E) viridifloric acid; second row: dicarboxylic necic acids: (F) senecic acid, and (G) isatinecic acid.
The combination of different necine bases with many necic acids results in the huge diversity of PAs [2]. The N-oxidation of the tertiary nitrogen of the necine base multiplies this diversity [4] (Figure 6). Hundreds of PAs have already been described, and new variants continue to be discovered [4].
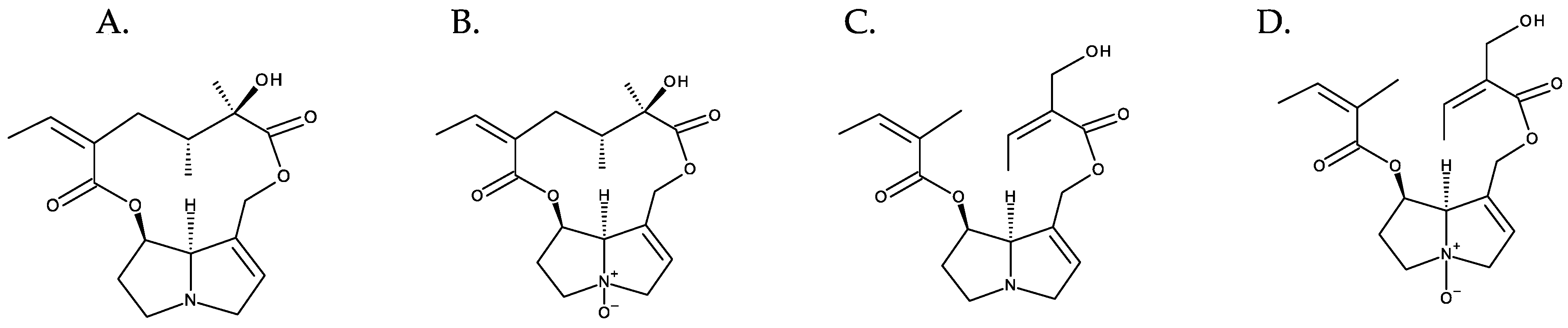
Figure 6. Diversity of PAs exemplified with retronecine derivatives. From left to right: (A) senecionine and (B) senecionine-N-oxide (cyclic diesters); (C) triangularine and (D) triangularine-N-oxide (open-chain diesters).
It is also common to group PAs into types defined by the binding patterns between necine bases and necic acids [4] or by the plant taxa where they were originally identified. According to Hartmann and Witthe, based on the combination of necine base and the necic acids, it is possible to classify them into five groups. The first and largest group are the senecionine-type PAs, which are found mainly in the Fabaceae family and the Senecionaeae genus (of the Asteraceae family). The triangularine-type PAs represent the second group typical of the Boraginaceae family and Senecioneae genus. The third type is the lycopsamine-type PAs that can be found in Boraginaceae. The fourth group is is monocrolatin-type PAs, predominant in the Fabaceae family. The phallenopsin-type PAs that can be found in some species of Boraginaceae [2][4][6] (Table 1).
Table 1. Examples of PA-producing plants and their PAs.
| Family | Genus | Species (e.g.) | Type of PA | PAs |
|---|---|---|---|---|
| Fabaceae | Crotalaria spp. | C. albida C. assamica C. pallida C. sessiliflora |
Monocrotalin-type | Monocrotaline Aucherine |
| Senecionine-type | Senecionine Platyphylline Rosmarinine Senecivernine Nemorensine |
|||
| Asteraceae | Senecio spp. | S. jacobaea S. vulgaris L. S. nemorensis L. S. argunensis S. integrifolius (L.) S. scandens S. longilobus |
Triangularine-type | Triangularine Sarracine Macrophylline |
| Eupatoriae spp. | E. cannabinum E. chinense E. japonicum E. fortunei |
Lycopsamine-type | Acetylechimidine and isomers Echimidine and isomers |
|
| Boraginaceae | Heliotropium spp. | H. arborescens H. indicum |
Lycopsamine-type Phalaenosine-type |
Europine Heliotrine Lasiocarpine |
The biosynthesis of necine bases begins with the decarboxylation of the amino acids L-arginine and L-ornithine, which leads to the formation of putrescine [2][4][5]. Next, the formation of the precursor of the necine bases—homospermidine—occurs, for which two theories have been proposed: one that argues that homospermidine results from the condensation of two putrescine molecules [2][5]; another that argues that the formation of homospermidine results from the reaction between a putrescine molecule and another spermidine molecule [4][7]. Regardless of the precursors used, the formation of homospermidine, a key step in the biosynthesis of PAs, is catalyzed by homospermidine synthase. Homospermidine is then cyclized to the corresponding iminium ion, which, in turn, is reduced and cyclized, giving rise to isoretronecanol, trachelantamidine, and rosmarinecine, which are all necine bases. Subsequently, through hydroxylation and dehydration, retronecin and heliotridine are obtained. From retronecin and via subsequent hydroxylations and methylations, otonecine is obtained [5] (Figure 7).
