You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rui Medeiros | -- | 1928 | 2024-02-06 22:58:50 | | | |
| 2 | Jessie Wu | Meta information modification | 1928 | 2024-02-07 01:27:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tavares, V.; Marques, I.S.; De Melo, I.G.; Assis, J.; Pereira, D.; Medeiros, R. Prevention and Diagnosis of Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/54820 (accessed on 25 December 2025).
Tavares V, Marques IS, De Melo IG, Assis J, Pereira D, Medeiros R. Prevention and Diagnosis of Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/54820. Accessed December 25, 2025.
Tavares, Valéria, Inês Soares Marques, Inês Guerra De Melo, Joana Assis, Deolinda Pereira, Rui Medeiros. "Prevention and Diagnosis of Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/54820 (accessed December 25, 2025).
Tavares, V., Marques, I.S., De Melo, I.G., Assis, J., Pereira, D., & Medeiros, R. (2024, February 06). Prevention and Diagnosis of Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/54820
Tavares, Valéria, et al. "Prevention and Diagnosis of Ovarian Cancer." Encyclopedia. Web. 06 February, 2024.
Copy Citation
Ovarian cancer (OC) is the female genital malignancy with the highest lethality. Patients present a poor prognosis mainly due to the late clinical presentation allied with the common acquisition of chemoresistance and a high rate of tumour recurrence. Effective screening, accurate diagnosis, and personalised multidisciplinary treatments are crucial for improving patients’ survival and quality of life.
ovarian neoplasms
biomarkers
early detection of cancer
1. Disease Aetiology and Prevention
Various aetiological determinants are thought to impact ovarian tumourigenesis showing heterogeneity depending on tumour histology [1][2][3]. The most impactful ones are advanced age, genetic predisposition, and a family history of cancer. These factors are particularly related to continuous ovulation, hormonal changes, cumulative genetic damage, and chronic inflammation [1][4][5][6]. Ovarian tumours are rare among young women, particularly those under the age of 30. After the age of 50, especially following menopause, OC risk drastically increases, with the average diagnosis occurring between 50 and 70 years [7]. Concerning the genetic component, OC is one of the most heritable tumours, mainly linked to germline genetic mutations associated with the hereditary breast and OC syndrome (predominantly mutations in BRCA1 and BRCA2) and hereditary nonpolyposis colorectal cancer syndrome (mutations in MLH1, MSH2, MSH6, and PMS2) [4][8]. Thus, a family history of breast, ovarian, and colorectal tumours, particularly at young ages, could be indicative of a high risk of OC onset [9][10]. For instance, while the risk of developing OC in the general population is <2%, women with BRCA1 and BRCA2 mutations have an overall lifetime risk of 20–40% and 10–20%, respectively [11].
Despite inconsistent data, reproductive factors such as early menarche, late menopause onset, long-term hormone replacement therapy, and nulliparity also constitute risk factors [12][13][14][15][16]. In opposition, pregnancy, breastfeeding, and the use of oral contraceptives are considered to be protective factors [17][18][19]. The impact of these determinants on the predisposition for OC is commonly attributed to the cumulative number of ovulatory cycles, as fewer cycles are associated with a lower OC risk [7][20][21]. Also, oestrogen exposure could be a contributing factor [22][23]. Other important risk determinants include lifestyle-related factors (e.g., diet, tobacco use, high body mass index, and obesity), a history of gynaecological conditions (e.g., endometriosis, ovarian cysts, and pelvic inflammatory disease), a personal history of endometrial, breast or colorectal cancers and ethnicity [24][25][26][27].
Identifying predisposing factors for OC development is important for tailoring prevention measures. However, there is no effective method for OC’s primary prevention. Nonetheless, tubal sterilisation and salpingo-oophorectomy for women at high risk, particularly those with hereditary syndromes, are possible prophylactic options. As such, according to the National Comprehensive Cancer Network (NCCN) guidelines (version 2.2021, 2021), genetic testing should be offered to women with a family history of the disease [28][29][30]. Furthermore, although conflicting, some studies have found that low-dose aspirin and other anti-inflammatory medications may decrease the risk of OC [20][31][32][33].
