
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claudio Baggiani | -- | 3189 | 2024-02-07 12:30:25 | | | |
| 2 | Sirius Huang | Meta information modification | 3189 | 2024-02-08 02:24:40 | | |
Video Upload Options
Mycotoxins are toxic metabolites of molds which can contaminate food and beverages. Because of their acute and chronic toxicity, they can have harmful effects when ingested or inhaled, posing severe risks to human health. Contemporary analytical methods have the sensitivity required for contamination detection and quantification, but the direct application of these methods on real samples is not straightforward because of matrix complexity, and clean-up and preconcentration steps are needed, more and more requiring the application of highly selective solid-phase extraction materials. Molecularly imprinted polymers (MIPs) are artificial receptors mimicking the natural antibodies that are increasingly being used as a solid phase in extraction methods where selectivity towards target analytes is mandatory. Herein, the state-of-the-art about molecularly imprinted polymers as solid-phase extraction materials in mycotoxin contamination analysis will be discussed.
1. Introduction
2. Solid-Phase Extraction with Commercial MIPs
3. Solid-Phase Extraction with Home-Made MIPs
4. On-Line Solid-Phase Extraction
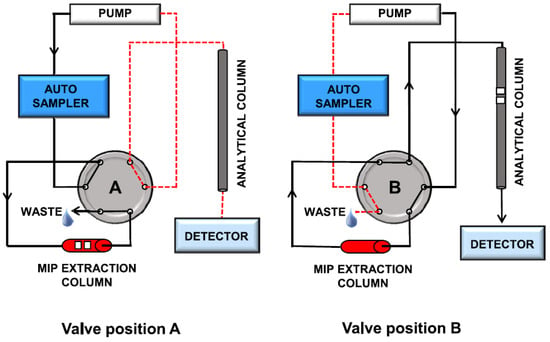
5. Dispersive Solid-Phase Microextraction
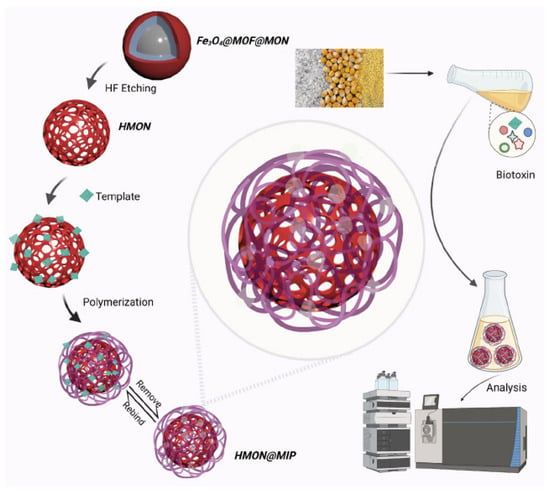
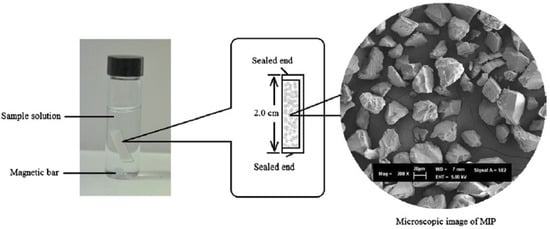
6. Magnetic Solid-Phase Extraction and Stir Bar Sorptive Extraction
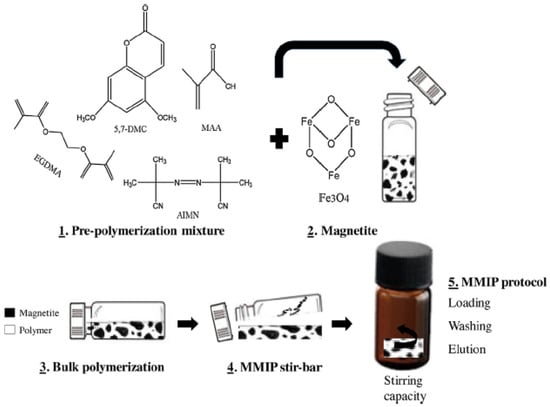
References
- Richard, J.L. Some major mycotoxins and their mycotoxicosis, an overview. Int. J. Food Microbiol. 2007, 119, 3–10.