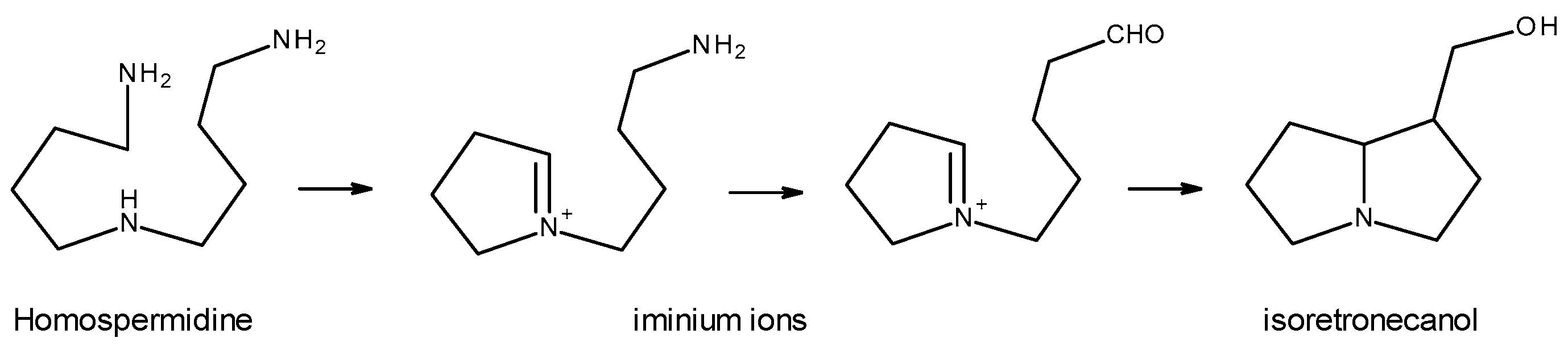
Figure 7. Necine-based biosynthesis (Adapted from: [5]).
Although necine bases are synthesized through a common pathway, necic acids can have distinct biosynthetic origins. Some of them, such as acetic acid, are products of the plant’s primary metabolism [4]. The others are mostly derived from aliphatic amino acids, such as L-threonine, L-valine, L-leucine, and L-isoleucine [7], the latter having a central role as a precursor of necic acids [8] (Figure 8). Regarding the formation of monocarboxylic acids, such as angelic, tiglic, or sarracinic acids, mainly threonine and isoleucine are involved. The biosynthesis of trachelantic, viridifloric, or senecioic acids mainly involves valine as a precursor. Finally, dicarboxylic acids, such as senecic acid, are mainly formed from isoleucine and threonine [2][4].
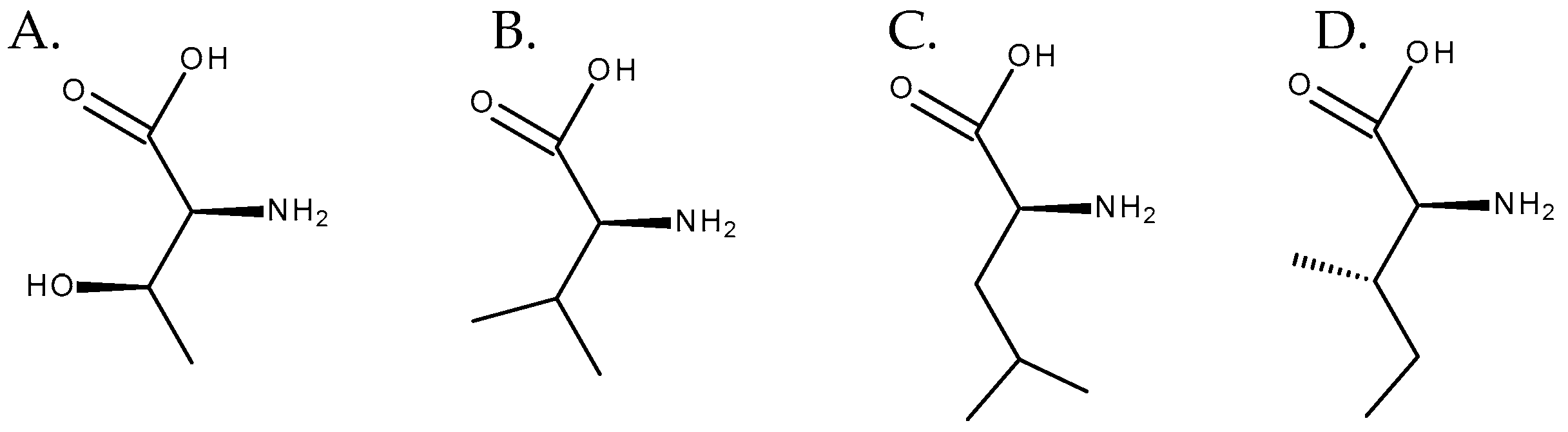
Figure 8. Amino acids involved in the biosynthesis of necic acids. From left to right: (A) L-threonine, (B) L-valine, (C) L-leucine, and (D) L-isoleucine.