The secondary prevention of OC, which refers to disease screening, has also been challenging [34][35]. Ideally, an adequate screening exam should be easy to conduct, steadily reliable, inexpensive, and induce minimal discomfort. Importantly, it must have high sensitivity and specificity. For instance, an adequate test to screen for OC should have a sensitivity and specificity superior to 75% and 99.6%, respectively, to reach a positive predictive value (PPV) of 10% [36]. Also, a suitable exam should target the subpopulation with the highest prevalence of this condition of interest to establish an adequate PPV. Lastly, it should improve the morbimortality rates in the target population [34][37]. Several potential methods for OC screening have been reviewed, including serum CA-125 measurement, a transvaginal ultrasound, colour Doppler ultrasonography, and pelvic examination. However, none of them have shown adequate performance in trials involving the general population and high-risk groups [37][38][39][40]. For instance, CA-125 (also known as mucin 16 or MUC16), which is widely used in the clinical setting for OC monitoring, exhibits limited sensitivity in early disease stages. Also, its levels can be elevated in benign conditions such as ovarian cysts and endometriosis [3][41][42][43]. More recently, novel molecular markers have been proposed, including HE4, CA 72-4, CA 19-9, folate receptor alpha (FRα), microRNA profiles, DNA methylation patterns, circulating tumour DNA and antibodies in liquid biopsies, particularly blood and cervical mucus and swabs [42][44][45][46][47][48]. The use of liquid biopsies in disease screening is attractive since they can capture the disease’s heterogeneity through minimally invasive sample collection and at a low cost. However, the tumour material in these biopsies is usually scarce and does not provide information about the tumour’s architecture or its primary site [48]. According to existing data, a multimodal approach combining several tests might be the most effective tool to screen OC accurately [49]. In this context, several multivariate index assays have been proposed to help detect early-stage OC, including the risk of malignancy index (RMI), OVA1, and risk of ovarian malignancy algorithm (ROMA) [50][51][52][53]. Another advancement in this field is the development of new imaging techniques, namely auto-fluorescence and magnetic relaxometry, which could help detect the disease at earlier stages, enabling timely therapeutic intervention and better outcomes [47]. Despite these improvements, screening for asymptomatic and average-risk women is still controversial, given the low prevalence of this disease and the high probability of false-positive findings, which may lead to excessive interventions [54][55]. Consequently, 60–70% of OC patients are diagnosed at advanced stages upon symptom presentation, which, as formerly mentioned, significantly impacts their prognosis [7][56][57]. Of note, the list of possible symptoms encompasses vaginal bleeding, diarrhoea, constipation, abdominal distension allied to pain, eating difficulties, urinary frequency, fatigue, nausea, anorexia, dyspepsia, and early satiety [3]. The time of presentation of these symptoms may vary depending on the histological nature of the disease [58].
Given their implications, education on the risk factors underlying OC onset is crucial to increase patients’ health awareness and self-advocacy.
2. Disease Diagnosis and Prognosis Assessment
Current strategies to diagnose OC include a medical history evaluation combined with the gynaecological exam, serum CA-125 quantification, and imaging tests (transvaginal ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET)), while also demanding a histopathological examination from either a diagnostic biopsy or, if possible, a surgical specimen for a definitive diagnosis and staging [59][60][61]. For MC, the evaluation of the tumour markers CEA and CA 19-9 is also recommended according to the European Society for Medical Oncology (ESMO) 2023 guidelines for OC management [3].
At diagnosis, the International Federation of Gynecology and Obstetrics (FIGO) staging system is one of the most important tools to predict the clinical outcomes of OC patients and evaluate their therapeutical options [62]. This system, first published in 1973 and last revised in 2021, includes four stages, each with subdivisions (Figure 1) [55][62][63]. Ovarian carcinomas can also be subclassified based on histologic grading, with two systems being applied [40]. For non-serous tumours, according to cell architecture, the disease can be deemed as GX (grade not determined), G1 (well differentiated), G2 (moderately differentiated), and G3 (poorly differentiated). On the other hand, serous carcinomas can be categorised as low or high grade based on their distinct cellular characteristics and behaviours [40][63].