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507.
- Vidal, A.; Mengelers, M.; Yang, S.P.; De Saeger, S.; De Boevre, M. Mycotoxin biomarkers of exposure: A comprehensive review. Comp. Rev. Food Sci. Food Safety 2018, 17, 1127–1155.
- van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157.
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Food Sci. Agric. 2013, 93, 2892–2899.
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia 2006, 161, 261–273.
- Dzantiev, B.B.; Byzova, N.A.; Urusov, A.E.; Zherdev, A.V. Immunochromatographic methods in food analysis. TrAC—Trend. Anal. Chem. 2014, 55, 81–93.
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180.
- Li, P.-W.; Zhang, Z.-W.; Hu, X.-F.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013, 32, 420–452.
- Pichon, V.; Delaunay-Bertoncini, M.; Hennion, M.C. Sampling and Sample Preparation for Field and Laboratory, 1st ed.; Pawliszyn, J., Ed.; Comprehensive Analytical Chemistry, Elsevier Science: Amsterdam, The Netherlands, 2002; Volume 37, pp. 1081–1100.
- Cichna-Markl, M. New strategies in sample clean-up for mycotoxin analysis. World Mycotox. J. 2011, 4, 203–215.
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518.
- Yang, Y.; Li, G.-L.; Wu, D.; Liu, J.-H.; Li, X.-T.; Luo, P.-J.; Hu, N.; Wang, H.-L.; Wu, Y.-N. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trend. Food Sci. Technol. 2020, 96, 233–252.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119.
- Reville, E.K.; Sylvester, E.H.; Benware, S.J.; Negic, S.S.; Berda, E.B. Customizable molecular recognition: Advancements in design, synthesis, and application of molecularly imprinted polymers. Polym. Chem. 2022, 13, 3387–3411.
- Pichon, V.; Delaunay, N.; Combès, A. Sample preparation using molecularly imprinted polymers. Anal. Chem. 2020, 92, 16–33.
- Baggiani, C.; Anfossi, L.; Giovannoli, C. Molecular imprinted polymers as synthetic receptors for the analysis of myco- and phyco-toxins. Analyst 2008, 133, 719–730.
- Available online: https://www.sigmaaldrich.com/IT/it/technical-documents/technical-article/analytical-chemistry/solid-phase-extraction/supelmip (accessed on 3 October 2023).
- Available online: https://www.affinisep.com (accessed on 3 October 2023).
- Bryla, M.; Jedrzejczak, R.; Roszko, M.; Szymczyk, K.; Obiedzinski, M.W.; Sekul, J.; Rzepkowska, M. Application of molecularly imprinted polymers to determine B-1, B-2, and B-3 fumonisins in cereal products. J. Sep. Sci. 2013, 36, 578–584.
- Cao, J.L.; Zhou, S.-J.; Kong, W.-J.; Yang, M.-H.; Wan, L.; Yang, S.-H. Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in ginger and LC-MS/MS confirmation. Food Contr. 2013, 33, 337–343.
- Cao, J.-L.; Kong, W.-J.; Zhou, S.-J.; Yin, L.-H.; Wan, L.; Yang, M.-H. Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in beer, red wine, and grape juice. J. Sep. Sci. 2013, 36, 1291–1297.
- Prelle, A.; Spadaro, D.; Denca, A.; Garibaldi, A.; Gullino, M.L. Comparison of clean-up methods for ochratoxin A on wine, beer, roasted coffee and chili commercialized in Italy. Toxins 2013, 5, 1827–1844.
- Mishra, R.K.; Catanante, G.; Hayat, A.; Marty, J.L. Evaluation of extraction methods for ochratoxin A detection in cocoa beans employing HPLC. Food Additiv. Contam. A 2016, 33, 500–508.
- Lucci, P.; Moret, S.; Bettin, S.; Conte, L. Selective solid-phase extraction using a molecularly imprinted polymer for the analysis of patulin in apple-based foods. J. Sep. Sci. 2017, 40, 458–465.
- Lucci, P.; Derrien, D.; Alix, F.; Perollier, C.; Bayoudh, S. Molecularly imprinted polymer solid-phase extraction for detection of zearalenone in cereal sample extracts. Anal. Chim. Acta 2010, 672, 15–19.
