Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silviya Abarova | -- | 1373 | 2024-02-07 14:32:21 | | | |
| 2 | Jessie Wu | + 2 word(s) | 1375 | 2024-02-08 03:43:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abarova, S.; Alexova, R.; Dragomanova, S.; Solak, A.; Fagone, P.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Kalfin, R.; Tancheva, L. Geranium sanguineum L.. Encyclopedia. Available online: https://encyclopedia.pub/entry/54854 (accessed on 07 February 2026).
Abarova S, Alexova R, Dragomanova S, Solak A, Fagone P, Mangano K, et al. Geranium sanguineum L.. Encyclopedia. Available at: https://encyclopedia.pub/entry/54854. Accessed February 07, 2026.
Abarova, Silviya, Ralitza Alexova, Stela Dragomanova, Ayten Solak, Paolo Fagone, Katia Mangano, Maria Cristina Petralia, Ferdinando Nicoletti, Reni Kalfin, Lyubka Tancheva. "Geranium sanguineum L." Encyclopedia, https://encyclopedia.pub/entry/54854 (accessed February 07, 2026).
Abarova, S., Alexova, R., Dragomanova, S., Solak, A., Fagone, P., Mangano, K., Petralia, M.C., Nicoletti, F., Kalfin, R., & Tancheva, L. (2024, February 07). Geranium sanguineum L.. In Encyclopedia. https://encyclopedia.pub/entry/54854
Abarova, Silviya, et al. "Geranium sanguineum L.." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Bloody cranesbill (Geranium sanguineum L.) is a flowering perennial herb with a natural range extending over Europe. The herb is used in the ethnopharmacological practice in Bulgaria. Infusions and decoctions from the roots and aerial parts can be used as a rinse for inflamed mucous membranes of the mouth and respiratory tract, a wash for wounds or skin eruptions, for its astringent, anti-inflammatory, antiviral, hypotensive, and immunostimulant activity, as well as for supportive treatment of diarrhea, dysentery, and enterocolitis.
viral infection
COVID-19
poliphenols
1. SARS-CoV-2 and COVID-19
SARS-CoV-2 is a beta coronavirus responsible for the COVID-19 pandemic which, as of September 2023, has caused over 770 million cumulative cases and over 6.9 million deaths (www.covid19.who.int; accessed on 8 November 2023). The virus recognizes angiotensin-converting enzyme 2 (ACE2) on the surface of host cells. This receptor is expressed on many human cells, including lung epithelia, kidney, and cardiomyocytes and explains the pathology of COVID-19 as a multi-organ disease with the upper respiratory tract as a primary target.
After ACE2 binding, the viral spike (S) glycoprotein requires proteolytic processing in order to enter the host cells. Cathepsin L is an endosomal cysteine protease needed to prime the S protein. Knockdown of cathepsin L in the lungs was successful in reducing experimental SARS-CoV-2 infectivity in a mouse model, and alleviated brain pathology as well [1]. TMPRSS2 is a serine protease present on the lung cell membrane which can cleave the spike protein and facilitate fusion with the host cell [2]. In addition to the host proteases, the virus also contains two cysteine proteases: the main protease (Mpro or 3CLpro) and a papain-like protease (PLPro). These proteases play a crucial role in the processing of the viral polyprotein, which is essential for the virus’s replication and lifecycle. Several monoclonal antibodies targeting the S protein (such as Bebtelovimab, Bamlanivimab, Etesevimab, Sotrovimab, Casirivimab, Imdevvimab, Regdanvimab, Tixagevimab, and Cilgavimab) have been approved by both the FDA and EMA for COVID-19 treatment. These antibodies work by preventing the virus from entering host cells.
Furthermore, various agents have been developed to inhibit viral replication. These include remdesivir, which acts as an inhibitor of the viral RNA polymerase; nirmatrelvir, an inhibitor of the viral main protease 3CLpro, typically used in combination with ritonavir; and molnupiravir, a nucleoside analog that induces mutations in the virus.