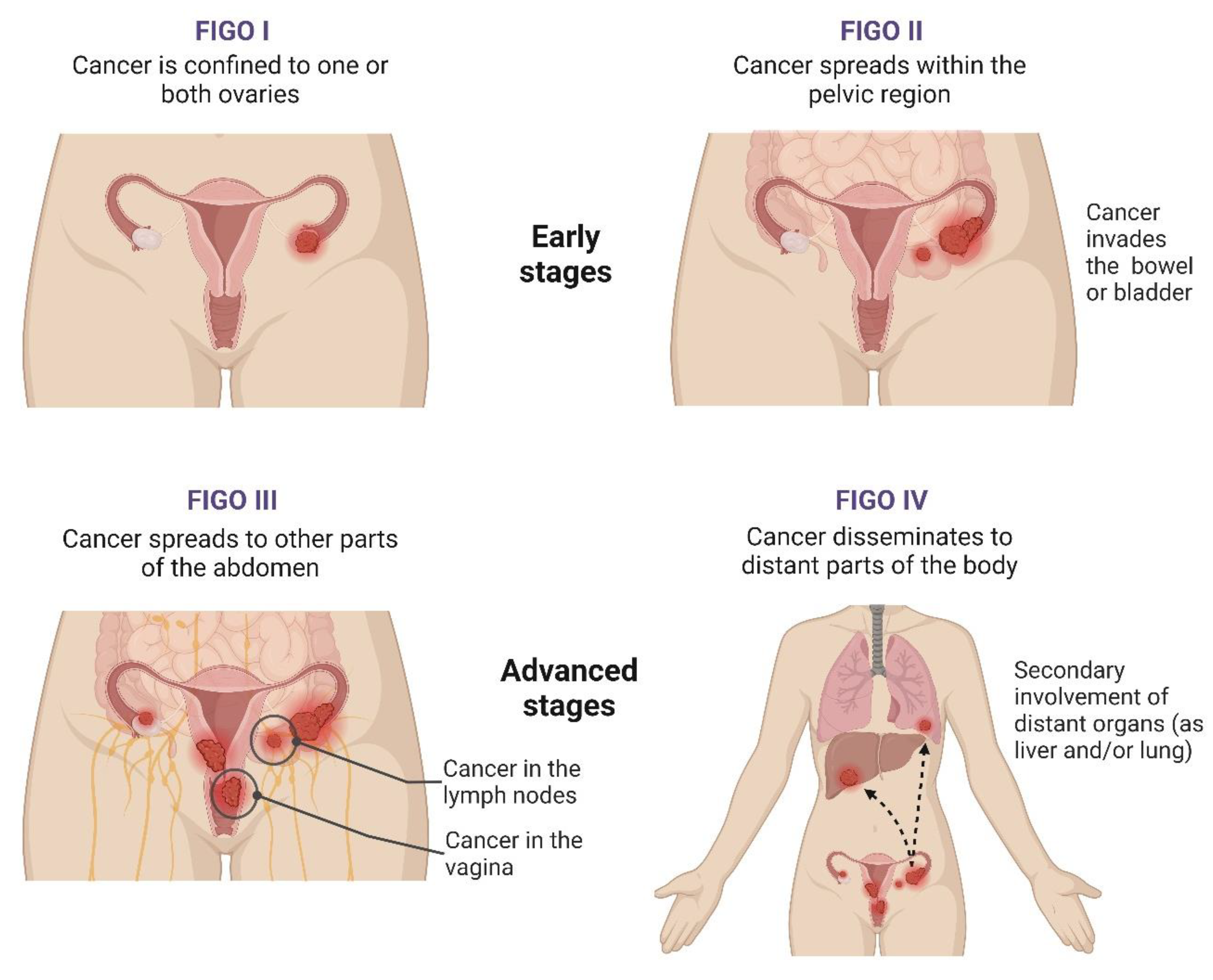
Figure 1. Stages of ovarian cancer. Figure created with BioRender.com (accessed on 28 December 2023). The FIGO staging system for ovarian cancer (OC) includes four stages that address the disease’s extent and severity by evaluating the tumour burden, dissemination within the abdomen, and the secondary involvement of distant organs. Stage I integrates tumours confined to either the ovary (one or both ovaries) or the fallopian tubes, while, at stage II, the tumour has already spread beyond the ovaries or fallopian tubes, with pelvic extension or primary peritoneal cancer. In stage III, OC cells spread to the peritoneum outside the pelvis, and there might be metastasis to the retroperitoneal lymph nodes. Lastly, stage IV is characterised by OC’s dissemination to other body parts beyond the pelvis and abdomen, namely the liver and lungs [63]. Abbreviations: FIGO, International Federation of Gynecology and Obstetrics.