- Lucci, P.; David, S.; Conchione, C.; Milani, A.; Moret, S.; Pacetti, D.; Conte, L. Molecularly imprinted polymer as selective sorbent for the extraction of zearalenone in edible vegetable oils. Foods 2020, 9, 1439.
- Lhotská, I.; Gajdošová, B.; Solich, P.; Šatínský, D. Molecularly imprinted vs. reversed-phase extraction for the determination of zearalenone: A method development and critical comparison of sample clean-up efficiency achieved in an on-line coupled SPE chromatography system. Anal. Bioanal. Chem. 2018, 410, 3265–3273.
- Kholova, A.; Lhotska, I.; Erben, J.; Chvojka, J.; Svec, F.; Solich, P.; Satínský, D. Comparison of nanofibers, microfibers, nano/microfiber graphene doped composites, molecularly imprinted polymers, and restricted access materials for on-line extraction and chromatographic determination of citrinin, zearalenone, and ochratoxin A in plant-based milk beverages. Microchem. J. 2023, 193, 108937.
- Kubo, T.; Otsuka, K. Recent progress in molecularly imprinted media by new preparation concepts and methodological approaches for selective separation of targeting compounds. TrAC—Trend. Anal. Chem. 2016, 81, 102–109.
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC—Trend. Anal. Chem. 2020, 128, 115923.
- Wei, S.; Liu, Y.; Yan, Z.; Liu, L. Molecularly imprinted solid phase extraction coupled to high performance liquid chromatography for determination of aflatoxin M1 and B1 in foods and feeds. RSC Adv. 2015, 5, 20951–20960.
- Meydan, I.; Bilici, M.; Turan, E.; Zengin, A. Selective extraction and determination of citrinin in rye samples by a molecularly imprinted polymer (MIP) using reversible addition fragmentation chain transfer precipitation polymerization (RAFTPP) with high-performance liquid chromatography (HPLC) detection. Anal. Lett. 2021, 54, 1697–1708.
- Wang, S.-W.; Shao, R.; Li, W.-W.; Li, X.; Sun, J.-L.; Jiao, S.-S.; Dai, S.-J.; Dou, M.-H.; Xu, R.-M.; Li, Q.-J.; et al. Three-dimensional ordered macroporous magnetic inverse photonic crystal microsphere-based molecularly imprinted polymer for selective capture of Aflatoxin B1. ACS Appl. Mater. Interf. 2022, 14, 18845–18853.
- Rui, C.; He, J.; Li, Y.; Liang, Y.; You, L.; He, L.; Li, K.; Zhang, S. Selective extraction and enrichment of aflatoxins from food samples by mesoporous silica FDU-12 supported aflatoxins imprinted polymers based on surface molecularly imprinting technique. Talanta 2019, 201, 342–349.
- Song, L.-X.; Wang, H.-G.; Rui, C.-F.; Liu, Q.; Zhang, Y.-X.; Cheng, Y.; He, J. Preparation and properties of aflatoxins imprinted polymer grafted onto the surface of mesoporous silica SBA-15 functionalized with double bonds. J. Sep. Sci. 2021, 44, 4181–4189.
- Liang, Y.; He, J.; Huang, Z.; Li, H.; Zhang, Y.; Wang, H.; Rui, C.; Li, Y.; You, L.; Li, K.; et al. An amino-functionalized zirconium-based metal-organic framework of type UiO-66-NH2 covered with a molecularly imprinted polymer as a sorbent for the extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from grain. Microchim. Acta 2020, 187, 32.
- Liang, G.; Zhai, H.; Huang, L.; Tan, X.; Zhou, Q.; Yu, X.; Lin, H. Title: Synthesis of carbon quantum dots-doped dummy molecularly imprinted polymer monolithic column for selective enrichment and analysis of aflatoxin B1 in peanut. J. Pharm. Biomed. Anal. 2018, 149, 258–264.
- Zhang, X.; He, J.; Wang, H.; Xu, P.; Wang, M.; Li, Y.; Chen, J.; He, L. Surface molecularly imprinted polymers based on NH2-MIL-53 for selective extraction ochratoxin A in real sample. Macromol. Res. 2022, 30, 719–730.