A third category of drugs for COVID-19 treatment focuses on modulating the host immune response to prevent severe organ damage caused by the hyperactivation of the immune system. These drugs include vilobelimab, which sequesters complement C5a; baricitinib, a Janus kinase inhibitor; tocilizumab, which binds to the IL-6 receptor; and anakinra, an antagonist of the IL-1 receptor. Notably, the latter three drugs have previously been employed to manage inflammation in rheumatoid arthritis. Long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC), is a complex and often debilitating condition that can affect individuals who have recovered from the acute phase of COVID-19. While the acute phase of the disease primarily involves respiratory symptoms, Long COVID encompasses a wide range of persistent and often unpredictable symptoms, extending far beyond the initial infection. These symptoms can affect various organ systems, including the respiratory, cardiovascular, neurological, and immune systems, and can significantly impact an individual’s quality of life. Long COVID remains an active area of research, and its full scope and underlying mechanisms are still being explored, making it a crucial focus in understanding the long-term consequences of the COVID-19 pandemic [3][4][5][6][7][8][9].
The persistent changes that COVID-19 induces in the body months after initial infection necessitate the availability of supportive therapies, which replenish the host’s defense systems and are non-toxic with long-term application. The novel SARS-CoV-2 mechanism in causing acute and Long COVID and emerging as a multi-organ dysfunction has spiked research activity in re-evaluating medicinal plants as a source for new pharmaceuticals. These efforts started early in the pandemic and have continued to the present day. Traditional medicine is a rich resource of knowledge on bioactive plant metabolites with pluripotent effects. Natural products often combine with antioxidant, antiviral and immunomodulatory effects [10].
2. Bloody cranesbill (Geranium sanguineum L.) in Traditional Medicine
Figure 1 presents plant efficacy according to the Bulgarian traditional medicine in various disorders. The extracts of bloody cranesbill have an antibacterial effect against S. aureus, E. coli, E. faecalis, K. pneumoniae, P. aeruginosa, and B. subtilis [11][12][13]. A polysaccharide extracted from the roots inhibits the growth of S. enterica [14]. The antibacterial properties of the essential oil from the flowers and the aerial parts of the herb have also been explored and more than 240 chemical components in the extracts have been identified [15][16]. An ethanol extract containing mainly anthocyanidins from the roots was reported to have antitumor activity in vitro and in a murine model of Ehrlich’s breast carcinoma [17].
