Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anis M. Limami | -- | 2548 | 2024-02-07 17:14:28 | | | |
| 2 | Jessie Wu | -11 word(s) | 2537 | 2024-02-08 03:31:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Morère-Le Paven, M.; Clochard, T.; Limami, A.M. NPF and NRT2 Potentially Involved in Nodule Functioning. Encyclopedia. Available online: https://encyclopedia.pub/entry/54863 (accessed on 07 February 2026).
Morère-Le Paven M, Clochard T, Limami AM. NPF and NRT2 Potentially Involved in Nodule Functioning. Encyclopedia. Available at: https://encyclopedia.pub/entry/54863. Accessed February 07, 2026.
Morère-Le Paven, Marie-Christine, Thibault Clochard, Anis M. Limami. "NPF and NRT2 Potentially Involved in Nodule Functioning" Encyclopedia, https://encyclopedia.pub/entry/54863 (accessed February 07, 2026).
Morère-Le Paven, M., Clochard, T., & Limami, A.M. (2024, February 07). NPF and NRT2 Potentially Involved in Nodule Functioning. In Encyclopedia. https://encyclopedia.pub/entry/54863
Morère-Le Paven, Marie-Christine, et al. "NPF and NRT2 Potentially Involved in Nodule Functioning." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Legumes are commonly used in sustainable agroecosystems because of their ability to tolerate low N fertilizer input due to their capacity to use atmospheric N2 through biological nitrogen fixation (BNF). The advantage of using legumes in agroecosystems is not limited to protecting soils from pollution caused by chemical fertilizers because once well-established legumes progressively fertilize the soil. Legumes, such as Pea (Pisum sativum), are nowadays introduced in cropping systems to provide ecological services i.e., limiting the usage of N fertilizer and decreasing herbicide input by competing with weeds for soil water, mineral nutrients and light, thus limiting their development. Competitive genotypes to fulfil this role should be selected on the basis of their ability to efficiently colonize the soil with deep-foraging, fast-growing and highly branched root systems. These traits are known to be under the control of rhizosphere factors, among which nitrate as a signal molecule, sensed by various nitrate transporters such as NPF (Nitrate Transporter1/Peptide transporter Family) and NRT2 (Nitrate Transporter 2), plays a major role. Paradoxically, if nitrate is necessary to ensure legumes’ seedling establishment before BNF starts, it is also a negative regulator of nodulation and BNF if it is provided at high concentrations.
nitrate transporter
nodules
NPF
NRT2
1. NPFs Playing a Role in Nodule Functioning
In L. japonicus, an in silico analysis showed that the expression of eight LjNPF genes was upregulated in mature N2-fixing nodules [1]. Two of these eight NPFs, LjNPF8.6 and LjNPF3.1, were studied in depth [2][3]. LjNPF8.6, whose expression is strongly induced in nodules compared to roots, is the first NPF for which a specific and positive role on nodule functioning has been shown [2]. LjNPF8.6 was found to be located in the central infection zone where N fixation takes place [4]. In addition, after inoculation of Ljnpf8.6 mutants by Mesorhizobium loti, an increase in superoxide content in the nodules accompanied by a reduction in N-fixation activity was observed with an accumulation of anthocyanin in stems and roots [2]. Anthocyanin accumulation in stems has been reported as a phenotype associated with nitrogen starvation condition associated with impaired nodule function or lack of nodulation ([3] and references therein). These observations suggest that LjNPF8.6 plays a role in the control of nodule functioning rather than in development. Furthermore, this transporter was shown to have a nitrate transport activity; it is thus tempting to suggest that LjNPF8.6 plays a role in the control of nodule functioning through the modulation of nitrate flux trough the peribacteroidal membrane [2]. Another interesting transporter in L. japonicus is LjNPF3.1 [1]. The LjNPF3 promoter was shown to be active in the cortical cells of inoculated hairy roots and at the base of the nodules [3]. Actually, its expression was more than 10-fold higher in nodules than in roots, while it was also expressed in leaves and mature flowers. In addition, inoculated Ljnpf3.1 mutants showed increased nodule biomass and anthocyanin accumulation in the stems, phenotypes that can be explained by a slight but significant decrease in the measured nitrogenase activity. Thus, LjNPF3.1 plays a positive role in efficient nodule functioning, possibly by transporting nitrate from the roots or from outside to the nodules [3]. However, the role of LjNPF3.1 would be limited to conditions of low external nitrate concentration that are not inhibitory for BNF.