The biosynthesis of PAs occurs fundamentally in roots [9]. However, it has also been described that the biosynthetic process can occur in specific young leaves [10]. These compounds are almost exclusively available and stored in the form of N-oxides, which, owing to their high-water solubility, can be easily transported to other plant organs. At any time, PANOs can be reduced to their respective tertiary amines.
It is estimated that approximately 6000 plant species have the capacity to produce one or more of the PAs and PANOs already identified. This fact probably makes PA-producing plants the most common group of toxic plants, capable of affecting both animals and humans. PAs are mostly found in Angiosperms, in the families Asteraceae (tribes Senecioneae—where the genus Senecio stands out—and Eupatorieae), Boraginaceae (all genera, highlighting the genus Heliotropium of the subfamily Heliotropioideae and the genera Echium and Symphytum of the subfamily Boraginoideae), Fabaceae (genus Crotalaria), Orchidaceae, and Apocynaceae [6][8][11].
Some of these plants are used as cover crops for soil improvement, ornamental plants or as animal feed. Among them, there are some species of the genera Heliotropium and Crotalaria, which are common weeds in crop fields, whereas others are particularly appreciated as melliferous plants (family Boraginaceae) [6].
Most PA-producing plants produce several different PAs that can be found in different concentrations. In a way, the different structural types of PAs are typical of certain taxa, although there are some overlaps. Senecionin-type PAs (such as jacobine, jacoline, jaconine, retrorsine, senecionine and seneciphylline) are characteristic of the tribe Senecioneae (of the Asteraceae family), but also of the Fabaceae family, particularly the genus Crotalaria. Lycopsamine-type PAs (which include echimidine, lycopsamine, and vulgarine) appear particularly in the Boraginaceae family, but also in the Eupatorieae tribe of the Asteraceae family. The heliotrine type (which has europine, heliothrine and lasiocarpine as Pas) is typical of the genus Heliotropium (from the Boraginaceae family. Monocrotaline-type (for example, 6 ulvene, monocrotaline, and tricodesmine) appear most frequently in the Fabaceae family, more precisely, in the genus Crotalaria. The triangularine-type is predominantly found in the Senecioneae tribe and the Boraginaceae family. Phalaenopsin-type PAs are found in the Orchidaceae family. With regard to necic acids, monocarboxylic acids are characteristic of the Boraginaceae family and macrocyclic diesters are characteristic of the Asteraceae family.
The biosynthesis of PAs, which is dependent on genetic variation and heredity, is regulated differently during plant development. The occurrence and number of PAs vary greatly, depending on the species and part of the plant. For example, in the genera Senecio and Eupatorium (family Asteraceae), PA synthesis is restricted to the roots. It is also influenced by other factors such as climate and soil properties, namely nutrients, water quantity, and herbivore infestations [4] (Figure 9).