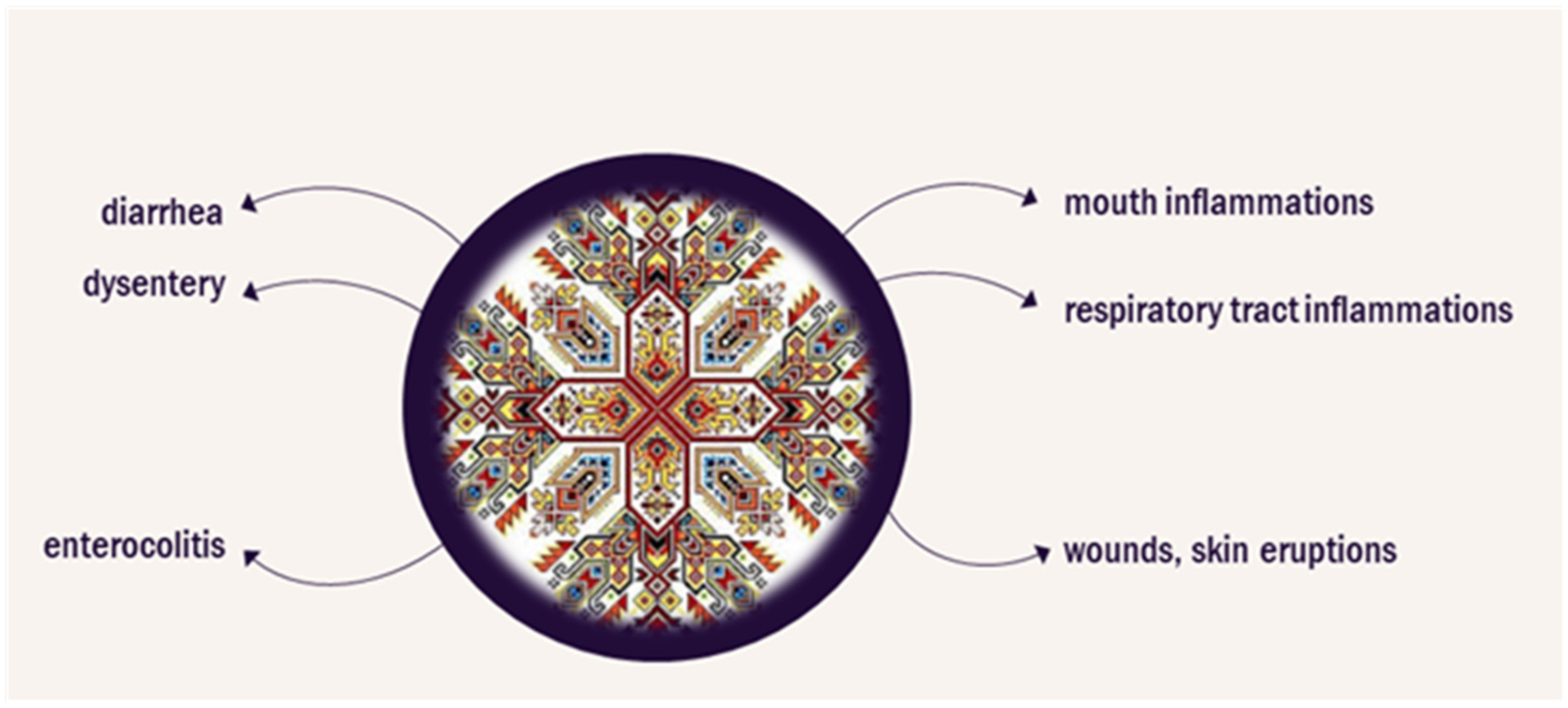
Figure 1. Application of Geranium sanguineum in Bulgarian traditional medicine.
An early survey of the Geranium spp. showed that G. sanguineum was among species with high ellagitannin content in the leaves [18]. The main polyphenol component according to Mavlyanov et al. [19] is bis-hexahydroxydiphenoyl-trigalloylglucose, similar to the condensed tannin from Geranium thunbergii.
The plant shares relatively high quercetin levels with other Geranium spp. but its content of myricetin is distinctive and not wide-spread in the genus [20]. This was more recently confirmed by Ivancheva and Petrova [21] who found myricetin, quercetin (including quercetin-3-glucoside and -galactoside), and kempferol. Whole-plant extracts identified quercetin and kempferol, as well as their glycosides (quercitrin, isoquercitrin, hyperoside, rutin), caftaric, and caffeic acid [22]. A combination of extraction methods achieved the identification of flavonoids, phenolic acids, and hydrolysable tannins including gallic acid, kaempferol, quercetin, rutin, 2-galloylglucose, 3-galloylglucose, and 2,3-digalloylglucose [19]. In the roots, polyphenols of the condensed type were dominant, including (+) catechin, (+/−) gallocatechin, and three protoanthocyanidins [23].
Another comparison of eight Geranium spp. detected the highest concentration of polyphenols and tannin content in G. sanguineum aerial parts [12]. While the amount of polyphenols in the leaves is 9–11%, it is even higher in the roots, at up to 18% [19]. Similar values for the total phenolics content in the leaves were obtained in a study by Maslennikov et al. [24], who identified G. sanguineum as the second most phenol-rich plant among 66 species of plants included in the study.
The later part of the 20th century saw systematic attempts to characterize the active components present in a standardized polyphenol complex (PPC) of G. sanguineum L. This extract from aerial roots yields a dark red water-soluble powder on lyophilization and contains 34.6% (w/w) total soluble phenolics with 16.15% represented by tannins, 0.126% flavonoids and 2.12 mg/kg catechins and proanthocyanidins [25][26][27][28]. Slightly higher values for tannins (19.7%) and flavonoids (0.22%) were obtained by Benzel et al. [11].