In M. truncatula, the expression of several MtNPFs is upregulated in nodules [5]. However only two NPFs playing a role in nodule functioning, MtNPF1.7 and MtNPF7.6, have been deeply studied in M. truncatula. MtNPF1.7 (also known as LATD/NIP) was functionally characterized as a high-affinity nitrate transporter [6], involved in root development [7][8], with an essential role in the formation and maintenance of nodule meristems and in rhizobial invasion [8]. Studies of different mutants, affected in MtNPF1.7, have shown that MtNPF1.7 is not necessary for the initial stages of rhizobial invasion into host roots but is required during further stages of nodulation [9][10][11]. Since MtNPF1.7 is expressed and required in both lateral root and nodule meristems, the corresponding protein could play a key role in the balance between development of lateral roots and nodules [8].
MtNPF7.6, the second NPF of M. truncatula studied in detail, was described as specifically expressed in nodule vasculature and localized in the plasma membrane of nodule transfer cells (NTCs) [12]. Using knockout mtnpf7.6 mutants, it has been shown that MtNPF7.6 modulates Lb expression, endogenous NO homeostasis and nitrogenase activity. MtNPF7.6 has been shown to play a role in nitrate-mediated regulation during root nodule symbiosis under both low- and high-nitrate conditions [12]. Under low nitrate (0.2 mM), MtNPF7.6, demonstrated as being a high-affinity nitrate transporter, functions in nitrate uptake from the environment and from the host root, as well as in nitrate transport to NTCs, promoting nodule growth. Under high-nitrate conditions (20 mM), MtNPF7.6 expression is induced, and an over-accumulation of nitrate due to MtNPF7.6-nitrate-transport inhibits nodule functioning. Interestingly, comparing the transcriptome of wild-type and mtnpf7.6 nodules, it has been shown that the expression patterns of four genes, encoding MtNRT2.1, MtNRT2.2, MtNRT2.3 and MtNPF6.5, were altered in the mutants, suggesting that MtNPF6.5 and MtNRT2s may be involved in the nutrient or signal exchange in nodule [12].
Concerning P. sativum, 69 PsNPFs were identified [13]. In addition, a full-length Unigene set of expressed sequences has been developed in P. sativum by sequencing 20 cDNA libraries produced from various plant organs harvested at various developmental stages from plants grown under different conditions [14] (https://urgi.versailles.inra.fr/download/pea/Pea_PSCAM_transcriptome, accessed on 15 March 2023). However, some NPFs mentioned in [14] were not identified previously [13]. Thus, to identify the complete PsNPF family in P. sativum, researchers performed a blastp search using PsNPF6.7 (Psat2g025760) as a query against P. sativum v1a genomic assembly [15]. Researchers were able to find 90 putative PsNPF sequences, among which researchers found the 69 previously identified [13] and 21 new ones distributed in the 8 clades (Figure 1) previously described [13].