Regarding the prognosis assessment, the FIGO stage, histologic subtype, grade, baseline serum CA-125 levels, the extent of debulking surgery, and chemotherapy schemes are traditionally deemed the most relevant independent prognostic factors of OC. For instance, those with early disease stages, type I tumours and lower baseline CA-125 levels usually have higher survival [7][64][65][66][67][68][69]. However, ongoing research has recently identified several molecular biomarkers associated with OC treatment response and prognosis, including mutations, gene expression patterns, and/or epigenetic changes [70][71][72]. This is particularly relevant given the high heterogeneity that characterises HGSC, with the predominant and most lethal OC subtype accounting for 70% of OC-related deaths [73]. Notably, Tothill et al., (2008) [74] were the first to propose HGSC subtypes based on the following genomic signatures: C1 (high stromal response), C2 (high immune signature), C4 (low stromal response) and C5 (mesenchymal). Next, Kurman and Shih (2010) [75] proposed the classic dualist model—type I vs. type II. Later, in 2011, data on histological structure and gene expression profile from the Cancer Genome Atlas (TCGA) Research Network led to the recognition of four HGSC subtypes: mesenchymal (with a gene expression profile that resembles mesenchymal tissues with increased cell motility and invasiveness), proliferative (displaying a molecular pattern indicative of high cell proliferation and limited inflammatory infiltration), differentiated (with a gene expression profile related to more specialised cell types) and immunoreactive (tumours with high infiltration of immune cells and with a gene expression profile characteristic of immune activation) [76][77]. Although not mutually exclusive, these subgroups correlate with prognosis. According to the “Classification of Ovarian Cancer” (CLOVAR) signature, the mesenchymal subtype is the most lethal with a related five-year OS of 18%, followed by the proliferative, differentiated, and, finally, the immunoreactive subtype, which is associated with a survival rate of 45% [78]. Importantly, these signatures also influence therapy response [79]. Since the proposal of these models, the integrative analysis of tumour (epi)genetic and molecular signatures has more or less confirmed the existence of these four HGSC subtypes with an impact on prognosis and/or treatment response (Table 1). This is anticipated to change OC management by facilitating personalised treatment [72].
Table 1. (Epi)genetic and molecular signatures of high-grade serous ovarian carcinoma (HGSC) with implications for therapy response and patients’ clinical outcomes.