- Zhao, D.-Y.; Jia, J.-F.; Yu, X.-L.; Sun, X.-J. Preparation and characterization of a molecularly imprinted polymer by grafting on silica supports: A selective sorbent for patulin toxin. Anal. Bioanal. Chem. 2011, 401, 2259–2273.
- Wang, H.; He, J.; Song, L.; Zhang, Y.; Xu, M.; Huang, Z.; Jin, L.; Ba, X.; Li, Y.; You, L.; et al. Etching of halloysite nanotubes hollow imprinted materials as adsorbent for extracting of zearalenone from grain samples. Microchem. J. 2020, 157, 104953.
- Huang, Z.-P.; He, J.; Li, Y.-Y.; Wu, C.-J.; You, L.-Q.; Wei, H.-L.; Li, K.; Zhang, S.-S. Preparation of dummy molecularly imprinted polymers for extraction of zearalenone in grain samples. J. Chromatogr. A 2019, 1602, 11–18.
- Zhang, Y.; He, J.; Song, L.; Wang, H.; Huang, Z.; Sun, Q.; Ba, X.; Li, Y.; You, L.; Zhang, S. Application of surface-imprinted polymers supported by hydroxyapatite in the extraction of zearalenone in various cereals. Anal. Bioanal. Chem. 2020, 412, 4045–4055.
- De Smet, D.; Dubruel, P.; Van Peteghem, C.; Schacht, E.; De Saeger, S. Molecularly imprinted solid-phase extraction of fumonisin B analogues in bell pepper, rice and corn flakes. Food Additiv. Contam. 2009, 26, 874–884.
- De Smet, D.; Monbaliu, S.; Dubruel, P.; Van Peteghem, C.; Schacht, E.; De Saeger, S. Synthesis and application of a T-2 toxin imprinted polymer. J. Chromatogr. A 2010, 1217, 2879–2886.
- Maier, N.M.; Buttinger, G.; Welhartizki, S.; Gavioli, E.; Lindner, W. Molecularly imprinted polymer-assisted sample clean-up of ochratoxin A from red wine: Merits and limitations. J. Chromatogr. B 2004, 804, 103–111.
- Palmieri, S.; Elfadil, D.; Fanti, F.; Della Pelle, F.; Sergi, M.; Amine, A.; Compagnone, D. Study on molecularly imprinted polymers obtained sonochemically for the determination of aflatoxins in food. Molecules 2023, 28, 703.
- Bayram, E.; Yilmaz, E.; Uzun, L.; Say, R.; Denizli, A. Multiclonal plastic antibodies for selective aflatoxin extraction from food samples. Food Chem. 2017, 221, 829–837.
- Abou-Hany, R.A.G.; Urraca, J.L.; Descalzo, A.B.; Gómez-Arribas, L.N.; Moreno-Bondi, M.C.; Orellana, G. Title: Tailoring molecularly imprinted polymer beads for alternariol recognition and analysis by a screening with mycotoxin surrogates. J. Chromatogr. A 2015, 1425, 231–239.
- Rico-Yuste, A.; Walravens, J.; Urraca, J.L.; Abou-Hany, R.A.G.; Descalzo, A.B.; Orellana, G.; Rychlik, M.; De Saeger, M.; Moreno-Bondi, M.C. Title: Analysis of alternariol and alternariol monomethyl ether in foodstuffs by molecularly imprinted solid-phase extraction and ultra-high-performance liquid chromatography tandem mass spectrometry. Food Chem. 2018, 243, 357–364.
- Moya-Cavas, T.; Navarro-Villoslada, F.; Urraca, J.L.; Serrano, L.A.; Orellana, G.; Moreno-Bondi, M.C. Simultaneous determination of zearalenone and alternariol mycotoxins in oil samples using mixed molecularly imprinted polymer beads. Food Chem. 2023, 412, 135538.
- Guo, B.-Y.; Wang, S.; Ren, B.; Li, X.; Qin, F.; Li, J. Citrinin selective molecularly imprinted polymers for SPE. J. Sep. Sci. 2010, 33, 1156–1160.
- Appell, M.; Jackson, M.A.; Wang, L.C.; Bosma, W.B. Determination of citrinin using molecularly imprinted solid phase extraction purification, HPLC separation, and fluorescence detection. J. Liq. Chromatogr. Rel. Technol. 2015, 38, 1815–1819.
