Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joanna Zawitkowska | -- | 3987 | 2024-02-07 01:28:50 | | | |
| 2 | Rita Xu | Meta information modification | 3987 | 2024-02-07 02:25:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Leśniak, M.; Lipniarska, J.; Majka, P.; Lejman, M.; Zawitkowska, J. Venetoclax Combination Therapies in Pediatric Hematological Malignancies. Encyclopedia. Available online: https://encyclopedia.pub/entry/54823 (accessed on 07 February 2026).
Leśniak M, Lipniarska J, Majka P, Lejman M, Zawitkowska J. Venetoclax Combination Therapies in Pediatric Hematological Malignancies. Encyclopedia. Available at: https://encyclopedia.pub/entry/54823. Accessed February 07, 2026.
Leśniak, Maria, Justyna Lipniarska, Patrycja Majka, Monika Lejman, Joanna Zawitkowska. "Venetoclax Combination Therapies in Pediatric Hematological Malignancies" Encyclopedia, https://encyclopedia.pub/entry/54823 (accessed February 07, 2026).
Leśniak, M., Lipniarska, J., Majka, P., Lejman, M., & Zawitkowska, J. (2024, February 07). Venetoclax Combination Therapies in Pediatric Hematological Malignancies. In Encyclopedia. https://encyclopedia.pub/entry/54823
Leśniak, Maria, et al. "Venetoclax Combination Therapies in Pediatric Hematological Malignancies." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Venetoclax is a strongly effective B-cell lymphoma-2 inhibitor (BCL-2) with an ability to selectively restore the apoptotic potential of cancerous cells. It has been proven that in combination with immunotherapy, targeted therapies, and lower-intensity therapies such as hypomethylating agents (HMAs) or low-dose cytarabine (LDAC), the drug can improve overall outcomes for adult patients with acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM), amongst other hematological malignancies.
venetoclax
Bcl-2 inhibitors
pediatric hematology
1. Introduction
1.1. Apoptosis
Apoptosis is a major form of programmed cell death, which is very important for the development and functioning of multi-cellular organisms. It regulates cell number, the removal of structures, and tissue sculpting and also protects against pathogens [1]. Apoptosis deregulation can lead to many diseases—cancer, autoimmune diseases, viral infections, or neurodegenerative disorders [2].
Caspases play a key role in the mechanism of apoptosis as they are both the initiators (caspase-8, -9, and -10) and executioners (caspase-3, -6, and -7) of the process. There are two main pathways by which caspases can be activated—the intrinsic (mitochondrial) pathway, initiated by microenvironmental disturbances, and the extrinsic (death receptor) pathway, activated by disturbances of the extracellular microenvironment [3]. The intrinsic pathway is initiated by, among others, genetic damage, oxidative stress, endoplasmic reticulum stress, hypoxia, or mitotic defects. This pathway is the result of increased mitochondrial penetrability and the release of proapoptotic molecules that normally reside in the mitochondrial intermembrane space into the cytoplasm [4].
1.2. B-Cell Lymphoma 2 (Bcl-2) Protein Family
The B-cell lymphoma 2 (Bcl-2) protein family regulates mitochondrial apoptosis. The members of the Bcl-2 family are classified into three subgroups, depending on the composition of typical BH (Bcl-2 Homology) domains, listed from BH1 to BH4, and their involvement in apoptosis regulation [5]. The BH1 and BH2 domains of Bcl-2 are needed for dimerization with proapoptotic proteins. The BH3 domain is of primary importance for the interaction between proapoptotic and antiapoptotic proteins and is present in all family members. The amino-terminal BH4 domain is mainly found in the antiapoptotic Bcl-2 family members [6].
The proteins are categorized into:
-
Antiapoptotic, e.g., BCL-2, BCL-XL, BCL-W, and MCL-1;
-
BH3-only (proapoptotic), e.g., BIM, BID, PUMA, NOXA, BIK, and BAD;
-
Pore-forming or ‘executioner’ (proapoptotic), e.g., BAX, BAK, and BOK.
Subfamily categorization is based on the BH and transmembrane domains, anti- or proapoptotic function status, and pore-forming ability [7][8].
The translocation of proapoptotic proteins BAX and BAK induces mitochondrial outer membrane permeabilization and cytochrome c release, followed by caspase activation (Figure 1). BH3-only proteins BID and BIM promote mitochondrial permeabilization via the activation of BAX and BAK, while BH3 proteins BAD, BIK, and PUMA bind and oppose the activation of antiapoptotic proteins. In contrast, antiapoptotic proteins prevent BAX/BAK-dependent mitochondrial outer membrane permeabilization via both the direct inactivation of BAK and BAX and via the sequestration of BH3-only proteins [9][10][11]. The proapoptotic proteins promote the mitochondrial release of cytochrome-c, whereas the antiapoptotic proteins regulate apoptosis by blocking this release. The delicate balance between these two groups determines whether a cell survives or dies [4]. Antiapoptotic proteins are often exploited by tumor cells to avoid death, thus playing an important role in carcinogenesis and in the acquisition of resistance to various therapeutic agents. Therefore, antiapoptotic proteins represent attractive targets in cancer therapy [12].