The standardized PPC extract contains caffeic acid, gallotannin, (+/−), catechin, (−) epicatechin, quercetin, hyperoside, apigenin, myricetin, morin, maltol, and additional unidentified flavonoids [27][28]. Thin-layer chromatography identified ellagic acid in the extract, but this was reported only in some later studies [28][29].
More recently, the polysaccharide components of G. sanguineum L. extracts have also been analyzed. The total polysaccharide content in leaves has been reported to be 27% (w/w) and in roots 56.8% (w/w) [14]. The lectin content in the roots of G. sanguineum L. is high, exceeding that of G. robertianum or G. sibiricum, but further studies on the lectin composition of the herb are lacking [30]. Documented biological effects of G. sanguineum extracts are summarized in Figure 2.
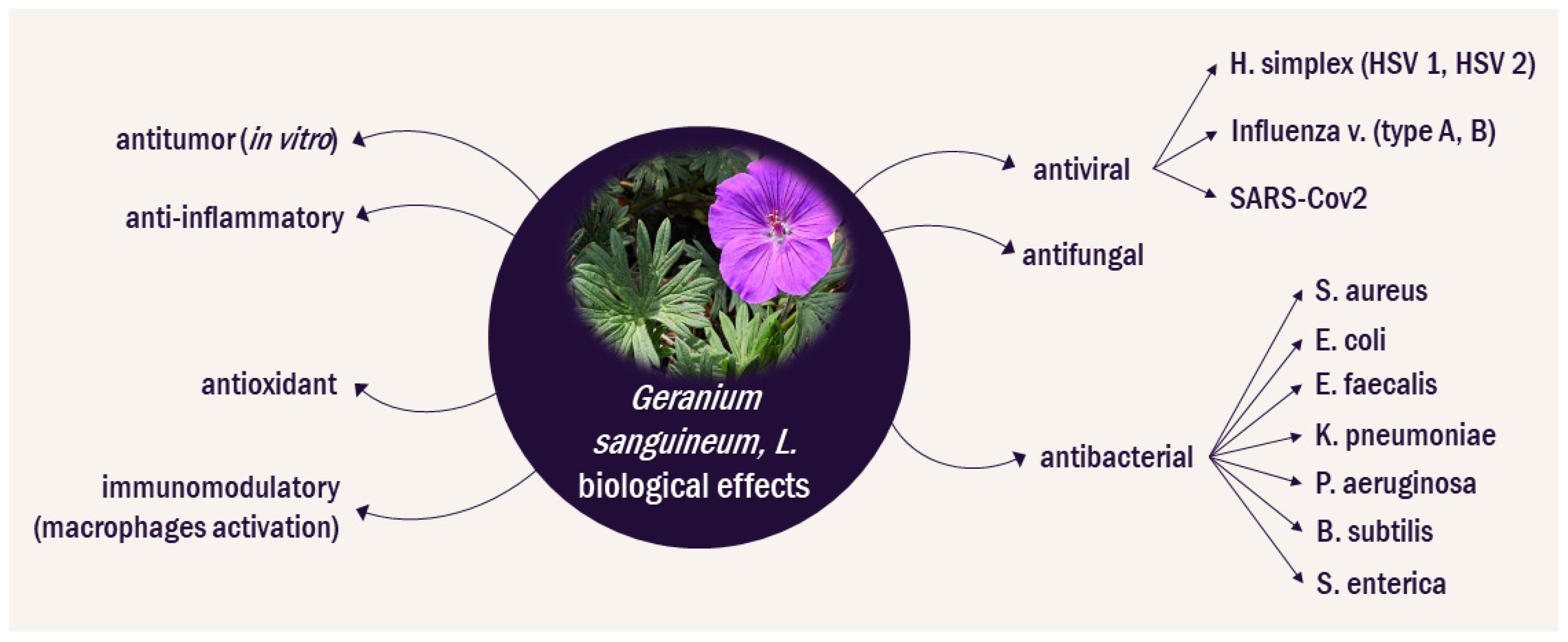
The standardized PPC from G.sanguineum L. has raised interest because of its activity against herpes and influenza virus both in vitro, as well as in in vivo rodent models [10]. The availability of a standardized extract has allowed for the mechanism of its bioactivity to be explored in a controlled fashion. There is an apparent strong synergy between the components of the extract. In addition, the extract seems to be not only directly antiviral, but also to be capable of modulating the oxidative and immune environment. Several studies on the G. sanguineum L. extracts have been conducted before the emergence of the SARS-CoV-2 pandemic. Researchers will now explore these studies within the context of the potential applications of G. sanguineum L. extract for the supportive treatment of both COVID-19 and its long-term consequences, known as Long COVID. This research seeks to shed light on how the findings from these earlier investigations may contribute to our understanding and management of the critical aspects of the past global health crisis, due to COVID-19 pandemic.