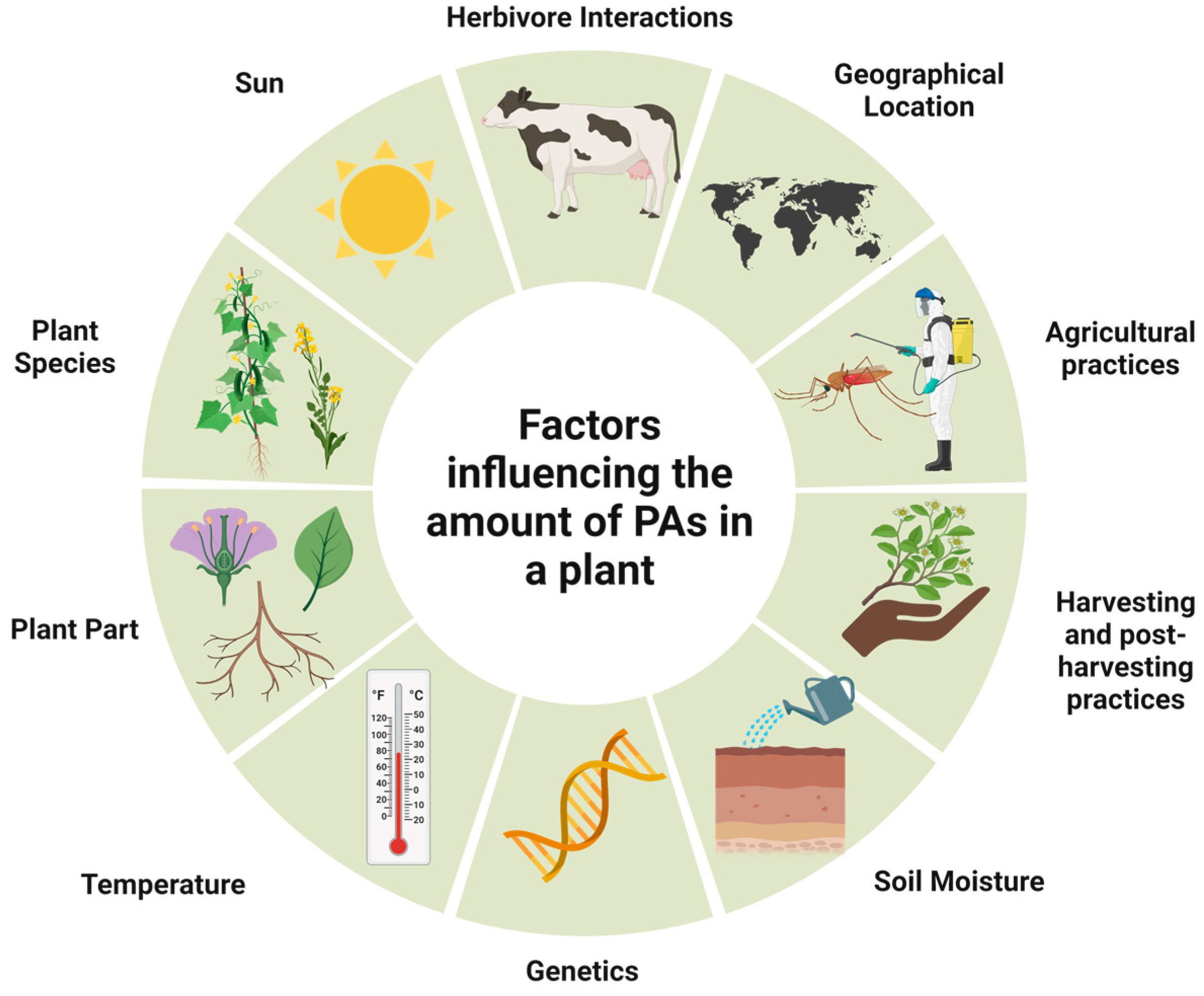
Figure 9. Main factors influencing PAs occurrence and contents in plant material.
2. Metabolism and Toxicity of PAs
It is estimated that approximately half of the known PAs are toxic [12], meaning that the consumption of plant products originating from numerous species or products derived from them may imply a risk of toxicity. In this regard, the genera Heliotropium and Crotalaria are particularly relevant, which include the species most commonly associated with poisoning in humans [6].
The toxicological effects of acute exposure of humans and livestock to PAs are known and well documented in the literature. The majority of cases reported in humans are related to the consumption of medicines and herbal dietary supplements, vegetable infusions, cereals, or products derived from them contaminated with seeds from plants producing PAs, honey, and dairy products. These events are mostly related to products produced in regions of Central Asia, Afghanistan, and India, with a direct relationship with periods of meteorological drought, which favor the development of weeds and contamination of crops [6].
Some of the documented cases are related to wheat flour contaminated with Heliotropium seeds and qurut—a goat’s milk cheese common in Central Asia—where PAs characteristic of Heliotropium and Crotalaria species were detected [13].