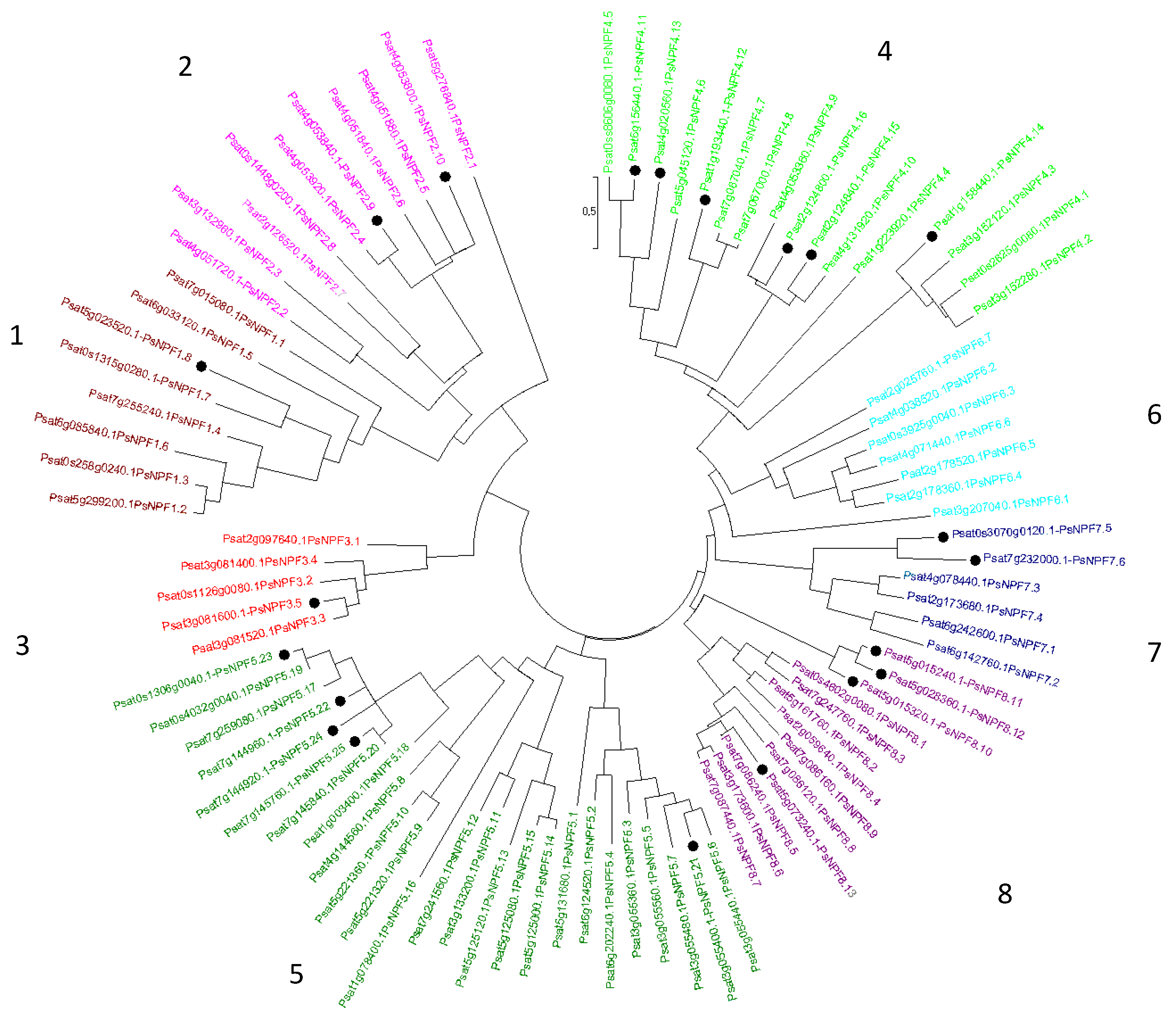
Figure 1. Phylogenetic tree of the NPF family from P. sativum. Ninety amino acid sequences were aligned with the CLUSTALW program. The evolutionary history was inferred using the Maximum Likelihood method based on the JTT matrix-based model [16]. Evolutionary analyses were conducted in MEGA7 [17]. The eight NPF clades, numbered from 1 to 8, are indicated by different colors [18]. The newly identified sequences are presented with a black point.
The new sequences are distributed as follows: one sequence belongs to the clade 1, two to the clade 2, one to the clade 3, six to the clade 4, five to the clade 5, two to the clade 7 and four to the clade 8. New PsNPF were annotated according to the two-number code previously established [18]. Then, researchers exported the expression data of the 90 PsNPF genes from the full-length Unigene set of P. sativum [14]. It should be noted that the length of PsNPF proteins ranged from 93 to 637 amino acids, with some protein sequences being much shorter than those of NPFs already described in the literature: they have been retained in this research because the corresponding genes are expressed (except PsNPF5.23), sometimes very significantly, as seen for PsNPF4.16 (233 amino acids), which is very strongly expressed in the peduncles of the C stage (20 days after the start of flowering) [14].
In a study [14], 842 genes were shown to be specifically expressed in nodules. Among them, 66 contigs encoded transporters of various families, of which 6 belonged to the NPF family (Figure 2A), and 3 showed significant expression in nodules but were also expressed in other organs (Figure 2B). One of them, PsNPF7.1, is the ortholog of MtNPF7.6 [12]. PsNPF7.1 is specifically and very strongly expressed in nodules (Figure 2, [14]). In a recent study, researchers investigated whether Rhizobium-derived signals interfere with nitrate signaling in P. sativum [19]. It appeared that PsNPF7.1 expression was induced in 12-day-old seedlings only in the presence of Rhizobium. In addition, PsNPF7.1 expression was upregulated by 1 mM nitrate and downregulated by 10 mM. Therefore, a possible role of PsNPF7.1 in nodule functioning dependent on environmental nitrate concentration seems interesting to be studied further. The orthologous genes of MtNPF1.7, LjNPF8.6 and LjNPF3.1 in P. sativum, PsNPF1.5, PsNPF8.4 and PsNPF3.1, respectively, would also be interesting to study. It is worth noting that some NPF genes produce different transcripts, as seen for AtNPF5.5 [20], among which PsNPF1.5 produces two different transcripts. It would be interesting to investigate whether the corresponding proteins are both functional and which role they fulfill.