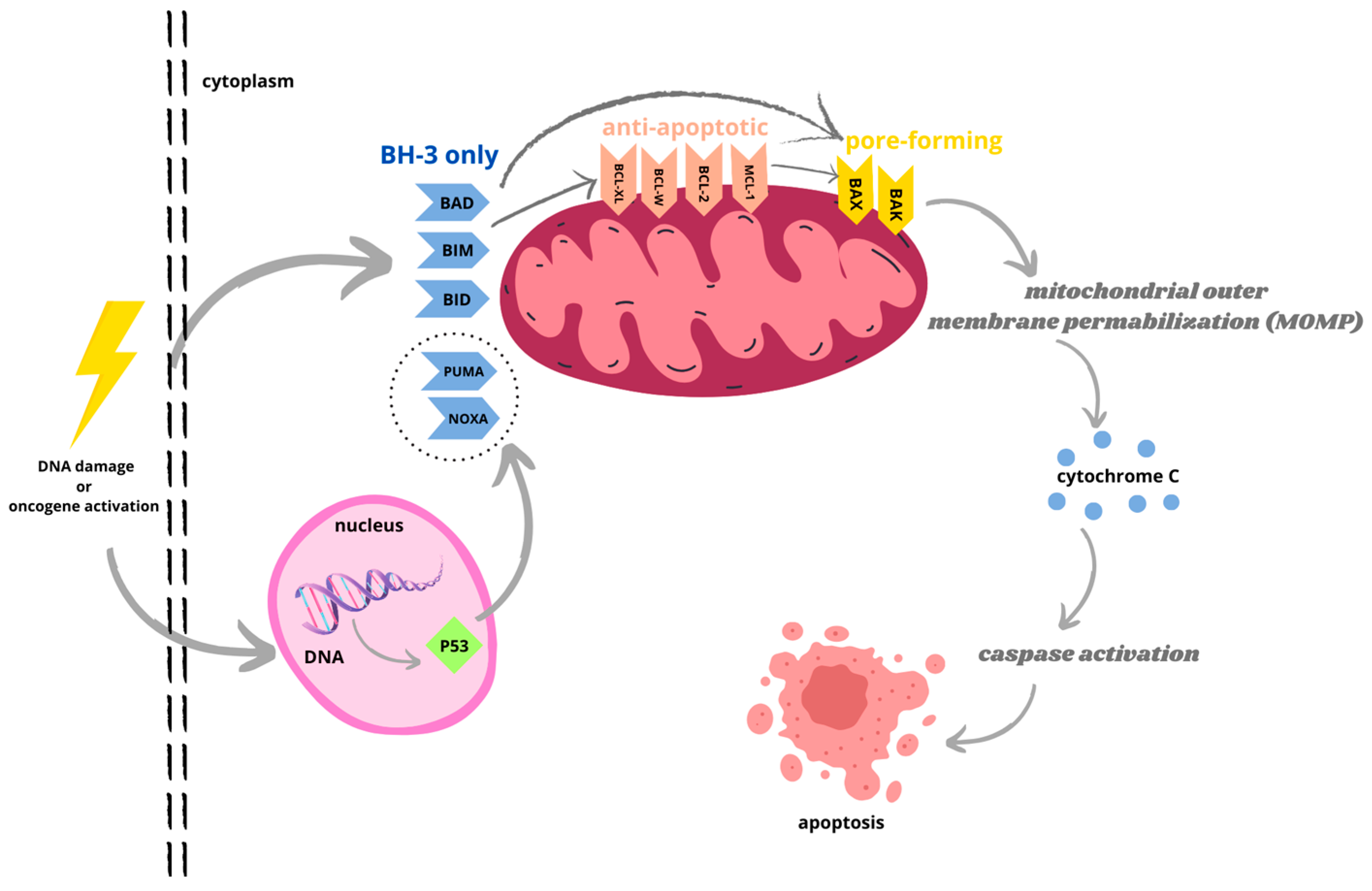
Figure 1. Bcl-2 protein family regulates mitochondrial apoptosis via intrinsic pathway initiated by toxic factors, genetic damage, or oncogene activation. The interaction between antiapoptotic and pro apoptotic Bcl-2 proteins prevents cell death. Activated BH3-only proteins bind to antiapoptotic proteins, located in outer mitochondrial membrane (OMM), resulting in release of pore-forming effector proteins BAX and BAK, which cycle between OMM and cytosol. Activated BH3-only proteins can also directly activate BAX and BAK. The translocation of BAX and BAK induces mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release, followed by formation of apoptosome and caspase activation. Caspase cascade causes destruction of apoptotic cells.
1.3. Bcl-2 Inhibitors
In recent years, work has been carried out to selectively target Bcl-2 proteins by creating BH3 mimetics that bind to hydrophobic grooves of the pro-survival proteins with high affinity. As a result, a number of Bcl-2 inhibitors have been developed [13].
The first highly selective Bcl-2 inhibitor, ABT-737, and its clinical derivative navitoclax (ABT-263) target BCL-2, BCL-W, and BCL-XL. Navitoclax is a BH3 mimetic drug which binds strongly to the BH3 domain of BCL-2 antiapoptotic members. Navitoclax binds to the BH3 binding groove of BCL-2 proteins located in the cytoplasm, causing the displacement of proapoptotic BH3-only protein BIM from BCL-2. The release of BIM causes apoptosis. Navitoclax alone successfully treats small-cell lung cancer and acute lymphocytic leukemia, whereas in combination therapy for solid tumors, it enhances the therapeutic effect of other chemotherapeutic agents [14][15][16].
Another advancement was the discovery of obatoclax (GX15-070). It is a less selective Bcl-2 inhibitor that antagonizes BCL-2, BCL-XL, BCL-W, and MCL-1. Obatoclax is a synthetic indole bipyrrole derivative of bacterial prodiginines. It is a small-molecule inhibitor of the antiapoptotic proteins of the Bcl-2 family [17][18][19]. Several phase I and II clinical trials have shown its only modest efficacy in the treatment of solid tumors and hematological malignancies [20]. Obatoclax may be more effective when used in combination with other anticancer therapeutics [21].
The next innovation was the development of venetoclax (VTX; ABT-199). It is a highly selective oral Bcl-2 inhibitor with high affinity to BCL-2 and lower affinity to BCL-W and BCL-XL—a molecule crucial for platelet survival. VTX shows activity in BCL-2-dependent hematologic malignancies, especially in chronic lymphocytic leukemia (CLL) [13][22]. The maximum plasma concentration is reached 5–8 h post-dose, and the elimination half-life ranges between 17 and 41 h after a single oral dose. Bioavailability is increased by food, and it is primarily metabolized via the CYP3A pathway and through the hepatic/fecal system [23].
1.4. Venetoclax in Adult Hematology
VTX has been successfully granted approval by the U.S. Food and Drug Administration (FDA) as a treatment option for previously untreated patients with CLL or small lymphocytic lymphoma (SLL), with or without 17p deletion, in combination with obinutuzumab [24][25], as well as for newly diagnosed acute myeloid leukemia (AML) patients who are 75 years or older, or who have comorbidities precluding intensive induction chemotherapy in combination with azacitidine, decitabine, or low-dose cytarabine (LDAC) [26].
In relapsed and refractory AML patients, the efficacy and safety of VTX monotherapy were first explored in a phase II study, which showed low efficacy with an overall response rate (ORR) of 19% [27], whereas VTX combination AML therapies with DNA methyltransferase inhibitors (DNMTis) or LDAC demonstrated promising results [28]. In a study of 145 patients over 60 years, VTX in combination with DNMTis was associated with complete remission (CR) or complete remission with incomplete hematologic recovery (CRi) in 67%, with a median overall survival of 17.5 months [29]. In 82 patients over 60 years receiving VTX in combination with LDAC, the CR/CRi rate was 54%, and the median overall survival (OS) was 10.1 months [30]. Despite those promising responses, primary resistance and clonal evolution leading to adaptive resistance remains an important theme in AML. Recent studies have illustrated the complex and polyclonal nature of resistance to targeted therapeutics [31]. TP53 mutations are especially related to inferior response rates, shorter disease response, and higher minimal residual disease positivity in newly diagnosed AML patients treated with a combination of VTX and decitabine [32].