In Europe, occurrences are rare [11] and the risk of acute poisoning due to PAs is considered low, both by the German Federal Institute for Risk Assessment (BfR) and the European Food Safety Authority (EFSA) [14][15]. However, the potential health risks from chronic exposure to low doses have been the cause of recent concern from European regulatory authorities—EFSA and the European Medicines Agency (EMA) [16], motivated, above all, by the detection of significant amounts of PAs in various foods, as well as in herbal medicines and food supplements [4].
This concern is also because there is little toxicological information on most PAs, and their molecular mechanisms of toxicity are not fully understood [17]. All this apprehension is reinforced by the knowledge, for several decades now, of the acute toxicity, genotoxicity, and carcinogenicity of some of these compounds in laboratory animals [6][15][18][19].
PAs are rapidly absorbed in the gastrointestinal tract, with cutaneous absorption estimated to be of little significance. Compartmental studies have revealed distribution mainly in red blood cells, liver, kidney, lung, and plasma [6]. Because of their lipophilic properties, some PAs are capable of crossing the placental barrier [17].
The catabolism of PAs involves three metabolic pathways.
In general, the metabolization pathways vary according to the necine base; therefore, for retronecine- and heliotridine-type PAs, there are three metabolization pathways:
-
Hydrolysis of the ester groups to form the necine base and the corresponding necic acids.
-
N-oxidation, for conversion to PANOs.
On the other hand, otonecine-type PAs have only two main metabolic pathways:
-
Hydrolysis of the ester groups to form the corresponding necine bases and necic acids.
The liver is the main organ responsible for metabolism, although small contributions from the lungs and kidneys have been observed. Hydrolysis, which may occur in the transport and distribution process via non-specific blood esterases, occurs mainly through the catalysis of hepatic microsomal carboxylesterases. The products, necine bases and necic acids, are non-toxic and can be immediately conjugated and excreted via urination [5][19].
However, most PAs are oxidized to the corresponding N-oxides through hepatic microsomal monooxygenases: flavin monooxygenase and microsomal cytochrome P450 monooxygenase (CYP450) [5][23]. This N-oxidation is exclusive to the bases of the retronecin and heliotridine types because nitrogen is methylated in the bases of the otonecine type. The N-oxide metabolites are very soluble in water and are therefore easily excreted in urine [14]. However, given that PANOs can be metabolically reconverted into PAs, a cyclical effect may occur [12].