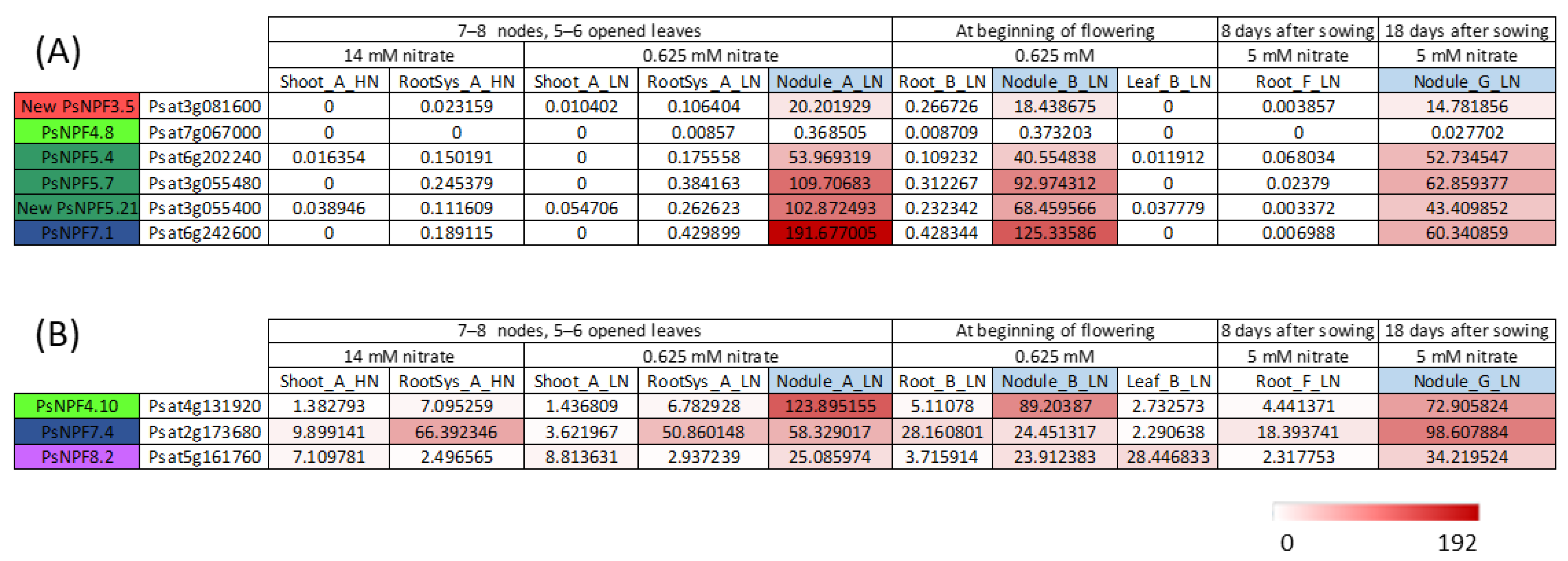
Figure 2. PsNPF genes that are expressed in nodules. Data were extracted from the full-length Unigene set of expressed sequences from P. sativum [14]. The expression of the 90 PsNPFs under all the conditions. (A) PsNPF exclusively expressed in nodules. (B) PsNPF genes highly expressed in nodules and other organs. Numbers are normalized count data.
2. NRT2s Playing a Role in Nodule Functioning
LjNRT2.4 was the first NRT2 to be thoroughly studied in L. japonicus. In contrast to the other LjNRT2 genes, a strong induction of LjNRT2.4 expression was observed in nodules compared to roots [21][22]. A positive role of LjNRT2.4 was reported in a nitrate-mediated nodule functioning pathway [22]. In fact, two Ljnrt2.4 mutants were impaired in nitrate content and nitrogenase activity in nodules. LjNRT2.4, whose tissue localization was shown to be the nodule vascular bundles and subcellular localization the plasma membrane, would transport nitrate into the N2-fixing cells of the nodule. Nitrite derived from nitrate reduction in the cytoplasm can be transported to the mitochondria where it serves as an electron acceptor in the respiratory chain, thus contributing to ATP synthesis [23][24][25]. Nitrate can also be reduced to nitrite by nitrate reductase in the bacteroid. LjNPF8.6, localized in the peribacteroidal membrane, would play a role in the regulation of nitrate flux between the plant cell and the bacteroid [2]. Thus, a model has been proposed in which LjNRT2.4 and LjNPF8.6 would be involved in nodule functioning in a complementary manner [22].