In view of its beneficial efficacy and tolerable toxicity profile, VTX has become a therapeutic option for the management of de novo and relapsed refractory CLL, demonstrating durable responses regardless of adverse prognostic features such as deletion (del) (17p) [33][34][35]. In a cohort of 158 patients, mainly with relapsed and refractory (RR) CLL with del (17p), treatment with VTX established promising tolerability and durable responses, including an ORR of 77%, undetectable minimal residual disease (uMRD) in peripheral blood (PB) of 30%, and estimated 24-month progression free survival (PFS) of 50% [36]. Retrospective data from 683 patients with CLL, treated with ibrutinib, idelalisib, or VTX after ibrutinib therapy failure, demonstrated better outcomes in those treated with VTX (ORR 79%) versus idelalisib (ORR 46%). Furthermore, in the case of kinase inhibitor (KI) failure, alternate KI or VTX therapy appears superior to chemoimmunotherapy variations [37]. Combination therapy with anti-CD20 monoclonal antibodies (mAbs) and other small molecules in CLL has been the subject of interest in order to achieve deeper and more durable responses and allow for fixed-duration therapy [33][38].
For many patients, especially those with high-risk disease, VTX-based therapy is better tolerated and more effective than traditional chemoimmunotherapy.
Moreover, there is the novel Bcl-2 inhibitory compound lisaftoclax (APG-2575) that is currently undergoing clinical evaluation upon FDA permission [39] and has been granted four Orphan Drug Designations (ODDs) by the FDA for the treatment of patients with AML, CLL, Waldenström macroglobulinemia (WM), and multiple myeloma (MM) [40].
2. Pediatric AML
Regardless of significant advances in the treatment of AML and the continuous expansion of new treatment regiments, there is a group of patients who are unqualified or impervious to intensive induction chemotherapy, resulting in a poor prognosis and restricted therapeutic options [41][42]. Children with relapsed or refractory acute myeloid leukemia have poor treatment outcomes and overall survival. Despite relapse prevention being the aim of initial therapy, around 30% of pediatric AML patients will later develop bone marrow relapse, with an overall survival of less than 40% [43]. Since it has been proven that some AML blast cells display high levels of BCL-2 protein, mainly amongst chemotherapy-resistant patients [44][45][46][47], antiapoptotic proteins are seen as a promising therapeutic target. Despite encouraging results being reported for VTX usage both in monotherapy and in combination therapies in clinical studies of adult patients with AML [27][47][48], not much is known about VTX administration and efficacy in younger patients. Due to the fairly low incidence of childhood cancers, conducting a pediatric clinical study faces many limitations including a small sample size, heterogeneous cohort, lack of a control group, short duration of follow-up, and occurrence of disease progression during single-agent studies.
2.1. Clinical Studies in Relapsed/Refractory AML
VTX is currently the subject of many ongoing phase I/II clinical trials to estimate the virtue and tolerability of this agent in this population. The most notable study to date was carried out by Karol and colleagues. They aimed to ascertain the tolerance of VTX in combination therapy with standard and high-dose chemotherapy in pediatric patients with relapsed/refractory AML or ambiguous lineage leukemia. The conducted phase I dose escalation study (NCT03194932) proposed the safety and activity of BCL-2 inhibitor in combined therapy. A total of 36 patients were given VTX in 28-day cycles at 240 mg/m2 or 360 mg/m2, in combination with cytarabine, with or without intravenous idarubicin. The recommended phase II dose was established at 360 mg/m2. The overall response was observed in 69% of the 35 patients assessed after the first cycle, with a notable 70% complete response rate among the 20 patients treated at the recommended phase II dose. Among the patients, 66% developed febrile neutropenia and 16% developed invasive fungal infections. The findings also highlight the need for such a treatment combination evaluation in newly diagnosed patients with high-risk AML [49]. These encouraging data prompted a study by Place et al. to establish the dose-limiting toxicity, pharmacokinetics, and preliminary efficacy of VTX monotherapy in an open-label, global phase I two-part study (EudraCT 2017–000439–14; NCT03236857) amongst pediatric and young adult patients (AYA) with relapsed/refractory malignancies. During part 1, younger patients with any relapsed/refractory tumor type who had no available curative options were enrolled. Participants were given VTX daily with a two- or three-day dose ramp up to 800 mg, weigh and age-adjusted, over the course of 9 months. After the first assessment, patients may have received VTX in combination with chemotherapy, beginning at week 4 in patients with hematologic malignancies. During part 2, patients with relapsed/refractory acute lymphoblastic leukemia (ALL), AML, non-Hodgkin’s lymphoma (NHL), or neuroblastoma were enrolled in four cohorts; the outcomes are yet to be presented [50].
2.2. Other Studies on Venetoclax Combination Therapies in AML
Recent studies support the usage of VTX-containing regimens in myeloid malignancies, especially as a linking therapy for allogeneic hematopoietic stem cell transplantation (HSCT) [51][52][53][54]. Niswander et al. analyzed 37 pediatric patients with relapsed/refractory acute leukemias, including many with high-risk cytomolecular genetic features treated with VTX in combination with hypomethylating agent (HMA) with or without CD33 antibody gemtuzumab ozogamicin. The median minimal residual disease (MRD) level was 0.5%, with 14 patients (n = 12 AML) achieving a CR with MRD-negative remission (38% of 37 treated patients). Patients’ responses to the regimen were typically achieved within one cycle of therapy or not at all. Successful remission induction was HSCT-enabling for 11 patients with AML [52]. A multicenter retrospective analysis evaluated VTX with HMA azacitidine or decitabine, or with a combination of cytotoxic agents, such as cytarabine, fludarabine, idarubicin, or doxorubicin, in 31 pediatric patients with high-risk myeloid malignancies who had received previous lines of therapy. The median dose of 350 mg/m2 VTX was administered daily within a median of two cycles. The response rate was satisfying, with an overall response rate of 71% and a CR of 51.6%. Twenty patients received allogeneic HSCT at a median time of 3.3 months from the start of treatment and were alive at the end of follow-up (7.7 months) [53]. Moreover, a retrospective report from Children’s Hospital Colorado by Winters et al. on the use of azacitidine (AZA)/VTX combination among six patients with AML revealed that all responders achieved minimal residual disease negativity and three of them proceeded to HSCT [54].
The most common adverse events (AEs) found were a prolonged depletion of all blood cell lines, especially neutropenia, and severe blood, pulmonary, and skin infections, including bacteriemia, which are consistent with other published data on VTX in pediatric patients with acute leukemias [55][56]. The overall response rate was comparable to that seen in heavily pretreated adult patients with AML who received similar VTX combination therapies [48]. Most patients received maximal benefit within one to two cycles of VTX-based therapy, and all durable responses were followed by HSCT, indicating those regimens are likely to become a bridge therapy for allogeneic hematopoietic stem cell transplantation rather than a definitive therapy.