| Authors (Year) | Number of Cases | Methods | HGSC Clusters and/or Main Features | Main Findings | References |
|---|---|---|---|---|---|
| Macintyre et al., (2018) | 132 patients 112 patients (Pan-Cancer Analysis of Whole Genomes) 415 patients (TCGA) |
Genome sequencing | Signatures of copy number variations: Signature 1—telomere shortening and RAS/MAPK activation; Signature 2—tandem duplication; Signature 3—BRCA1/2-related HRR deficiency; Signature 4—whole genome duplication; Signature 5—subclonal catastrophic chromothriptic-like events; Signature 6—focal amplification; Signature 7—non-BRCA1/2-related HRR deficiency. |
Signature 1—platinum-resistant recurrence and poor survival; Signature 2—poor survival; Signatures 3 and 7—prolonged survival; Signatures 4, 5 and 6—unclear implications. |
[80] |
| Harris et al., (2019) | Not reported | Tumour xenografting DNA and RNA NGS DNA fingerprinting Immunohistochemistry |
DNA alternations in genes involved in the ERBB2 pathway. | Deregulation in the ERBB2 pathway—favourable results by combining platinum-based chemotherapy with anti-HER2 drugs. | [81] |
| Li et al., (2019) | Seven patients | Tumour xenografting RNA and whole exome sequencing Immunohistochemistry |
Deregulation of AKT3, HLA-DPA1, PIK3R5 and SAP25 expression; POLR2A and TMEM205 mutations. |
Features associated with the acquisition of chemoresistance to carboplatin and paclitaxel. | [82] |
| McDonald et al., (2019) | 450 patients with chemoresistance (TCGA) | Genome-wide cluster analysis Pathway enrichment analysis |
Cluster 1—growth factor signalling; Cluster 2—cell survival; Cluster 3—cellular senescence. |
Best therapeutic options: Cluster 1—tyrosine kinases or angiokinase inhibitors; Cluster 2—mTOR inhibitors; Cluster 3—deacetylase inhibitors. |
[83] |
| Hao et al., (2021) | Two patients (four matched pair samples of primary and metastatic tumours) |
Single-cell RNA sequencing | Cluster EC1— glycolysis/gluconeogenesis; Cluster EC2—cytokine–cytokine receptor interaction; Cluster EC3—nucleotide and amino acid metabolism; Cluster EC4—immune response Cluster EC5—DNA repair and drug metabolism |
Cluster EC5 may be most aggressive and resistant to chemotherapy and PARP inhibitors. | [84] |
| Li et al., (2021) | 66 tumour cells 568 tumour samples and 7 normal ovary samples (TCGA) |
Single-cell RNA sequencing | Differently expressed genes | Low expression of ANP32E, EGFL6, GPRC5A, PMP22 and STAT1—prolonged survival; Low expression of ANP32E, CYB5R3 and FBXO21—prolonged PFS |
[85] |
Abbreviations: HRR, homologous recombination repair; NGS, next-generation sequencing; PFS, progression-free survival; TCGA, The Cancer Genome Atlas.
References
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299.
- Wentzensen, N.; Poole, E.M.; Trabert, B.; White, E.; Arslan, A.A.; Patel, A.V.; Setiawan, V.W.; Visvanathan, K.; Weiderpass, E.; Adami, H.-O. Ovarian cancer risk factors by histologic subtype: An analysis from the ovarian cancer cohort consortium. J. Clin. Oncol. 2016, 34, 2888.
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.; Pothuri, B.; Ray-Coquard, I.; Tan, D.; Bellet, E.; Oaknin, A. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848.
- Flaum, N.; Crosbie, E.J.; Edmondson, R.J.; Smith, M.J.; Evans, D.G. Epithelial ovarian cancer risk: A review of the current genetic landscape. Clin. Genet. 2020, 97, 54–63.
- Hunn, J.; Rodriguez, G.C. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23.
- Singla, A. Epidemiology and Risk Factors for Ovarian Cancer. In Preventive Oncology for the Gynecologist; Springer: Singapore, 2019; pp. 223–231.
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 2019, 35, 151–156.
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Physician 2009, 80, 609–616.
- Bergfeldt, K.; Rydh, B.; Granath, F.; Grönberg, H.; Thalib, L.; Adami, H.-O.; Hall, P. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: A population-based cohort study. Lancet 2002, 360, 891–894.
- Malander, S.; Rambech, E.; Kristoffersson, U.; Halvarsson, B.; Ridderheim, M.; Borg, Å.; Nilbert, M. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol. Oncol. 2006, 101, 238–243.
- Liao, Y.; Tu, C.; Song, X.; Cai, L. Case report: Analysis of BRCA1 and BRCA2 gene mutations in a hereditary ovarian cancer family. J. Assist. Reprod. Genet. 2020, 37, 1489–1495.
- Pajenga, E.; Rexha, T.; Çeliku, S.; Bejtja, G.; Pisha, M. Hormonal risk factors for ovarian cancer in the Albanian case-control study. Bosn. J. Basic Med. Sci. 2013, 13, 89.