LjNRT2.1 has also been studied in depth. Using Ljnrt2.1 mutants, it has been shown how LjNRT2.1 control root nodule symbiosis in a nitrate-rich environment in L. japonicus [26]. The authors proposed a model in which LjNRT2.1 acts in the same signaling pathway as LjNLP1 and LjNLP4 for the nitrate-induced control of nodulation. In the presence of nitrate, the LjNLP1 transcription factor induced LjNRT2.1 expression. LjNRT2.1 transports nitrate from the soil to the root. The increase of nitrate in the root triggers the nuclear localization of LjNLP4, which inhibits nodulation through the regulation of gene expression. As LjNLP1 is activated by nitrate, it has been suggested that another nitrate transporter than LjNRT2.1 should be involved in the model to allow the first step, which is nitrate transport and LjNLP1 activation [26]. In addition, LjNIN, a positive regulator of nodulation, whose expression is induced by rhizobial infection [27][28], would negatively regulate the expression of LjNRT2.1 resulting in a reduction of nitrate uptake. Thus, LjNRT2.1 would be at the center of a strategy used by the plant regarding nitrate acquisition, switching from dependence on soil nitrate to symbiotic fixation [26].
Among the three MtNRT2 of M. truncatula, only the role of MtNRT2.1 in nodulation has been addressed [29]. Some similarities between MtNRT2.1 and LjNRT2.1 have been observed. In fact, MtNRT2.1 expression, like that of LjNRT2.1, is activated by MtNLP1. Using Mtnrt2.1 mutants, it has been shown that MtNRT2.1 encodes a high-affinity nitrate transporter responsible for the majority of nitrate taken up by the plant in the 0.5–5 mM nitrate concentration range [29]. In addition, MtNRT2.1’s ability to uptake nitrate in Xenopus laevis oocytes requires MtNAR2. MtNRT2.1 was also shown to play a dual role in nitrate regulation of nodulation in M. truncatula as it is required for nodule establishment under low-nitrate conditions and necessary for repression of nodulation under high-nitrate conditions [29]. Accordingly, a model has been proposed in which low nitrate induces MtCEP1 expression, which systemically induces MtNRT2.1 expression through MtCRA2, resulting in an enhancement in nodulation and nitrate uptake. MtNLP1, whose localization in the nucleus is limited under low nitrate, is increased by high nitrate in the nucleus, leading to the activation of the expression of CLE5, which negatively regulates nodulation [29]. Thus, MtNRT2 has been shown to play a role in nodule functioning in M. truncatula as well as MtNAR2 which seems necessary for nitrate transport [29]. The importance of MtNAR2 in nodules seems to be confirmed by its expression in this organ [30].
In the pea genome, only one full-length PsNRT2, named PsNRT2.3 (Ps4g113000), was identified [13]. Two more PsNRT2 genes exist, PsNRT2.1 (Psat4g155600) and PsNRT2.2 (Psat7g149120), but both corresponding proteins are short with only three transmembrane domains against eight in NRT2 in general. A possible loss of nitrate transport function has been suggested for these two proteins [13]. Researchers have made a phylogenetic tree to establish PsNRT2 relationship with NRT2 of M. truncatula and L. japonicus (Figure 3). It shows a clustering of PsNRT2.1/2.2 with MtNRT2.1/2.2 and LjNRT2.1/2.2 on the one hand, and a clustering of PsNRT2.3 with MtNRT2.3 and LjNRT2.3 on the other hand. Researchers confirm that LjNRT2.4 appears isolated in the phylogenetic tree, having no ortholog in M. truncatula [22] and having no ortholog in P. sativum either (Figure 3).