2.3. Genetic Sensitivity and Resistance to Venetoclax
With acute myeloid leukemia being a molecularly heterogeneous disorder, genetic lesions have been linked to particular clinical features, therapy response, and patients’ outcomes, leading to improvements in risk stratification. There are favorable (RUNX1-RUNX1T1, CBFB-MYH11, NPM1, and CEBPA bZIP) and unfavorable (MECOM, DEK-NUP214, KMT2A, NUP98, FLT3/ITD, WT1, monosomy 7, monosomy 5, and TP53) pediatric genetic markers that are being used to guide practitioners through patients’ management [57][58]. Amongst the available data discussing clinical experience with VTX in children, the most recurrent structural rearrangements or sequence variants observed were KMT2A rearrangements, FLT3 alterations, and NPM1 mutations; many patients who obtained CR had a particular molecular subtype of the malignancy or a cancer predisposition syndrome [49][51][52][53][54][55][56][59]. It is important to establish which molecular subtypes of pediatric malignancies might display specific VTX vulnerability, despite no evident mechanistic links to BH3 mimetic responses. KMT2A is a frequently rearranged gene in leukemias [60][61], mostly in pediatric and infant AML [62][63]. Maseti and colleagues identified eight pediatric patients with KMT2A rearrangements, six of whom achieved CR and one of whom achieved a partial response (PR) [53]. During another study, in a cohort of 17 relapsed patients with KMT2A, 40% (n = 6) achieved CR/CRi after a median of one cycle of a VTX-including regimen [55]. Preclinical data suggest a significant antiapoptotic dependence and responsiveness to VTX in in vitro models of KMT2A-rearranged myeloid and B-cell lymphoblastic leukemia [64][65]. The combination of VTX with novel KMT2A-r identified drugs, such as I-BET151, sunitinib, or thioridazine, drastically decreases leukemic cell count, which provides a rationale for targeting the mitochondrial pathway as a strategy to sensitize resistant AML to VTX [65]. Moreover, initial results from a retrospective adult KMT2A-rearranged cohort presented a high response rate with VTX and HMA combined therapy [66]. Recent studies concerning adult AML showed increased responses to VTX amongst NPM1- [29][67][68][69], IDH1/2-, TET2-, and relapsed or refractory RUNX1-mutated patients, compared to the other cases [68][69]. Therapy for older patients with NPM1+ AML was associated with CR rates > 85% and an OS of 80% after a median follow-up of 1 year [69]. Somatic mutations of the NPM1 gene are found in less than 10% of pediatric AML patients, much rarer than in adults [70][71]. Trabal et al. identified six patients with mutation profiles, including NPM1, IDH1/2, or TET2 mutations; three of those children achieved CR/Cri [55]. As of yet, there are no data specific for children regarding markers of VTX sensitivity.
On the contrary, adult AML patients displaying FLT3, TP53, RAS, or PTPN11 mutations, monocytic AML, or AML cases pretreated with HMAs showed decreased receptivity to VTX-based therapies [48][68][72]. During the study by Karol and colleagues, not one of the five patients with FLT3 alterations responded to treatment [49]. The FLT3-tyrosine kinase receptor is vital for normal hematopoietic development. Present in around 30% of cases, somatic activating mutations in this gene are among the most frequent somatic alterations in pediatric AML [73][74]. FLT3-internal tandem duplication mutations have been linked to VTX resistance in some experimental models [75]. Gilteritinib, a highly specific inhibitor of FLT3 mutations, has been certified as a single-agent treatment of relapsed or refractory AML in the United States and Europe [76]. There are emerging clinical trials combining VTX with a tyrosine kinase inhibitor for relapsed and refractory FLT3+ patients with AML that have demonstrated efficacy [77]. Testing FLT3 signaling first might be beneficial when identifying a targeted AML population who might respond well to this innovative treatment approach. Further to this, VTX was not efficient in two cases of infant AML with GLIS fusions [56], which are correlated with a highly refractory phenotype in pediatric AML subtypes [78][79]. Newly released preclinical data supported VTX resistance in murine models of CBFA2T3–GLIS2 pediatric acute megakaryoblastic leukemia, but the models displayed sensitivity to navitoclax, a BCL-XL inhibitor, hinting at a potential path for targeting this high-risk infant leukemia [80].
3. Pediatric ALL
Comprehensive Treatment with Venetoclax
In a retrospective study by Gibson et al. involving pediatric and AYA patients with relapsed or recurrent hematologic malignancies, including ALL and lymphoblastic lymphoma (LBL), VTX was used as an addition to conventional cytotoxic chemotherapy. Patients received various combinations of cyclophosphamide, vincristine, dexamethasone, doxorubicin, methotrexate, cytarabine, decitabine, nelarabine, pegylated asparaginase, fludarabine, idarubicin, etoposide, gemtuzumab, and rituximab. VTX doses ranged between 100 and 400 mg per day. A total of 61% of patients responded with complete remission. In this study, combination therapy with VTX proved its effectiveness in both first-diagnosed pediatric T-ALL and RR, as well as T-cell LBL [81].
A retrospective observation made by Marinoff et al. showed the efficiency of VTX combined with hypomethylating drugs and chemotherapy in B-ALL patients as well. However, a limitation of that study was that the cohort primarily consisted of relapsed and refractory patients, 70% of whom had received three or more prior lines of therapy. Moreover, the study showed that special attention should be paid to significant side effects such as infections, which were more serious when VTX was combined with conventional chemotherapeutic agents such as vincristine, fludarabine, or cytarabine compared to the combination of VTX with hypomethylating agents such as azacitidine, cytarabine, and decitabine [56]. In addition to infectious side effects, almost 90% of patients presented substantial thrombocytopenia. Most common AEs reported in other studies were severe neutropenia, hyperbilirubinemia, sepsis, aspartate aminotransferase elevation, and disseminated intravascular coagulation [81]. Moreover, as the duration of VTX therapy increased, the thrombocytopenia and neutropenia periods expanded [82]. A similar relationship occurred when increasing the dosage of VTX, despite it not being statistically significant during later observations [82].