- Giovannoli, C.; Passini, C.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Determination of ochratoxin A in italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J. Agric. Food Chem. 2014, 62, 5220–5225.
- De Smet, D.; Dubruel, P.; Van Peteghem, C.; De Saeger, S. Development of a molecularly imprinted polymer for patulin in apple juice. World Mycotox. J. 2011, 4, 375–383.
- Zhao, M.-J.; Shao, H.; He, Y.-H.; Li, H.; Yan, M.-M.; Jiang, Z.-J.; Wang, J.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Yan, F.-Y.; et al. The determination of patulin from food samples using dual-dummy molecularly imprinted solid-phase extraction coupled with LC-MS/MS. J. Chromatogr. B 2019, 1125, 121714.
- Khorrami, A.R.; Taherkhani, M. Synthesis and evaluation of a molecularly imprinted polymer for pre-concentration of patulin from apple juice. Chromatographia 2011, 73, S151–S156.
- Anene, A.; Hosni, K.; Chevalier, Y.; Kalfat, R.; Hbaieb, S. Molecularly imprinted polymer for extraction of patulin in apple juice samples. Food Contr. 2016, 70, 90–95.
- Urraca, J.L.; Marazuela, M.D.; Moreno-Bondi, M.C. Molecularly imprinted polymers applied to the clean-up of zearalenone and α-zearalenol from cereal and swine feed sample extracts. Anal. Bioanal. Chem. 2006, 385, 1155–1161.
- Urraca, J.L.; Marazuela, M.D.; Merino, E.R.; Orellana, G.; Moreno-Bondi, M.C. Molecularly imprinted polymers with a streamlined mimic for zearalenone analysis. J. Chromatogr. A 2006, 1116, 127–134.
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barcelò, D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A 2007, 1152, 97–115.
- Lhotska, I.; Holznerova, A.; Solich, P.; Satinsky, D. Critical comparison of the on-line and off-line molecularly imprinted solid-phase extraction of patulin coupled with liquid chromatography. J. Sep. Sci. 2017, 40, 4599–4609.
- Yu, J.C.C.; Krushkova, S.; Lai, E.P.C.; Dabek-Zlotorzynska, E. Molecularly-imprinted polypyrrole-modified stainless steel frits for selective solid phase preconcentration of ochratoxin A. Anal. Bioanal. Chem. 2005, 382, 1534–1540.
- Yu, J.C.C.; Lai, E.P.C. Molecularly imprinted polypyrrole modified carbon nanotubes on stainless steel frit for selective micro solid phase pre-concentration of ochratoxin A. React. Funct. Polym. 2006, 66, 702–711.
- Yu, J.C.C.; Lai, E.P.C. Determination of ochratoxin A in red wines by multiple pulsed elutions from molecularly imprinted polypyrrole. Food Chem. 2007, 105, 301–310.
- Liu, Y.; Su, Z.; Wang, J.; Gong, Z.; Lyu, H.; Xie, Z. Molecularly imprinted polymer with mixed-mode mechanism for selective extraction and on-line detection of ochratoxin A in beer sample. Microchem. J. 2021, 170, 106696.
- Lyu, H.; Sun, H.; Zhu, Y.; Wang, J.; Xie, Z.; Li, J. A double-recognized aptamer-molecularly imprinted monolithic column for high-specificity recognition of ochratoxin A. Anal. Chim. Acta 2020, 1103, 97–105.
- Zhou, S.-N.; Lai, E.P.C.; Miller, J.D. Analysis of wheat extracts for ochratoxin A by molecularly imprinted solid-phase extraction and pulsed elution. Anal. Bioanal. Chem. 2004, 378, 1903–1906.
- Vidal, J.C.; Duato, P.; Bonel, L.; Castillo, J.R. Molecularly imprinted on-line solid-phase extraction coupled with fluorescence detection for the determination of ochratoxin a in wheat samples. Anal. Lett. 2012, 45, 51–62.
- Lhotska, I.; Kholova, A.; Machynakova, A.; Hrobonova, K.; Solich, P.; Svec, F.; Satinsky, D. Preparation of citrinin-selective molecularly imprinted polymer and its use for on-line solid-phase extraction coupled to liquid chromatography. Anal. Bioanal. Chem. 2019, 411, 2395–2404.