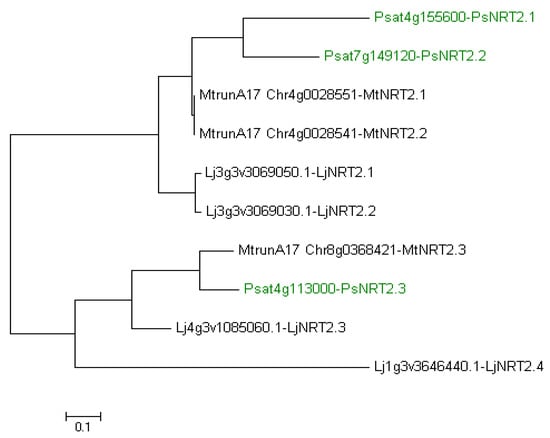
Figure 3. Phylogenetic tree of NRT2 from Lotus japonicus, Medicago truncatula and Pisum sativum. Ten amino acid sequences were aligned with the CLUSTALW program. The evolutionary history was inferred using the Maximum Likelihood method based on the JTT matrix-based model [16]. Evolutionary analyses were conducted in MEGA7 [17]. NRT2 from P. sativum are indicated in green. Lj, L. japonicus; Mt, M. truncatula; Ps, P. sativum.
The omics data [14] allow visualization of the expression of the three PsNRT2 genes and of the PsNAR2 (Psat4g061680) gene under different conditions in different tissues (Figure 4).
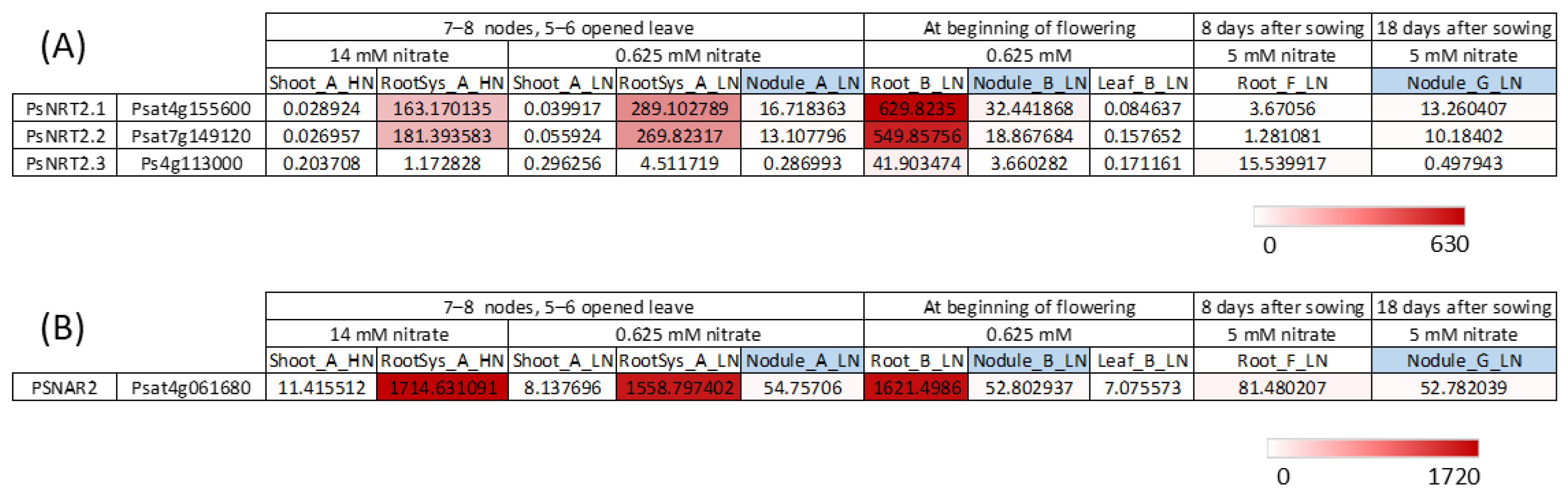
Figure 4. PsNRT2 and PsNAR2 genes expression. Data were extracted from the full-length Unigene set of expressed sequences from P. sativum resulting from de novo assembly of RNA-seq data [14]. (A) PsNRT2 genes expression. (B) PsNAR2 gene expression. Numbers are normalized count data.