The effect of combined therapies with VTX in B-cell ALL seems to exceed the effectiveness of VTX in T-cell ALL. Pullarkat et al., in the evaluation of both pediatric and adult patients with relapsed or recurrent disease after using a combination of VTX, navitoclax, and chemotherapy, noted that the estimated median OS was longer in patients with B-cell ALL than T-cell ALL (9.7 months versus 6.6 months) [83]. It is difficult to determine whether this is solely the effect of VTX due to the fact that, in most clinical trials, VTX is used in combination with other substances to intensify therapy. In another study, after a dosage of VTX (100 mg/kg/for 21 days) in most of the tested pediatric ALL xenografts, an objective response and delay in the development of the disease were achieved. In this case, the treatment contributed to the prolongation of event-free survival (EFS) in B-cell ALL as opposed to T-cell ALL [84]. In a study conducted by Diamanti et al., VTX used in vitro proved to be efficient in most of the samples tested, except for CD34−/CD19− cells, but the in vitro study showed its lack of effectiveness in T-cell ALL. It is important to note that lower BCL-2 expression was observed in T-cell ALL patients, and some authors consider this phenomenon to be a possible cause of lower sensitivity in these patients to VTX [85]. However, the available literature has data on a more successful effect of VTX on T-cell ALL as, in the already-mentioned study by Gibson et al., VTX showed its efficacy in both T-cell ALL and T-cell LBL, where 77% of the patients achieved CR or CRi [81].
Brown et al. paid particular attention to VTX increasing the effectiveness of azacitidine in xenografts of neonatal ALL with KMT2A rearrangements in a randomized controlled trial. Despite each of these agents having a positive antileukemic effect on its own, it was their combination that proved to be the best option for targeting leukemia stem cells. This highlights the importance of VTX in new therapies, especially in cases of infantile leukemia, which is more aggressive, with event-free survival being much lower than in older children with ALL [86].
Currently, most research focuses on the use of VTX in polytherapy, which will bring more spectacular effects in the fight against cancer, especially in severe cases. Combining VTX, which is a BCL-2-specific inhibitor, with navitoclax, a pan-Bcl-2 inhibitor [85], seems to not only maximize the effectiveness of treatment but also increase clinical tolerance. These conclusions were reached by researchers assessing pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia or lymphoblastic lymphoma in a multicenter, phase I study (NCT03181126). The study evaluated the safety and preliminary efficacy of VTX with low-dose navitoclax and chemotherapy in 47 patients, 12 of whom were under 18 years of age. The results indicated the validity of combining VTX with low doses of navitoclax in patients with B-cell acute lymphoblastic leukemia, T-cell acute lymphoblastic leukemia, and lymphoblastic lymphoma. The simultaneous inhibition of BCL-2 and BCL-XL led to a reduction in thrombocytopenia and other side effects induced by navitoclax, which ensured treatment effectiveness [83].
There are reports suggesting that BH-3 mimetics are not sufficient in the treatment of childhood leukemias and combining BH-3 mimetics with inhibitors of survival pathways is not a dispensable option [85]. It was proven that the usage of VTX alone, despite a positive response, left leukemic blasts in the liver and spleen and positive minimal residual disease in the bone marrow of mice at the end of therapy [87]. Therefore, a substance that enhances the action of VTX was sought. Researchers identified dinaciclib, which is an inhibitor of cyclin-dependent kinases 1, 2, 5, 9, and 16 showing synergy with VTX, obtaining a 97% inhibition of the growth of leukemic cells in hypodiploid ALL [87]. Such results are promising, but further research in this direction is needed.
Recently, another substance, i.e., a receptor of the tyrosine kinase inhibitor MRX-2843, was discovered, showing synergy with VTX. Combining MRX-2843 with BCL-2 inhibitor showed positive effects in early T-precursor ALL (ETP ALL). This type of leukemia is associated with a high rate of treatment failures. The intensity of action increased in direct proportion to the doses of drugs. Nevertheless, when these substances were used in monotherapy, MRX-2843 showed better effects in ETP ALL [88].
Despite being less effective in monotherapy, VTX should be considered as an agent that intensifies the action of other substances supporting the treatment of hematological cancers. In addition to the dose, different methods of administering VTX with other substances should be considered combinatorial. Richter et al. compared the co-incubation of the new drug MK-2206 with VTX to the administration of one ingredient after the other. In this case, the most satisfactory effect was achieved when VTX was administered second [89]. Such dependencies may occur when other substances are used; therefore, it is important to expand research in this direction.
References
- Voss, A.K.; Strasser, A. The Essentials of Developmental Apoptosis. F1000Research 2020, 9.
- Thompson, C.B. Apoptosis in the Pathogenesis and Treatment of Disease. Science 1995, 267, 1456–1462.
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541.
- Lomonosova, E.; Chinnadurai, G. BH3-Only Proteins in Apoptosis and beyond: An Overview. Oncogene 2008, 27 (Suppl. S1), S2–S19.
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619.
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63.
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 177.
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714.
- Kuwana, T.; Bouchier-Hayes, L.; Chipuk, J.E.; Bonzon, C.; Sullivan, B.A.; Green, D.R.; Newmeyer, D.D. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol. Cell 2005, 17, 525–535.
- Cheng, E.H.; Wei, M.C.; Weiler, S.; Flavell, R.A.; Mak, T.W.; Lindsten, T.; Korsmeyer, S.J. BCL-2, BCL-X(L) Sequester BH3 Domain-Only Molecules Preventing BAX- and BAK-Mediated Mitochondrial Apoptosis. Mol. Cell 2001, 8, 705–711.
- Senichkin, V.V.; Pervushin, N.V.; Zuev, A.P.; Zhivotovsky, B.; Kopeina, G.S. Targeting Bcl-2 Family Proteins: What, Where, When? Biochemistry 2020, 85, 1210–1226.
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208.
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428.
- Park, C.-M.; Bruncko, M.; Adickes, J.; Bauch, J.; Ding, H.; Kunzer, A.; Marsh, K.C.; Nimmer, P.; Shoemaker, A.R.; Song, X.; et al. Discovery of an Orally Bioavailable Small Molecule Inhibitor of Prosurvival B-Cell Lymphoma 2 Proteins. J. Med. Chem. 2008, 51, 6902–6915.
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An Inhibitor of Bcl-2 Family Proteins Induces Regression of Solid Tumours. Nature 2005, 435, 677–681.
- Konopleva, M.; Watt, J.; Contractor, R.; Tsao, T.; Harris, D.; Estrov, Z.; Bornmann, W.; Kantarjian, H.; Viallet, J.; Samudio, I.; et al. Mechanisms of Antileukemic Activity of the Novel Bcl-2 Homology Domain-3 Mimetic GX15-070 (Obatoclax). Cancer Res. 2008, 68, 3413–3420.
- Trudel, S.; Li, Z.H.; Rauw, J.; Tiedemann, R.E.; Wen, X.Y.; Stewart, A.K. Preclinical Studies of the Pan-Bcl Inhibitor Obatoclax (GX015-070) in Multiple Myeloma. Blood 2007, 109, 5430–5438.