C-oxidation, particularly of carbon-α, catalyzed by the microsomal monooxygenase CYP450, will convert PAs to reactive toxic pyrrolic metabolites—pyrrolic esters or dehydropyrrolizidine alkaloids (DHPAs) (Figure 10).
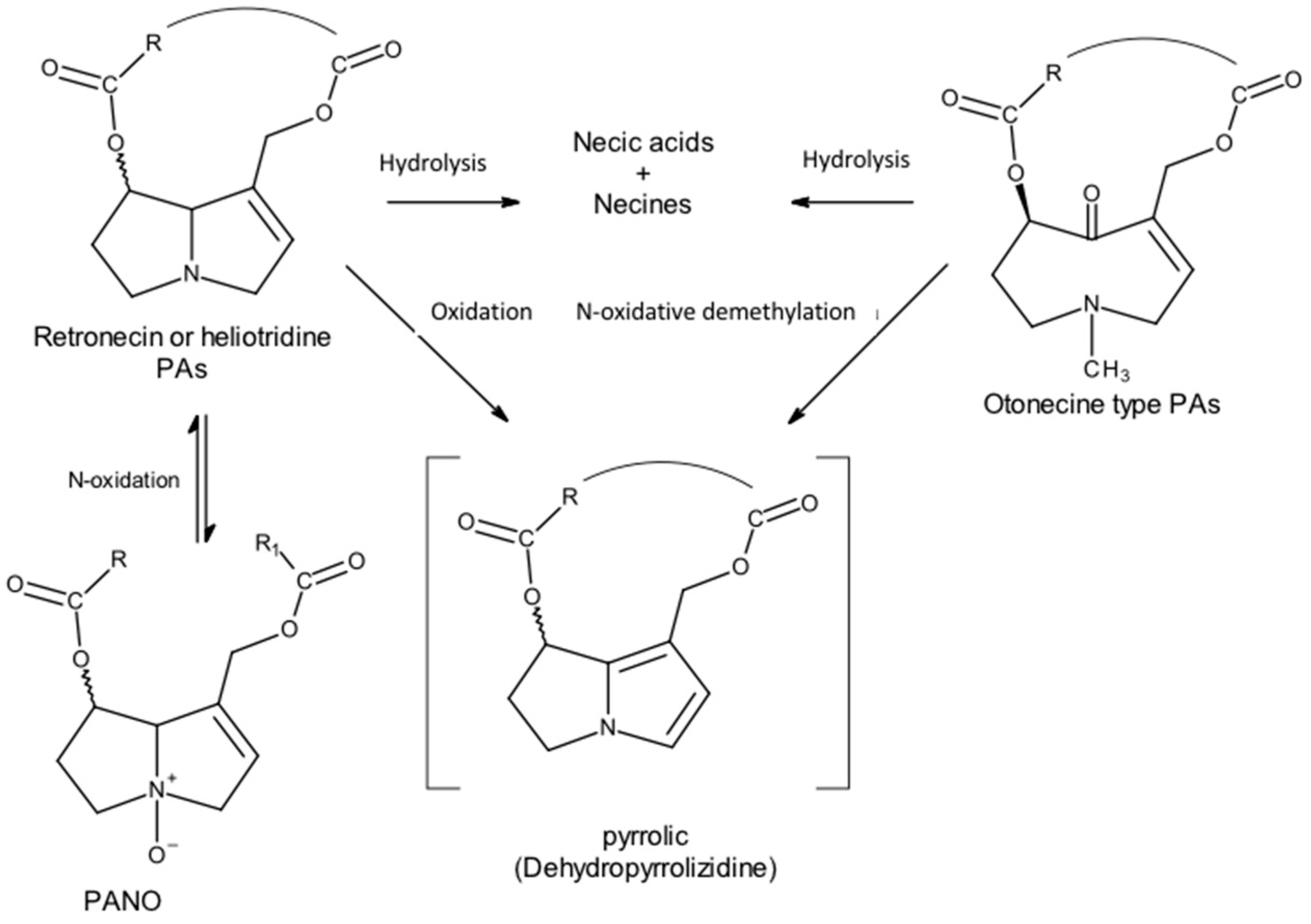
Figure 10. PA metabolism leads to pyrrolic derivatives (Adapted from: [12]).
PA metabolism is rapid, and metabolites can be detected in the liver and lungs 30–120 min after ingestion [24].
Approximately 80% of ingested PAs are excreted unchanged, predominantly through urine in feces and milk [2].
Not all PAs are toxic, and those that exhibit toxicity have common structural characteristics, among which the double bond between the 1 and 2 positions stands out, being unsaturated PAs (1,2-unsaturated). Therefore, the PAs of the retronecine, heliotridine, and otonecine types stand out as being toxic, and those of the platinecine type as non-toxic. The presence of this double bond is responsible for the level of toxicity in the liver because these compounds need to undergo metabolic activation, to form the highly reactive pyrrole intermediates. The pyrrole intermediates can penetrate the nucleus and react with DNA to form adducts and DNA-protein cross-links that can cause damage, particularly to hepatocytes. Hepatic veno-occlusive disease is caused by the damage that these toxic metabolites cause to the hepatocytes. Briefly, after oral ingestion of PAs, they are rapidly absorbed in the gastrointestinal tract and undergo metabolic activation in the liver [2][25]. Saturated PAs do not undergo metabolic activation in the liver and form water-soluble compounds that are easily excreted [6].
As noted earlier, the liver is the main organ affected by PAs toxicity. HVOD is the main clinical manifestation and is considered a marker of PA toxicity. Some symptoms included vomiting, hepatomegaly, and bloody diarrhea. Poisoning can present itself in three forms: acute, subacute, and chronic, and the point of distinction is the symptoms presented. The acute one is characterized by hemorrhagic necrosis, hepatomegaly, and ascites; in the subacute one, both the ascites and the liver are enlarged, and there is blockage of the hepatic sinuses leading to Sinusiodal Obstruction Syndrome (HSOS); finally, in the chronic one, necrosis, fibrosis and cirrhosis, liver failure, and death are present symptoms [2].
Therefore, for PAs to be considered toxic, they must have three minimum structural requirements: the presence of a double bond at positions 1 and 2; a hydroxymethyl substituent at position C1, preferably with a second hydroxyl group at position C7; and the presence of a branched necic acid (mono- or dicarboxylic) with at least five carbon atoms [26] (Figure 11).