- Moreno-González, D.; Jáč, P.; Riasová, P.; Nováková, L. In-line molecularly imprinted polymer solid phase extraction-capillary electrophoresis coupled with tandem mass spectrometry for the determination of patulin in apple-based food. Food Chem. 2021, 334, 127607.
- Khezeli, T.; Daneshfar, A. Development of dispersive micro-solid phase extraction based on micro and nano sorbents. TrAC—Trends Anal. Chem. 2017, 89, 99–118.
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. TrAC—Trends Anal. Chem. 2019, 118, 793–809.
- Fan, L.; Zhang, Q.; Wang, F.; Yang, H. Dummy molecularly imprinted solid-phase extraction-SERS determination of AFB1 in peanut. Spectrochim. Acta A 2023, 288, 122130.
- Jayasinghe, G.D.T.M.; Dominguez-Gonzalez, R.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Miniaturized vortex assisted-dispersive molecularly imprinted polymer micro-solid phase extraction and HPLC-MS/MS for assessing trace aflatoxins in cultured fish. Anal. Methods 2020, 12, 4351–4362.
- Thati, R.; Seetha, B.S.; Alegete, P.; Mudiam, M.K.R. Molecularly imprinted dispersive micro solid-phase extraction coupled with high-performance liquid chromatography for the determination of four aflatoxins in various foods. Food Chem. 2024, 433, 137342.
- Yang, L.; Wang, J.; Li, C.Y.; Liu, Q.; Wang, J.; Wu, J.; Lv, H.; Ji, X.M.; Liu, J.M.; Wang, S. Hollow-structured molecularly imprinted polymers enabled specific enrichment and highly sensitive determination of aflatoxin B1 and sterigmatocystin against complex sample matrix. J. Hazard. Mater. 2023, 451, 131127.
- Lee, T.P.; Saad, B.; Khayoon, W.S.; Salleh, B. Molecularly imprinted polymer as sorbent in micro-solid phase extraction of ochratoxin A in coffee, grape juice and urine. Talanta 2012, 88, 129–135.
- Chmangui, A.; Jayasinghe, G.; Driss, M.R.; Touil, S.; Bermejo-Barrera, P.; Bouabdallah, S.; Moreda-Pineiro, A. Assessment of trace levels of aflatoxins AFB1 and AFB2 in non-dairy beverages by molecularly imprinted polymer based micro solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Meth. 2021, 13, 3433–3443.
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC—Trend. Anal. Chem. 2014, 59, 50–58.
- Cui, Y.; Ding, L.; Ding, J. Recent advances of magnetic molecularly imprinted materials: From materials design to complex sample pretreatment. TrAc—Trends Anal. Chem. 2022, 147, 116514.
- Wu, C.-J.; He, J.; Li, Y.-Y.; Chen, N.-N.; Huang, Z.-P.; You, L.-Q.; He, L.-J.; Zhang, S.-S. Solid-phase extraction of aflatoxins using a nanosorbent consisting of a magnetized nanoporous carbon core coated with a molecularly imprinted polymer. Microchim. Acta 2018, 185, 515.
- Suo, L.-L.; Hu, M.-H. One-step synthesis of polydopamine-coated dummy molecularly imprinted polymers on the surface of Fe3O4 for selective extraction and enrichment of aflatoxin B in foods prior to high-performance liquid chromatography detection. J. Anal. Chem. 2023, 78, 213–221.
- Pezeshkpur, M.; Tadayon, F.; Sohrabi, M.R. A molecularly imprinted polymer based on Fe3O4@Au nanoparticles for detection of Aflatoxin B1 in food samples. ChemistrySelect 2023, 8, e202300112.
- Erdem, V.Z.; Basegmez, H.I.O.; Pesint, G.B. AFB1 recognition from liver tissue via AFB1 imprinted magnetic nanoparticles. J. Chromatogr. B 2022, 1210, 123453.