References
- Cui, Z.; Zeng, C.; Huang, F.; Yuan, F.; Yan, J.; Zhao, Y.; Zhou, Y.; Hankey, W.; Jin, V.X.; Huang, J.; et al. Cas13d knockdown of lung protease Ctsl prevents and treats SARS-CoV-2 infection. Nat. Chem. Biol. 2022, 18, 1056–1064.
- Palestra, F.; Poto, R.; Ciardi, R.; Opromolla, G.; Secondo, A.; Tedeschi, V.; Ferrara, A.L.; Di Crescenzo, R.M.; Galdiero, M.R.; Cristinziano, L.; et al. SARS-CoV-2 Spike Protein Activates Human Lung Macrophages. Int. J. Mol. Sci. 2023, 24, 3036.
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86014.
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86002.
- Fernández-Lázaro, D.; Garrosa, M. Identification, mechanism, and treatment of skin lesions in COVID-19: A review. Viruses 2021, 13, 1916.
- Imran, M.; Jin, X.; Ali, M.; Tapfumaneyi, P.; Lelasseur, P.; Carlo, L.; Jude, A.; Bourg, A.L.; Panchal, B.; Dick, A.; et al. The Pandemic and Your Skin—Direct and Indirect Impact of COVID-19. Cosmetics 2023, 10, 34.
- Martora, F.; Villani, A.; Fabbrocini, G.; Battista, T. COVID-19 and cutaneous manifestations: A review of the published literature. J. Cosmet. Dermatol. 2023, 22, 4–10.
- Bohnacker, S.; Hartung, F.; Henkel, F.; Quaranta, A.; Kolmert, J.; Priller, A.; Ud-Dean, M.; Giglberger, J.; Kugler, L.M.; Pechtold, L.; et al. Mild COVID-19 imprints a long-term inflammatory eicosanoid- and chemokine memory in monocyte-derived macrophages. Mucosal Immunol. 2022, 15, 515–524.
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847.
- Tancheva, L.P. Drug metabolism and oxidative stress during Influenza Virus Infection. In Experimental Approaches for Antioxidant Protection; Eagle: Silistra, Bulgaria, 2019; p. 111. ISBN 978-619-7424-22-5.
- Benzel, I.L.; Hordiienko, O.I.; Hroshovyi, T.A.; Benzel, L.V.; Pokryshko, O.V. Obtaining of Geranium sanguineum phytoextracts and study of their anti-microbial properties. Int. J. Green. Pharm. 2018, 12, 142–147.
- Ilic, M.; Samardzic, S.; Kotur-Stevuljevic, J.; Usjak, D.; Milenkovic, M.; Kovacevic, N.; Drobac, M. Polyphenol rich extracts of Geranium L. species as potential natural antioxidant and antimicrobial agents. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6283–6294.
- Ivancheva, S.; Manolova, N.; Serkedjieva, J.; Dimov, V.; Ivanovska, N. Polyphenols from Bulgarian Medicinal Plants with Anti-Infectious Activity. In Plant Polyphenols: Synthesis, Properties, Significance, Hemingway; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 717–728.