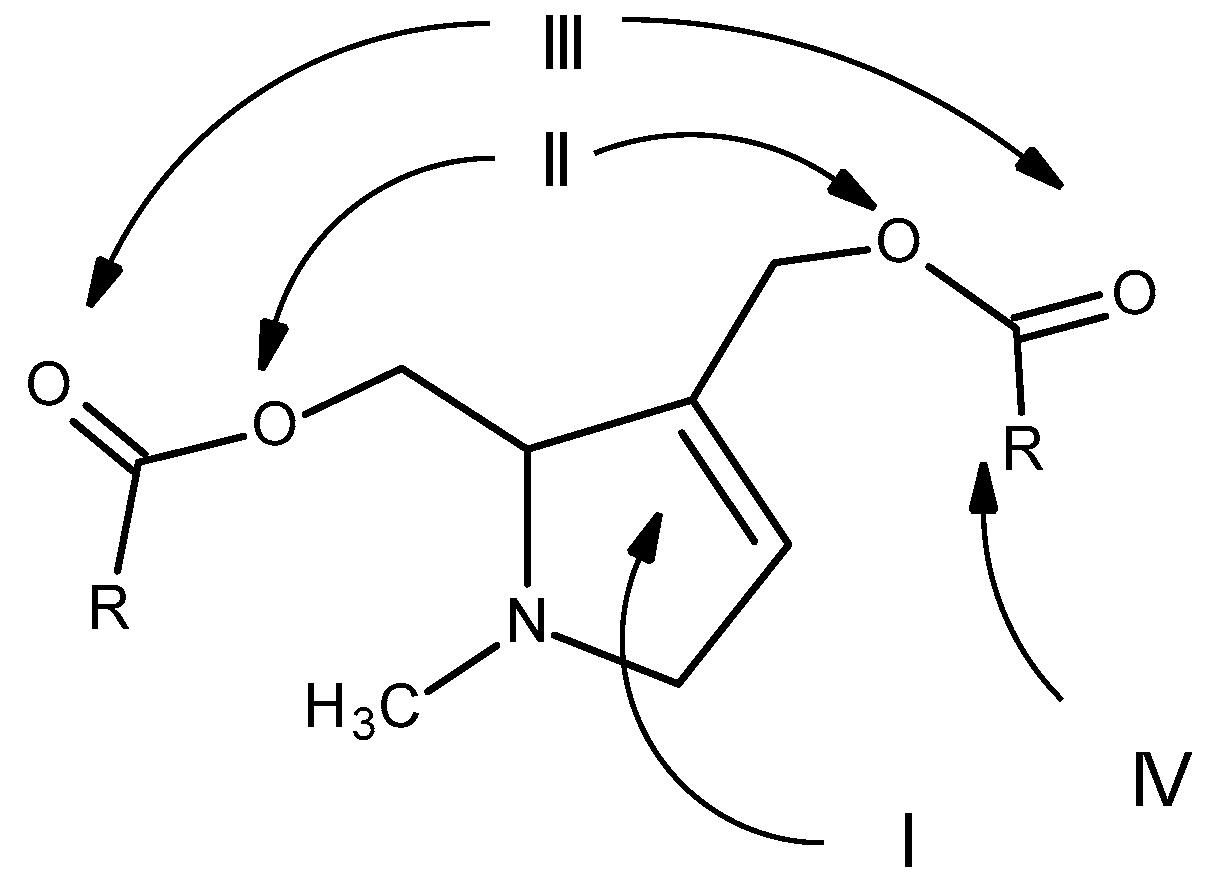
Figure 11. Structural features essential for the toxicity of PAs: (I) double bond at positions 1 and 2; (II) hydroxymethyl substituent at position C1; (III) hydroxyl group at position C7 (III); (IV) presence of a branched necic acid (mono- or dicarboxylic) with at least five carbon atoms (Adapted from: [23]).
References
- Avula, B.; Sagi, S.; Wang, Y.H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Characterization and Screening of Pyrrolizidine Alkaloids and N-Oxides from Botanicals and Dietary Supplements Using UHPLC-High Resolution Mass Spectrometry. Food Chem. 2015, 178, 136–148.
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668.