- Budiana, I.N.G.; Angelina, M.; Pemayun, T.G.A. Ovarian cancer: Pathogenesis and current recommendations for prophylactic surgery. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 47.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842.
- Liu, Y.; Ma, L.; Yang, X.; Bie, J.; Li, D.; Sun, C.; Zhang, J.; Meng, Y.; Lin, J. Menopausal hormone replacement therapy and the risk of ovarian cancer: A meta-analysis. Front. Endocrinol. 2019, 10, 801.
- Toufakis, V.; Katuwal, S.; Pukkala, E.; Tapanainen, J.S. Impact of parity on the incidence of ovarian cancer subtypes: A population-based case–control study. Acta Oncol. 2021, 60, 850–855.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet 2008, 371, 303–314.
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V.; Collaborators, M.W.S. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289.
- Feng, L.P.; Chen, H.L.; Shen, M.Y. Breastfeeding and the risk of ovarian cancer: A meta-analysis. J. Midwifery Women’s Health 2014, 59, 428–437.
- Mallen, A.; Soong, T.R.; Townsend, M.K.; Wenham, R.M.; Crum, C.P.; Tworoger, S.S. Surgical prevention strategies in ovarian cancer. Gynecol. Oncol. 2018, 151, 166–175.
- Walker, J.L.; Powell, C.B.; Chen, L.m.; Carter, J.; Bae Jump, V.L.; Parker, L.P.; Borowsky, M.E.; Gibb, R.K. Society of Gynecologic O ncology recommendations for the prevention of ovarian cancer. Cancer 2015, 121, 2108–2120.
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen signaling and its potential as a target for therapy in ovarian cancer. Cancers 2020, 12, 1647.
- Lukanova, A.; Kaaks, R. Endogenous hormones and ovarian cancer: Epidemiology and current hypotheses. Cancer Epidemiol. Biomark. Prev. 2005, 14, 98–107.
- Guo, H.; Guo, J.; Xie, W.; Yuan, L.; Sheng, X. The role of vitamin D in ovarian cancer: Epidemiology, molecular mechanism and prevention. J. Ovarian Res. 2018, 11, 71.
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47.
- Tanha, K.; Mottaghi, A.; Nojomi, M.; Moradi, M.; Rajabzadeh, R.; Lotfi, S.; Janani, L. Investigation on factors associated with ovarian cancer: An umbrella review of systematic review and meta-analyses. J. Ovarian Res. 2021, 14, 153.
- Qu, X.-L.; Fang, Y.; Zhang, M.; Zhang, Y.-Z. Phytoestrogen intake and risk of ovarian cancer: A meta-analysis of 10 observational studies. Asian Pac. J. Cancer Prev. 2014, 15, 9085–9091.
- Hoskins, P.J.; Gotlieb, W.H. Missed therapeutic and prevention opportunities in women with BRCA-mutated epithelial ovarian cancer and their families due to low referral rates for genetic counseling and BRCA testing: A review of the literature. CA A Cancer J. Clin. 2017, 67, 493–506.
- Berliner, J.L.; Cummings, S.A.; Boldt Burnett, B.; Ricker, C.N. Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes—Practice resource of the National Society of Genetic Counselors. J. Genet. Couns. 2021, 30, 342–360.
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102.
- Baandrup, L.; Kjær, S.K.; Olsen, J.; Dehlendorff, C.; Friis, S. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann. Oncol. 2015, 26, 787–792.
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377.
- Baandrup, L.; Faber, M.T.; Christensen, J.; Jensen, A.; Andersen, K.K.; Friis, S.; Kjær, S.K. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: Systematic review and meta-analysis of observational studies. Acta Obstet. Et Gynecol. Scand. 2013, 92, 245–255.
- Raffle, A.E.; Gray, J.M. Screening: Evidence and Practice; Oxford University Press: New York, NY, USA, 2019.
- Temming, L.A.; Macones, G.A. What is prenatal screening and why to do it? Semin. Perinatol. 2016, 40, 3–11.