- Pan, S.D.; Ye, M.-J.; Gao, G.-S.; He, Q.; Wang, L.; Chen, X.-H.; Qiu, Q.-L.; Jin, M.-C. Synthesis of a monodisperse well-defined core–shell magnetic molecularly-imprinted polymer prior to LC-MS/MS for fast and sensitive determination of mycotoxin residues in rice. Anal. Methods 2017, 9, 5281–5292.
- Hu, M.-H.; Huang, P.-C.; Suo, L.-L.; Wu, F.-Y. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim. Acta 2018, 185, 300.
- Fu, H.; Xu, W.; Wang, H.-X.; Liao, S.-H.; Chen, G.-T. Preparation of magnetic molecularly imprinted polymer for selective identification of patulin in juice. J. Chromatogr. B 2020, 1145, 122101.
- Zhao, M.; Shao, H.; Ma, J.; Li, H.; He, Y.; Wang, M.; Jin, F.; Wang, J.; Abd El-Aty, A.M.; Hacımüftüoglu, H.; et al. Preparation of core-shell magnetic molecularly imprinted polymers for extraction of patulin from juice samples. J. Chromatogr. A 2020, 1615, 460751.
- Du, Q.; Zhang, Y.; Yu, L.; He, H. Surface molecularly imprinted polymers fabricated by differential UV–vis spectra and reverse prediction method for the enrichment and determination of sterigmatocystin. Food Chem. 2022, 367, 130715.
- Cavaliere, C.; Antonelli, M.; Cerrato, A.; La Barbera, G.; Laganà, A.; Laus, M.; Piovesana, S.; Capriotti, A.L. A novel magnetic molecular imprinted polymer for selective extraction of zearalenone from cereal flours before liquid chromatography-tandem mass spectrometry determination. Toxins 2019, 11, 493.
- Fu, H.; Xu, W.; Wang, H.; Liao, S.; Chen, G. Preparation of magnetic molecularly imprinted polymers for the identification of zearalenone in grains. Anal. Bioanal. Chem. 2020, 412, 4725–4737.
- Fu, H.; Liu, J.; Xu, W.; Wang, H.; Liao, S.; Chen, G. A new type of magnetic molecular imprinted material combined with beta-cyclodextrin for the selective adsorption of zearalenone. J. Mater. Chem. B 2020, 8, 10966–10976.
- Wang, M.-Y.; He, J.; Zhang, Y.-X.; Tian, Y.; Xu, P.-F.; Zhang, X.; Li, Y.-Y.; Chen, J.; He, L.-J. Application of magnetic hydroxyapatite surface-imprinted polymers in pretreatment for detection of zearalenone in cereal samples. J. Chromatogr. B 2022, 1201, 123297.
- Hu, M.; Ge, W.; Liu, X.; Zhu, Y. Preconcentration and determination of zearalenone in corn oil by a one-step prepared polydopamine-based magnetic molecularly imprinted polymer (MIP) with high-performance liquid chromatography with fluorescence (HPLC-FLD) detection. Anal. Lett. 2022, 55, 343–354.
- Huang, Z.; He, J.; Li, H.; Zhang, M.; Wang, H.; Zhang, Y.; Li, Y.; You, L.; Zhang, S. Synthesis and application of magnetic-surfaced pseudo molecularly imprinted polymers for zearalenone pretreatment in cereal samples. Food Chem. 2020, 308, 125696.
- Zhang, Y.; Liu, D.; Peng, J.; Cui, Y.; Shi, Y.; He, H. Magnetic hyperbranched molecularly imprinted polymers for selective enrichment and determination of zearalenone in wheat proceeded by HPLC-DAD analysis. Talanta 2020, 209, 120555.
- David, F.; Ochiai, N.; Sandra, P. Two decades of stir bar sorptive extraction: A retrospective and future outlook. TrAC—Trends Anal. Chem. 2019, 112, 102–111.
- Díaz-Bao, M.; Regal, P.; Barreiro, R.; Fente, C.A.; Cepeda, A. A facile method for the fabrication of magnetic molecularly imprinted stir-bars: A practical example with aflatoxins in baby foods. J. Chromatogr. A 2017, 1471, 51–59.
- Regal, P.; Diaz-Bao, M.; Barreiro, R.; Fente, C.; Cepeda, A. Design of a molecularly imprinted stirbar for isolation of patulin in apple and LC-MS/MS detection. Separations 2017, 4, 11.