It can be noted that despite the smaller size of PsNRT2.1 and PsNRT2.2 proteins, corresponding genes were expressed (Figure 4A). PsNRT2.1, PsNRT2.2 and PsNAR2 were very strongly expressed in the roots at two stages (7–8 nodes, 5–6 opened leaves and at beginning of flowering), while PsNRT2.3 was much less expressed at those stages. The results indicate that PsNRT2.1 and PsNRT2.2 were also expressed in nodules but much less than in roots (at least 18-times less), and PsNRT2.3 was almost not expressed in nodules. The question arises as to which of these PsNRT2 would play a role in nodule functioning in P. sativum. In P. sativum, no NRT2 gene is as strongly expressed in nodules as LjNRT2.4 in L. japonicus nodules [21], and there is no orthologous gene of LjNRT2.4 in this species (Figure 3). Among the three PsNRT2 genes in pea, PsNRT2.1 was the most highly expressed in nodules (Figure 4A). As we have seen, PsNRT2.1 orthologs in M. truncatula and L. japonicus play an important role in nodule functioning. The spatial expression pattern of LjNRT2.1 was precisely studied during nodule development using pLjNRT2.1:GUS reporter gene [26]. At the initial developmental stages of nodulation, LjNRT2.1 was expressed within cortical cells, while at later stages, LjNRT2.1 was expressed in the outer regions of nodules, including the epidermis. The spatial expression pattern of MtNR2.1 during nodulation was also determined using pMtNRT2.1:GUS transformed M. truncatula hairy roots. MtNRT2.1 is expressed in root vascular tissues and nodule meristem. In conclusion researchers propose that further studies would be necessary to study PsNRT2.1 and PsNRT2.2 expressions in detail during nodule development and to see if either or both proteins, PsNRT2.1 and PsNRT2.2, have a role in the regulation of nodulation despite the protein’s smaller size compared with other NRT2s.
References
- Valkov, V.T.; Chiurazzi, M. Nitrate Transport and Signaling. In The Lotus japonicus Genome: Compendium of Plant Genomes; Tabata, S., Stougaard, J., Eds.; Springer: Berlin, Germany, 2014; pp. 125–136.
- Valkov, V.T.; Rogato, A.; Alves, L.M.; Sol, S.; Noguero, M.; Léran, S.; Lacombe, B.; Chiurazzi, M. The Nitrate Transporter Family Protein LjNPF8.6 Controls the N-Fixing Nodule Activity. Plant Physiol. 2017, 175, 1269–1282.
- Vittozzi, Y.; Nadzieja, M.; Rogato, A.; Radutoiu, S.; Valkov, V.T.; Chiurazzi, M. The Lotus japonicus NPF3.1 Is a Nodule-Induced Gene That Plays a Positive Role in Nodule Functioning. Front. Plant Sci. 2021, 12, 688187.
- Takanashi, K.; Takahashi, H.; Sakurai, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Nakazono, M.; Yazaki, K. Tissue-Specific Transcriptome Analysis in Nodules of Lotus japonicus. Mol. Plant-Microbe Interact. 2012, 25, 869–876.
- Pellizzaro, A.; Alibert, B.; Planchet, E.; Limami, A.M.; Morère-Le Paven, M.C. Nitrate Transporters: An Overview in Legumes. Planta 2017, 246, 585–595.
- Bagchi, R.; Salehin, M.; Adeyemo, O.S.; Salazar, C.; Shulaev, V.; Sherrier, D.J.; Dickstein, R. Functional Assessment of the Medicago truncatula NIP/LATD Protein Demonstrates That It Is a High-Affinity Nitrate Transporter. Plant Physiol. 2012, 160, 906–916.
- Yendrek, C.R.; Lee, Y.C.; Morris, V.; Liang, Y.; Pislariu, C.I.; Burkart, G.; Meckfessel, M.H.; Salehin, M.; Kessler, H.; Wessler, H.; et al. A Putative Transporter Is Essential for Integrating Nutrient and Hormone Signaling with Lateral Root Growth and Nodule Development in Medicago truncatula. Plant J. 2010, 62, 100–112.
- Harris, J.M.; Dickstein, R. Control of Root Architecture and Nodulation by the LATD/NIP Transporter. Plant Signal. Behav. 2010, 5, 1365–1369.
- Veereshlingam, H.; Haynes, J.G.; Penmetsa, R.V.; Cook, D.R.; Sherrier, D.J.; Dickstein, R. Nip, a Symbiotic Medicago truncatula Mutant That Forms Root Nodules with Aberrant Infection Threads and Plant Defense-like Response. Plant Physiol. 2004, 136, 3692–3702.
- Bright, L.J.; Liang, Y.; Mitchell, D.M.; Harris, J.M. The LATD Gene of Medicago truncatula Is Required for Both Nodule and Root Development. Mol. Plant-Microbe Interact. 2005, 18, 521–532.
- Teillet, A.; Garcia, J.; De Billy, F.; Gherardi, M.; Huguet, T.; Barker, D.G.; De Carvalho-Niebel, F.; Journet, E.P. Api, a Novel Medicago truncatula Symbiotic Mutant Impaired in Nodule Primordium Invasion. Mol. Plant-Microbe Interact. 2008, 21, 535–546.