- Urtishak, K.A.; Edwards, A.Y.Z.; Wang, L.-S.; Hudome, A.; Robinson, B.W.; Barrett, J.S.; Cao, K.; Cory, L.; Moore, J.S.; Bantly, A.D.; et al. Potent Obatoclax Cytotoxicity and Activation of Triple Death Mode Killing across Infant Acute Lymphoblastic Leukemia. Blood 2013, 121, 2689–2703.
- Goard, C.A.; Schimmer, A.D. An Evidence-Based Review of Obatoclax Mesylate in the Treatment of Hematological Malignancies. Core Evid. 2013, 8, 15–26.
- Han, Z.; Liang, J.; Li, Y.; He, J. Drugs and Clinical Approaches Targeting the Antiapoptotic Protein: A Review. BioMed Res. Int. 2019, 2019, 1212369.
- Guerra, V.A.; DiNardo, C.; Konopleva, M. Venetoclax-Based Therapies for Acute Myeloid Leukemia. Best Pract. Res. Clin. Haematol. 2019, 32, 145–153.
- VENCLEXTA (Venetoclax Tablets) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf (accessed on 10 October 2023).
- US Food and Drug Administration. FDA Approves Venetoclax for CLL or SLL, with or without 17 p Deletion, after One Prior Therapy. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm (accessed on 10 October 2023).
- US Food and Drug Administration. FDA Approves Venetoclax for CLL and SLL. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll (accessed on 10 October 2023).
- US Food and Drug Administration. FDA Approves Venetoclax in Combination for AML in Adults. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-venetoclax-combination-untreated-acute-myeloid-leukemia (accessed on 10 October 2023).
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117.
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.A.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and Preliminary Efficacy of Venetoclax with Decitabine or Azacitidine in Elderly Patients with Previously Untreated Acute Myeloid Leukaemia: A Non-Randomised, Open-Label, Phase 1b Study. Lancet Oncol. 2018, 19, 216–228.
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax Combined with Decitabine or Azacitidine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia. Blood 2019, 133, 7–17.
- Wei, A.H.; Strickland, S.A.; Hou, J.-Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284.
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019, 9, 1050–1063.
- Kim, K.; Maiti, A.; Kadia, T.M.; Ravandi, F.; Daver, N.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; Short, N.J.; et al. Outcomes of TP53-Mutant Acute Myeloid Leukemia with Venetoclax and Decitabine. Blood 2020, 136, 33–36.
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukaemia: A Phase 1b Study. Lancet Oncol. 2017, 18, 230–240.
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 Selective Inhibitor Venetoclax Induces Rapid Onset Apoptosis of CLL Cells in Patients via a TP53-Independent Mechanism. Blood 2016, 127, 3215–3224.
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322.
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia with 17p Deletion: Results from the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980.
- Mato, A.R.; Hill, B.T.; Lamanna, N.; Barr, P.M.; Ujjani, C.S.; Brander, D.M.; Howlett, C.; Skarbnik, A.P.; Cheson, B.D.; Zent, C.S.; et al. Optimal Sequencing of Ibrutinib, Idelalisib, and Venetoclax in Chronic Lymphocytic Leukemia: Results from a Multicenter Study of 683 Patients. Ann. Oncol. 2017, 28, 1050–1056.
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Muñoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; MacDonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J. Clin. Oncol. 2019, 37, 2722–2729.
- Ascentage Pharma. Ascentage Pharma Received Clearance from U.S. FDA to Proceed with Global Registrational Phase III Clinical Trial for Lisaftoclax (APG-2575) in Previously Treated Patients with CLL/SLL. Available online: https://ascentage.com/ascentage-pharma-received-clearance-from-u-s-fda-to-proceed-with-global-registrational-phase-iii-clinical-trial-for-lisaftoclax-apg-2575-in-previously-treated-patients-with-cll-sll/ (accessed on 12 November 2023).
- Ascentage Pharma. Ascentage Pharma Announces Its 9th Orphan Drug Designation from the US FDA in 2020, Setting a Record for Chinese Biopharmaceutical Companies. Available online: https://ascentage.com/ascentage-pharma-announces-its-9th-orphan-drug-designation-from-the-us-fda-in-2020-setting-a-record-for-chinese-biopharmaceutical-companies/ (accessed on 12 November 2023).
- Hunger, S.P.; Raetz, E.A. How I Treat Relapsed Acute Lymphoblastic Leukemia in the Pediatric Population. Blood 2020, 136, 1803–1812.
- Zarnegar-Lumley, S.; Caldwell, K.J.; Rubnitz, J.E. Relapsed Acute Myeloid Leukemia in Children and Adolescents: Current Treatment Options and Future Strategies. Leukemia 2022, 36, 1951–1960.
- Rasche, M.; Zimmermann, M.; Steidel, E.; Alonzo, T.; Aplenc, R.; Bourquin, J.-P.; Boztug, H.; Cooper, T.; Gamis, A.S.; Gerbing, R.B.; et al. Survival Following Relapse in Children with Acute Myeloid Leukemia: A Report from AML-BFM and COG. Cancers 2021, 13, 2336.
- Bradbury, D.A.; Zhu, Y.M.; Russell, N.H. Bcl-2 Expression in Acute Myeloblastic Leukaemia: Relationship with Autonomous Growth and CD34 Antigen Expression. Leuk Lymphoma 1997, 24, 221–228.
- Bensi, L.; Longo, R.; Vecchi, A.; Messora, C.; Garagnani, L.; Bernardi, S.; Tamassia, M.G.; Sacchi, S. Bcl-2 Oncoprotein Expression in Acute Myeloid Leukemia. Haematologica 1995, 80, 98–102.
- Pan, R.; Hogdal, L.J.; Benito, J.M.; Bucci, D.; Han, L.; Borthakur, G.; Cortes, J.; DeAngelo, D.J.; Debose, L.; Mu, H.; et al. Selective BCL-2 Inhibition by ABT-199 Causes on-Target Cell Death in Acute Myeloid Leukemia. Cancer Discov. 2014, 4, 362–375.
- Bogenberger, J.M.; Kornblau, S.M.; Pierceall, W.E.; Lena, R.; Chow, D.; Shi, C.-X.; Mantei, J.; Ahmann, G.; Gonzales, I.M.; Choudhary, A.; et al. BCL-2 Family Proteins as 5-Azacytidine-Sensitizing Targets and Determinants of Response in Myeloid Malignancies. Leukemia 2014, 28, 1657–1665.
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-Day Decitabine with Venetoclax for Newly Diagnosed Intensive Chemotherapy Ineligible, and Relapsed or Refractory Acute Myeloid Leukaemia: A Single-Centre, Phase 2 Trial. Lancet Haematol. 2020, 7, e724–e736.