- Mathis, J.; Jellouli, M.A.; Sabiani, L.; Fest, J.; Blache, G.; Mathevet, P. Ovarian cancer screening in the general population. Horm. Mol. Biol. Clin. Investig. 2019, 41, 20190038.
- Patni, R. Screening for ovarian cancer: An update. J. Mid-Life Health 2019, 10, 3.
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E. Screening for ovarian cancer: US preventive services task force recommendation statement. Jama 2018, 319, 588–594.
- Stirling, D.; Evans, D.G.R.; Pichert, G.; Shenton, A.; Kirk, E.N.; Rimmer, S.; Steel, C.M.; Lawson, S.; Busby-Earle, R.C.; Walker, J. Screening for familial ovarian cancer: Failure of current protocols to detect ovarian cancer at an early stage according to the international Federation of gynecology and obstetrics system. J. Clin. Oncol. 2005, 23, 5588–5596.
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R. Reclassification of serous ovarian carcinoma by a 2-tier system: A gynecologic oncology group study. Cancer 2012, 118, 3087–3094.
- Jacob, F.; Meier, M.; Caduff, R.; Goldstein, D.; Pochechueva, T.; Hacker, N.; Fink, D.; Heinzelmann-Schwarz, V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol. Oncol. 2011, 121, 487–491.
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28.
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265.
- Anastasi, E.; Manganaro, L.; Granato, T.; Benedetti Panici, P.; Frati, L.; Porpora, M.G. Is CA72-4 a useful biomarker in differential diagnosis between ovarian endometrioma and epithelial ovarian cancer? Dis. Markers 2013, 35, 331–335.
- Guo, J.; Yu, J.; Song, X.; Mi, H. Serum CA125, CA199 and CEA combined detection for epithelial ovarian cancer diagnosis: A meta-analysis. Open Med. 2017, 12, 131–137.
- Kurosaki, A.; Hasegawa, K.; Kato, T.; Abe, K.; Hanaoka, T.; Miyara, A.; O’Shannessy, D.J.; Somers, E.B.; Yasuda, M.; Sekino, T. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int. J. Cancer 2016, 138, 1994–2002.
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel approaches to ovarian cancer screening. Curr. Oncol. Rep. 2019, 21, 75.
- Biskup, E.; Wils, R.S.; Hogdall, C.; Hogdall, E. Prospects of improving early ovarian cancer diagnosis using cervical cell swabs. Anticancer Res. 2022, 42, 1–12.
- Cragun, J.M. Screening for ovarian cancer. Cancer Control 2011, 18, 16–21.
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 922–929.
- Bristow, R.E.; Smith, A.; Zhang, Z.; Chan, D.W.; Crutcher, G.; Fung, E.T.; Munroe, D.G. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol. Oncol. 2013, 128, 252–259.
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46.
- Zhang, R.; Siu, M.K.; Ngan, H.Y.; Chan, K.K. Molecular biomarkers for the early detection of ovarian cancer. Int. J. Mol. Sci. 2022, 23, 12041.
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA A Cancer J. Clin. 2019, 69, 184–210.
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78.
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388.
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705.
- Lataifeh, I.; Marsden, D.E.; Robertson, G.; Gebski, V.; Hacker, N.F. Presenting symptoms of epithelial ovarian cancer. Aust. N. Z. J. Obstet. Gynaecol. 2005, 45, 211–214.
- Mustafin, C.; Vesnin, S.; Turnbull, A.; Dixon, M.; Goltsov, A.; Goryanin, I. Diagnostics of Ovarian Tumors in Postmenopausal Patients. Diagnostics 2022, 12, 2619.
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Kemppainen, J.; Hynninen, J.; Virtanen, J.; Seppänen, M. PET/CT for evaluation of ovarian cancer. Semin. Nucl. Med. 2019, 49, 484–492.
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian Cancer, the Revised FIGO Staging System, and the Role of Imaging. Am. J. Roentgenol. 2016, 206, 1351–1360.
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85.
- Cooper, B.C.; Sood, A.K.; Davis, C.S.; Ritchie, J.M.; Sorosky, J.I.; Anderson, B.; Buller, R.E. Preoperative CA 125 levels: An independent prognostic factor for epithelial ovarian cancer. Obstet. Gynecol. 2002, 100, 59–64.