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer Cells Mediate Nitrate Uptake to Control Root Nodule Symbiosis. Nat. Plants 2020, 6, 800–808.
- Gu, B.; Chen, Y.; Xie, F.; Murray, J.D.; Miller, A.J. Inorganic Nitrogen Transport and Assimilation in Pea (Pisum sativum). Genes 2022, 13, 158.
- Alves-Carvalho, S.; Aubert, G.; Carrère, S.; Cruaud, C.; Brochot, A.L.; Jacquin, F.; Klein, A.; Martin, C.; Boucherot, K.; Kreplak, J.; et al. Full-Length de Novo Assembly of RNA-Seq Data in Pea (Pisum sativum L.) Provides a Gene Expression Atlas and Gives Insights into Root Nodulation in This Species. Plant J. 2015, 84, 1–19.
- Kreplak, J.; Madoui, M.A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A Reference Genome for Pea Provides Insight into Legume Genome Evolution. Nat. Genet. 2019, 51, 1411–1422.
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Bioinformatics 1992, 8, 275–282.
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874.
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A Unified Nomenclature of Nitrate Transporter 1/Peptide Transporter Family Members in Plants. Trends Plant Sci. 2014, 19, 5–9.
- Boeglin, L.; Morère Le-Paven, M.C.; Clochard, T.; Fustec, J.; Limami, A.M. Pisum sativum Response to Nitrate as Affected by Rhizobium leguminosarum-Derived Signals. Plants 2022, 11, 1966.
- Leran, S.; Garg, B.; Boursiac, Y.; Corratge-Faillie, C.; Brachet, C.; Tillard, P.; Gojon, A.; Lacombe, B. AtNPF5.5, a Nitrate Transporter Affecting Nitrogen Accumulation in Arabidopsis Embryo. Sci. Rep. 2015, 5, 7962.
- Criscuolo, G.; Valkov, V.T.; Parlati, A.; Alves, L.M.; Chiurazzi, M. Molecular Characterization of the Lotus japonicus NRT1(PTR) and NRT2 Families. Plant Cell Environ. 2012, 35, 1567–1581.
- Valkov, V.T.; Sol, S.; Rogato, A.; Chiurazzi, M. The Functional Characterization of LjNRT2.4 Indicates a Novel, Positive Role of Nitrate for an Efficient Nodule N2-Fixation Activity. New Phytol. 2020, 228, 682–696.
- Lepetit, M.; Brouquisse, R. Control of the Rhizobium–Legume Symbiosis by the Plant Nitrogen Demand Is Tightly Integrated at the Whole Plant Level and Requires Inter-Organ Systemic Signaling. Front. Plant Sci. 2023, 14, 1114840.
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both Plant and Bacterial Nitrate Reductases Contribute to Nitric Oxide Production in Medicago truncatula Nitrogen-Fixing Nodules. Plant Physiol. 2011, 155, 1023–1036.
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen Metabolism in Plants under Low Oxygen Stress. Planta 2014, 239, 531–541.
- Misawa, F.; Ito, M.; Nosaki, S.; Nishida, H.; Watanabe, M.; Suzuki, T.; Miura, K.; Kawaguchi, M.; Suzaki, T. Nitrate Transport via NRT2.1 Mediates NIN-LIKE PROTEIN-Dependent Suppression of Root Nodulation in Lotus japonicus. Plant Cell 2022, 34, 1844–1862.
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A Plant Regulator Controlling Development of Symbiotic Root Nodules. Nature 1999, 402, 191–195.
- Suzaki, T.; Kim, C.S.; Takeda, N.; Szczyglowski, K.; Kawaguchi, M. TRICOT Encodes an AMP1-Related Carboxypeptidase That Regulates Root Nodule Development and Shoot Apical Meristem Maintenance in Lotus japonicus. Development 2013, 140, 353–361.
- Luo, Z.; Wang, J.; Li, F.; Lu, Y.; Fang, Z.; Fu, M.; Mysore, K.S.; Wen, J.; Gong, J.; Murray, J.D.; et al. The Small Peptide CEP1 and the NIN-like Protein NLP1 Regulate NRT2.1 to Mediate Root Nodule Formation across Nitrate Concentrations. Plant Cell 2023, 35, 776–794.
- Pellizzaro, A.; Clochard, T.; Planchet, E.; Limami, A.M.; Morère-Le Paven, M.C. Identification and Molecular Characterization of Medicago truncatula NRT2 and NAR2 Families. Physiol. Plant. 2015, 154, 256–269.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
444
Revisions:
2 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