- Karol, S.E.; Alexander, T.B.; Budhraja, A.; Pounds, S.B.; Canavera, K.; Wang, L.; Wolf, J.; Klco, J.M.; Mead, P.E.; Gupta, S.D.; et al. Venetoclax in Combination with Cytarabine with or without Idarubicin in Children with Relapsed or Refractory Acute Myeloid Leukaemia: A Phase 1, Dose-Escalation Study. Lancet Oncol. 2020, 21, 551–560.
- Place, A.E.; Goldsmith, K.; Bourquin, J.-P.; Loh, M.L.; Gore, L.; Morgenstern, D.A.; Sanzgiri, Y.; Hoffman, D.; Zhou, Y.; Ross, J.A.; et al. Accelerating Drug Development in Pediatric Cancer: A Novel Phase I Study Design of Venetoclax in Relapsed/Refractory Malignancies. Future Oncol. 2018, 14, 2115–2129.
- Pfeiffer, T.; Li, Y.; Ashcraft, E.; Karol, S.E.; Rubnitz, J.E.; Epperly, R.; Madden, R.; Mamcarz, E.; Obeng, E.; Qudeimat, A.; et al. Venetoclax-Based Therapy as a Bridge to Allogeneic Hematopoietic Cell Transplantation in Children with Relapsed/Refractory AML. Bone Marrow Transplant. 2023, 58, 328–331.
- Niswander, L.M.; Chung, P.; Diorio, C.; Tasian, S.K. Clinical Responses in Pediatric Patients with Relapsed/Refractory Leukemia Treated with Azacitidine and Venetoclax. Haematologica 2020, 108, 3142.
- Masetti, R.; Baccelli, F.; Leardini, D.; Gottardi, F.; Vendemini, F.; Di Gangi, A.; Becilli, M.; Lodi, M.; Tumino, M.; Vinci, L.; et al. Venetoclax-Based Therapies in Pediatric Advanced MDS and Relapsed/Refractory AML: A Multicenter Retrospective Analysis. Blood Adv. 2023, 7, 4366–4370.
- Winters, A.C.; Maloney, K.W.; Treece, A.L.; Gore, L.; Franklin, A.K. Single-Center Pediatric Experience with Venetoclax and Azacitidine as Treatment for Myelodysplastic Syndrome and Acute Myeloid Leukemia. Pediatr. Blood Cancer 2020, 67, e28398.
- Trabal, A.; Gibson, A.; He, J.; McCall, D.; Roth, M.; Nuñez, C.; Garcia, M.; Buzbee, M.; Toepfer, L.; Bidikian, A.; et al. Venetoclax for Acute Myeloid Leukemia in Pediatric Patients: A Texas Medical Center Experience. Cancers 2023, 15, 1983.
- Marinoff, A.E.; Aaronson, K.; Agrawal, A.K.; Braun, B.S.; Golden, C.; Huang, B.J.; Michlitsch, J.; Southworth, E.; Thrall, A.; Vo, K.T.; et al. Venetoclax in Combination with Chemotherapy as Treatment for Pediatric Advanced Hematologic Malignancies. Pediatr. Blood Cancer 2023, 70, e30335.
- Conneely, S.E.; Rau, R.E. The Genomics of Acute Myeloid Leukemia in Children. Cancer Metastasis Rev. 2020, 39, 189–209.
- Zafar, N.; Ghias, K.; Fadoo, Z. Genetic Aberrations Involved in Relapse of Pediatric Acute Myeloid Leukemia: A Literature Review. Asia-Pac. J. Clin. Oncol. 2021, 17, e135–e141.
- Bobeff, K.; Pastorczak, A.; Urbanska, Z.; Balwierz, W.; Juraszewska, E.; Wachowiak, J.; Derwich, K.; Samborska, M.; Kalwak, K.; Dachowska-Kalwak, I.; et al. Venetoclax Use in Paediatric Haemato-Oncology Centres in Poland: A 2022 Survey. Children 2023, 10, 745.
- Winters, A.C.; Bernt, K.M. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front. Pediatr. 2017, 5, 4.
- Meyer, C.; Burmeister, T.; Gröger, D.; Tsaur, G.; Fechina, L.; Renneville, A.; Sutton, R.; Venn, N.C.; Emerenciano, M.; Pombo-de-Oliveira, M.S.; et al. The MLL Recombinome of Acute Leukemias in 2017. Leukemia 2018, 32, 273–284.
- Creutzig, U.; Zimmermann, M.; Reinhardt, D.; Rasche, M.; von Neuhoff, C.; Alpermann, T.; Dworzak, M.; Perglerová, K.; Zemanova, Z.; Tchinda, J.; et al. Changes in Cytogenetics and Molecular Genetics in Acute Myeloid Leukemia from Childhood to Adult Age Groups. Cancer 2016, 122, 3821–3830.
- Harrison, C.J.; Hills, R.K.; Moorman, A.V.; Grimwade, D.J.; Hann, I.; Webb, D.K.H.; Wheatley, K.; de Graaf, S.S.N.; van den Berg, E.; Burnett, A.K.; et al. Cytogenetics of Childhood Acute Myeloid Leukemia: United Kingdom Medical Research Council Treatment Trials AML 10 and 12. J. Clin. Oncol. 2010, 28, 2674–2681.
- Cheung, L.C.; Aya-Bonilla, C.; Cruickshank, M.N.; Chiu, S.K.; Kuek, V.; Anderson, D.; Chua, G.-A.; Singh, S.; Oommen, J.; Ferrari, E.; et al. Preclinical Efficacy of Azacitidine and Venetoclax for Infant KMT2A-Rearranged Acute Lymphoblastic Leukemia Reveals a New Therapeutic Strategy. Leukemia 2023, 37, 61.
- Tregnago, C.; Benetton, M.; Da Ros, A.; Borella, G.; Longo, G.; Polato, K.; Francescato, S.; Biffi, A.; Pigazzi, M. Novel Compounds Synergize with Venetoclax to Target KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. Front. Pharmacol. 2022, 12, 820191.
- Ball, B.J.; Arslan, S.; Koller, P.; Ngo, D.; Afkhami, M.; Salhotra, A.; Al-Malki, M.; Aribi, A.; Ali, H.; Sandhu, K.; et al. Clinical Experience with Venetoclax and Hypomethylating Agents (HMA) in Patients with Newly Diagnosed and Relapsed or Refractory KMT2A-Rearranged Acute Myeloid Leukemia (AML). Leuk. Lymphoma 2022, 63, 3232–3236.
- Bisaillon, R.; Moison, C.; Thiollier, C.; Krosl, J.; Bordeleau, M.-E.; Lehnertz, B.; Lavallée, V.-P.; MacRae, T.; Mayotte, N.; Labelle, C.; et al. Genetic Characterization of ABT-199 Sensitivity in Human AML. Leukemia 2020, 34, 63–74.