- Meinhold-Heerlein, I.; Hauptmann, S. The heterogeneity of ovarian cancer. Arch. Gynecol. Obstet. 2014, 289, 237–239.
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C.; Bouzbid, S.; Hamdi-Chérif, M.; Zaidi, Z.; et al. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413.
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. Int. J. Gynecol. Obstet. 2006, 95, S161–S192.
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061.
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586.
- Opławski, M.; Średnicka, A.; Niewiadomska, E.; Boroń, D.; Januszyk, P.; Grabarek, B.O. Clinical and molecular evaluation of patients with ovarian cancer in the context of drug resistance to chemotherapy. Front. Oncol. 2022, 12, 954008.
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.; Chen, S.; Elatre, W.; Gilks, B. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 2020, 31, 1240–1250.
- Wilczyński, J.; Paradowska, E.; Wilczyński, M. Personalization of Therapy in High-Grade Serous Tubo-Ovarian Cancer—The Possibility or the Necessity? J. Pers. Med. 2023, 14, 49.
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679.
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208.
- Kurman, R.J.; Shih, I.-M. The origin and pathogenesis of epithelial ovarian cancer—A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433.
- Network, C.G.A.R. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609.
- Verhaak, R.G.; Tamayo, P.; Yang, J.-Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2012, 123, 517–525.
- Konecny, G.E.; Wang, C.; Hamidi, H.; Winterhoff, B.; Kalli, K.R.; Dering, J.; Ginther, C.; Chen, H.-W.; Dowdy, S.; Cliby, W. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J. Natl. Cancer Inst. 2014, 106, dju249.
- Dion, L.; Carton, I.; Jaillard, S.; Nyangoh Timoh, K.; Henno, S.; Sardain, H.; Foucher, F.; Leveque, J.; de la Motte Rouge, T.; Brousse, S. The landscape and therapeutic implications of molecular profiles in epithelial ovarian cancer. J. Clin. Med. 2020, 9, 2239.
- Macintyre, G.; Goranova, T.E.; De Silva, D.; Ennis, D.; Piskorz, A.M.; Eldridge, M.; Sie, D.; Lewsley, L.-A.; Hanif, A.; Wilson, C. Copy number signatures and mutational processes in ovarian carcinoma. Nat. Genet. 2018, 50, 1262–1270.
- Harris, F.R.; Zhang, P.; Yang, L.; Hou, X.; Leventakos, K.; Weroha, S.J.; Vasmatzis, G.; Kovtun, I.V. Targeting HER 2 in patient-derived xenograft ovarian cancer models sensitizes tumors to chemotherapy. Mol. Oncol. 2019, 13, 132–152.
- Li, L.Y.; Kim, H.J.; Park, S.A.; Lee, S.H.; Kim, L.K.; Lee, J.Y.; Kim, S.; Kim, Y.T.; Kim, S.W.; Nam, E.J. Genetic profiles associated with chemoresistance in patient-derived xenograft models of ovarian cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 1117–1127.
- McDonald, M.E.; Salinas, E.A.; Devor, E.J.; Newtson, A.M.; Thiel, K.W.; Goodheart, M.J.; Bender, D.P.; Smith, B.J.; Leslie, K.K.; Gonzalez-Bosquet, J. Molecular characterization of non-responders to chemotherapy in serous ovarian cancer. Int. J. Mol. Sci. 2019, 20, 1175.
- Hao, Q.; Li, J.; Zhang, Q.; Xu, F.; Xie, B.; Lu, H.; Wu, X.; Zhou, X. Single-cell transcriptomes reveal heterogeneity of high-grade serous ovarian carcinoma. Clin. Transl. Med. 2021, 11, e500.
- Li, Y.; Wang, J.; Wang, F.; Gao, C.; Cao, Y.; Wang, J. Identification of specific cell subpopulations and marker genes in ovarian cancer using single-cell RNA sequencing. BioMed Res. Int. 2021, 2021, 1005793.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
730
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