- Louisse, J.; Rijkers, D.; Stoopen, G.; Holleboom, W.J.; Delagrange, M.; Molthof, E.; Mulder, P.P.J.; Hoogenboom, R.L.A.P.; Audebert, M.; Peijnenburg, A.A.C.M. Determination of Genotoxic Potencies of Pyrrolizidine Alkaloids in HepaRG Cells Using the ΓH2AX Assay. Food Chem. Toxicol. 2019, 131, 110532.
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498.
- Roeder, E. Analysis of pyrrolizidine alkaloids. Curr. Org. Chem. 1999, 3, 557–576.
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406.
- Hartmann, T. Chemical ecology of pyrrolizidine alkaloids. Planta 1999, 207, 483–495.
- Langel, D.; Ober, D.; Pelser, P. The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem. Rev. 2011, 10, 3–74.
- Ehmke, A.; von Borstel, K.; Hartmann, T. Alkaloid N-oxides as transport and vacuolar storage compounds of pyrrolizidine alkaloids in Senecio vulgaris L. Planta 1988, 176, 83–90.
- Kruse, L.H.; Stegemann, T.; Sievert, C.; Ober, D. Identification of a Second Site of Pyrrolizidine Alkaloid Biosynthesis in Comfrey to Boost Plant Defense in Floral Stage. Plant Physiol. 2017, 174, 47–55.
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.J.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.M.C.M.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine Alkaloids in Food and Phytomedicine: Occurrence, Exposure, Toxicity, Mechanisms, and Risk Assessment—A Review. Food Chem. Toxicol. 2020, 136, 111107.
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of Pyrrolizidine Alkaloids. J. Appl. Toxicol. 2010, 30, 183–196.
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; Van Egmond, H.P.; Omar, M.F.; Mofleh, J. An Outbreak of Hepatic Veno-Occlusive Disease in Western Afghanistan Associated with Exposure to Wheat Flour Contaminated with Pyrrolizidine Alkaloids. J. Toxicol. 2010, 2010, 313280.
- Bundesinstitut Für Risikobewertung (BfR). Pyrrolizidine Alkaloids in Herbal Teas and Teas; Bundesinstitut Für Risikobewertung (BfR): Berlin, Germany, 2013.
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements. EFSA J. 2017, 15, e04908.
- Avila, C.; Breakspear, I.; Hawrelak, J.; Salmond, S.; Evans, S. A Systematic Review and Quality Assessment of Case Reports of Adverse Events for Borage (Borago Officinalis), Coltsfoot (Tussilago Farfara) and Comfrey (Symphytum Officinale). Fitoterapia 2020, 142, 104519.
- Luckert, C.; Hessel, S.; Lenze, D.; Lampen, A. Disturbance of Gene Expression in Primary Human Hepatocytes by Hepatotoxic Pyrrolizidine Alkaloids: A Whole Genome Transcriptome Analysis. Toxicol. Vitr. 2015, 29, 1669–1682.
- European Medicines Agency (EMA). Public Statement on Contamination of Herbal Medicinal Products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids; EMA: Amsterdam, The Netherlands, 2016.
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine Alkaloids—Genotoxicity, Metabolism Enzymes, Metabolic Activation, and Mechanisms. Drug Metab. Rev. 2004, 36, 1–55.
- Fu, P.P. Pyrrolizidine Alkaloids: Metabolic Activation Pathways Leading to Liver Tumor Initiation. Chem. Res. Toxicol. 2017, 30, 81–93.
- Kolrep, F.; Numata, J.; Kneuer, C.; Preiss-Weigert, A.; Lahrssen-Wiederholt, M.; Schrenk, D.; These, A. In vitro biotransformation of pyrrolizidine alkaloids in different species. Part I: Microsomal degradation. Arch Toxicol 2018, 92, 1089–1097.
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine Alkaloids: An Update on Their Metabolism and Hepatotoxicity Mechanism. Liver Res. 2019, 3, 176–184.
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.B.; Seawright, A.A. Pyrrolizidine Alkaloids in Human Diet. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 53–67.
- Mativcks, A.R.; Driver, H.E. Metabolism and toxicity of anacrotine, a pyrrolizidinic alkaloid, in rats. Chem. Biol. Interact. 1987, 63, 91–104.
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The Concerning Food Safety Issue of Pyrrolizidine Alkaloids: An Overview. Trends Food Sci. Technol. 2022, 120, 123–139.
- European Medicines Agency (EMA). EMA/HMPC/893108/2011 Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs) Including Recommendations Regarding Contamination of Herbal Medicinal Products with Pas. Sci. Med. Health 2021, 1–36.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