- Georgiev, Y.N.; Dzhambazov, B.M.; Batsalova, T.G.; Vasicek, O.; Dobreva, L.I.; Denev, P.N.; Danova, S.T.; Simova, S.D.; Wold, C.W.; Ognyanov, M.H.; et al. Structural characterization of polysaccharides from Geranium sanguineum L. and their immunomodulatory effects in response to inflammatory agents. J. Ethnopharmacol. 2022, 294, 115390.
- Hammami, I.; Triki, M.A.; Rebai, A. Chemical compositions, antibacterial and antioxidant activities of essential oil and various extracts of Geranium sanguineum L. flowers. Arch. Appl. Sci. Res. 2011, 3, 135–144.
- Radulović, N.; Dekić, M.; Stojanović-Radić, Z. Chemical composition and antimicrobial activity of the volatile oils of Geranium sanguineum L. and G. robertianum L. (Geraniaceae). Med. Chem. Res. 2012, 21, 601–615.
- Dimitrova, M.; Todorova, K.; Iliev, I.; Sulikovska, I.; Kirazov, L.; Ivanov, I. Effects of Geranium sanguineum Ethanol Extract After i.p. Application in a Mouse Model of Ehrlich’s Breast Cancer. Pathology and Anthropology with Museum Bulgarian Anatomical Society. Acta Morphol. Et Anthropol. 2022, 29, 3–4.
- Bate-Smith, E.C. Ellagitannin content of leaves of Geranium Species. Phytochemistry 1972, 11, 1755–1757.
- Manolova, N.; Gegova, G.; Serkedzhieva Iu Maksimova-Todorova, V.; Uzunov, S. Antiviral action of a polyphenol complex isolated from the medicinal plant Geranium sanguineum L. I. Its inhibiting action on the reproduction of the influenza virus. Acta Microbiol. Bulg. 1986, 18, 73–77.
- Bate-Smith, E.C. Chemotaxonomy of Geranium. Bot. J. Linn. Soc. 1973, 67, 347–359.
- Ivancheva, S.; Petrova, A. A chemosystematic study of eleven Geranium species. Biochem. Syst. Ecol. 2000, 28, 255–260.
- Leucuta, S.; Vlase, L.; Gocan, S.; Radu, L.; Fodorea, C. Determination of phenolic compounds from Geranium sanguineum by HPLC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 3109–3117.
- Mavlyanov, S.M.; Islambekov, S.Y.; Kamaev, F.G.; Abdullaev, U.A.; Karimdzhanov, A.K.; Ismailov, A.I. Tannins of Geranium sanguineum. Transl. Khimiya Prir. Soedin. 1997, 33, 238–246.
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N. The content of phenolic compounds in medicinal plants of a botanical garden (Kaliningrad oblast). Biol. Bull. 2014, 41, 133–138.
- Abarova, S.; Tancheva, L.; Nikolov, R.; Serkedjieva, J.; Pavlova, E.; Bramanti, A.; Nicoletti, F.; Tzvetkov, N.T. Preventive effect of a polyphenol-rich extract from Geranium sanguineum L. on hepatic drug metabolism in influenza infected mice. Sci. Pharm. 2020, 88, 45.
- Ivanova, E.; Toshkova, R.; Serkedjieva, J. A plant polyphenol-rich extract restores the suppressed functions of phagocytes in influenza virus-infected mice. Microbes Infect. 2005, 7, 391–398.
- Pantev, A.; Ivancheva, S.; Staneva, L.; Serkedjieva, J. Biologically Active Constituents of a Polyphenol Extract from Geranium sanguineum L. with Anti-Influenza Activity. Z. Naturforschung C 2006, 61, 508–516.
- Serkedjieva, J.; Manolova, N. Plant Polyphenolic Complex Inhibits the Reproduction of Influenza and Herpes Simplex Viruses. In Plant Polyphenols: Synthesis, Properties, Significance; Springer: Boston, MA, USA, 1992; pp. 705–715.