- Griffioen, M.S.; de Leeuw, D.C.; Janssen, J.J.W.M.; Smit, L. Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies. Cancers 2022, 14, 3456.
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of Older Patients with NPM1-Mutated AML: Current Treatments and the Promise of Venetoclax-Based Regimens. Blood Adv. 2020, 4, 1311–1320.
- Hollink, I.H.I.M.; Zwaan, C.M.; Zimmermann, M.; Arentsen-Peters, T.C.J.M.; Pieters, R.; Cloos, J.; Kaspers, G.J.L.; de Graaf, S.S.N.; Harbott, J.; Creutzig, U.; et al. Favorable Prognostic Impact of NPM1 Gene Mutations in Childhood Acute Myeloid Leukemia, with Emphasis on Cytogenetically Normal AML. Leukemia 2009, 23, 262–270.
- Brown, P.; McIntyre, E.; Rau, R.; Meshinchi, S.; Lacayo, N.; Dahl, G.; Alonzo, T.A.; Chang, M.; Arceci, R.J.; Small, D. The Incidence and Clinical Significance of Nucleophosmin Mutations in Childhood AML. Blood 2007, 110, 979–985.
- Stevens, B.M.; Jones, C.L.; Winters, A.; Dugan, J.; Abbott, D.; Savona, M.R.; Fesik, S.W.; Pollyea, D.A.; Jordan, C.T. PTPN11 Mutations Confer Unique Metabolic Properties and Increase Resistance to Venetoclax and Azacitidine in Acute Myelogenous Leukemia. Blood 2018, 132, 909.
- Bolouri, H.; Farrar, J.E.; Triche, T.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The Molecular Landscape of Pediatric Acute Myeloid Leukemia Reveals Recurrent Structural Alterations and Age-Specific Mutational Interactions. Nat. Med. 2018, 24, 103–112.
- Tarlock, K.; Alonzo, T.A.; Loken, M.R.; Gerbing, R.B.; Ries, R.E.; Aplenc, R.; Sung, L.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; et al. Disease Characteristics and Prognostic Implications of Cell Surface FLT3 Receptor (CD135) Expression in Pediatric Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Clin. Cancer Res. 2017, 23, 3649–3656.
- Chyla, B.; Daver, N.; Doyle, K.; McKeegan, E.; Huang, X.; Ruvolo, V.; Wang, Z.; Chen, K.; Souers, A.; Leverson, J.; et al. Genetic Biomarkers of Sensitivity and Resistance to Venetoclax Monotherapy in Patients with Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, E202–E205.
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740.
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2022, 40, 4048–4059.
- Smith, S.M.; Lee, A.; Tong, S.; Leung, S.; Hongo, H.; Rivera, J.; Sweet-Cordero, A.; Michlitsch, J.; Stieglitz, E. Detection of a GLIS3 Fusion in an Infant with AML Refractory to Chemotherapy. Cold Spring Harb. Perspect. Biol. 2022, 8, a006220.
- Smith, J.L.; Ries, R.E.; Hylkema, T.; Alonzo, T.A.; Gerbing, R.B.; Santaguida, M.T.; Eidenschink Brodersen, L.; Pardo, L.; Cummings, C.L.; Loeb, K.R.; et al. Comprehensive Transcriptome Profiling of Cryptic CBFA2T3-GLIS2 Fusion-Positive AML Defines Novel Therapeutic Options: A COG and TARGET Pediatric AML Study. Clin. Cancer Res. 2020, 26, 726–737.
- Gress, V.; Roussy, M.; Boulianne, L.; Bilodeau, M.; Cardin, S.; El-Hachem, N.; Lisi, V.; Khakipoor, B.; Rouette, A.; Farah, A.; et al. CBFA2T3::GLIS2 Pediatric Acute Megakaryoblastic Leukemia Is Sensitive to BCL-XL Inhibition by Navitoclax and DT2216. Blood Adv. 2023.
- Gibson, A.; Trabal, A.; McCall, D.; Khazal, S.; Toepfer, L.; Bell, D.H.; Roth, M.; Mahadeo, K.M.; Nunez, C.; Short, N.J.; et al. Venetoclax for Children and Adolescents with Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancers 2021, 14, 150.
- Richard-Carpentier, G.; Jabbour, E.; Short, N.J.; Rausch, C.R.; Savoy, J.M.; Bose, P.; Yilmaz, M.; Jain, N.; Borthakur, G.; Ohanian, M.; et al. Clinical Experience with Venetoclax Combined with Chemotherapy for Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2020, 20, 212–218.
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov. 2021, 11, 1440–1453.
- Khaw, S.L.; Suryani, S.; Evans, K.; Richmond, J.; Robbins, A.; Kurmasheva, R.T.; Billups, C.A.; Erickson, S.W.; Guo, Y.; Houghton, P.J.; et al. Venetoclax Responses of Pediatric ALL Xenografts Reveal Sensitivity of MLL-Rearranged Leukemia. Blood 2016, 128, 1382–1395.
- Diamanti, P.; Ede, B.C.; Dace, P.E.; Barendt, W.J.; Cox, C.V.; Hancock, J.P.; Moppett, J.P.; Blair, A. Investigating the Response of Paediatric Leukaemia-Propagating Cells to BCL-2 Inhibitors. Br. J. Haematol. 2021, 192, 577–588.
- Brown, P.; Pieters, R.; Biondi, A. How I Treat Infant Leukemia. Blood 2019, 133, 205–214.
- Pariury, H.; Fandel, J.; Bachl, S.; Ang, K.K.; Markossian, S.; Wilson, C.G.; Braun, B.S.; Popescu, B.; Wohlfeil, M.; Beckman, K.; et al. Venetoclax and Dinaciclib Elicit Synergistic Preclinical Efficacy against Hypodiploid Acute Lymphoblastic Leukemia. Haematologica 2023, 108, 1272–1283.
- Summers, R.J.; Jain, J.; Vasileiadi, E.; Smith, B.; Chimenti, M.L.; Yeung, T.Y.; Kelvin, J.; Wang, X.; Frye, S.V.; Earp, H.S.; et al. Therapeutic Targeting of MERTK and BCL-2 in T-Cell and Early T-Precursor Acute Lymphoblastic Leukemia. Cancers 2022, 14, 6142.
- Richter, A.; Fischer, E.; Holz, C.; Schulze, J.; Lange, S.; Sekora, A.; Knuebel, G.; Henze, L.; Roolf, C.; Murua Escobar, H.; et al. Combined Application of Pan-AKT Inhibitor MK-2206 and BCL-2 Antagonist Venetoclax in B-Cell Precursor Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 2771.
More
Information
Subjects:
Hematology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