- Serkedjieva, J.; Gegova, G.; Mladenov, K. Protective efficacy of an aerosol preparation, obtained from Geranium sanguineum L., in experimental influenza infection. Pharmazie 2008, 63, 160–163.
- Rybak, L.; Rudik, G. Research on Quantitative Content of Lectins in Plants of the Geranium L. Genus. Pharma Innov. 2013, 2, 7725.
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142.
- Ivancheva, S.; Stantcheva, B. Ethnobotanical inventory of medicinal plants in Bulgaria. J. Ethnopharmacol. 2000, 69, 165–172.
- Serkedjieva, J.; Ivancheva, S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J. Ethnopharmacol. 1999, 64, 59–68.
- Serkedjieva, J. Combined antiinfluenza virus effect of a plant preparation and a bacterial protease inhibitor. Biotechnol. Biotechnol. Equip. 2009, 23, 589–593.
- Serkedjieva, J.; Krumova, E.; Stefanova, T.; Tancheva, L. Pulmonary protection of a plant polyphenol extract in influenza virus-infected mice. J. Infect. 2009, 59, S426.
- Serkedjieva, J.; Toshkova, R.; Antonova-Nikolova, S.; Stefanova, T.; Teodosieva, A.; Ivanova, I. Effect of a plant polyphenol-rich extract on the lung protease activities of influenza-virus-infected mice. Antivir. Chem. Chemother. 2007, 18, 75–82.
- Abarova, S.; Dimova, I.; Tancheva, L.; Pavlova, E.; Serkedjieva, J.; Ivancheva, S. Antioxidant mechanisms in prevention of experimental influenza virus infection by polyphenols, isolated from Geranium sanguineum L. In Polyphenols Communications; Antti Hoikkala, O.S., Ed.; Gummerus Printing: Helsinki, Finland, 2004; pp. 103–106.
- Tantcheva, L.; Abarova, S.; Dimova, I.; Serkedjieva, J.; Pavlova, E. Prooxidant and antioxidant activity of extract from Geranium sanguineum L. Diverse effect on lipid peroxidation and antioxidant activity in healthy and infected mice. Toxicol. Lett. 2005, 158S, 14–17.
- Pavlova, E.; Simeonova, L.; Serkedjieva, J. Antioxidant activities of Geranium sanguineum L. polyphenolic extract in chemiluminescent model systems. Inorg. Chem. Commun. 2019, 108, 107518.
- Sokmen, M.; Angelova, M.; Krumova, E.; Pashova, S.; Ivancheva, S.; Sokmen, A.; Serkedjieva, J. In Vitro antioxidant activity of polyphenol extracts with antiviral properties from Geranium sanguineum L. Life Sci. 2005, 76, 2981–2993.
- Murzakhmetova, M.; Moldakarimov, S.; Tancheva, L.; Abarova, S.; Serkedjieva, J. Antioxidant and prooxidant properties of a polyphenol-rich extract from Geranium sanguineum L. in vitro and in vivo. Phytother. Res. 2008, 22, 746–751.
- Toshkova, R.; Stefanova, T.; Nikolova, N.; Serkedjieva, J. A plant extract ameliorates the disfunctions of alveolar macrophages in influenza virus-infected mice. PharmacologyOnLine 2006, 3, 778–784.
- Su, H.; Yao, S.; Zhao, W.; Zhang, Y.; Liu, J.; Shao, Q.; Wang, Q.; Li, M.; Xie, H.; Shang, W.; et al. Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat. Commun. 2021, 12, 3623.
- Pan, H.; He, J.; Yang, Z.; Yao, X.; Zhang, H.; Li, R.; Xiao, Y.; Zhao, C.; Jiang, H.; Liu, Y.; et al. Myricetin possesses the potency against SARS-CoV-2 infection through blocking viral-entry facilitators and suppressing inflammation in rats and mice. Phytomedicine 2023, 116, 154858.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
552
Revisions:
2 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




