
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edward Araujo Júnior | -- | 1206 | 2024-02-05 23:20:20 | | | |
| 2 | Wendy Huang | Meta information modification | 1206 | 2024-02-07 07:45:20 | | |
Video Upload Options
Cytomegalovirus (CMV) is an enveloped DNA virus that, like other members of the herpes virus family, establishes a lifelong latency period after primary infection and becomes resident in monocytes and granulocytes. For this reason, vertical transmission can occur through primary infection, reactivation of the disease, or even contamination with another strain. CMV infection is spread through contact with contaminated bodily secretions (such as urine, saliva, genital secretions, and breast milk) and generally causes few symptoms in immunocompetent individuals, but can cause serious damage in immunosuppressed individuals, including fetuses. CMV infection is the most common congenital infection worldwide, affecting between 0.7% and 1% of all live births. Approximately 11% of infected newborns are symptomatic at birth, and between 30% and 40% of these are at risk of developing long-term neurological sequelae.
1. Introduction
2. Contamination
3. Symptomatology
4. Screening
5. Serologies and Interpretations
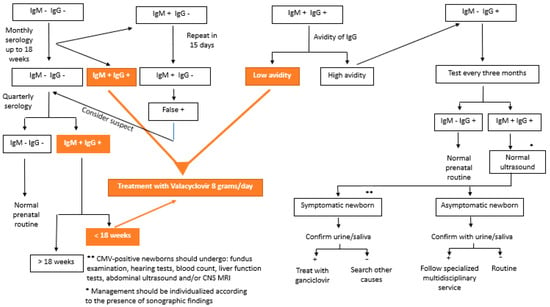
| Type of Infection | Definition |
|---|---|
| Confirmed primary infection | IgG and IgM− previously, showing serum conversion during pregnancy * |
| Presumed primary infection | CMV IgG+, with low avidity ** and IgM+, in the first trimester or CMV IgG and IgM+, with undetermined IgG avidity, with detection of CMV-DNA in at least 1 body fluid (blood, urine, or saliva) during pregnancy |
| False positive | IgM+ and IgG− in paired tests with a difference of at least 2 weeks * |
| Confirmed non-primary infection | CMV IgG+ before pregnancy or CMV IgG+ and IgM− in the 1st trimester |
| Presumed non-primary infection | CMV IgM− and 1- IgG+ before 12 weeks with unknown IgM or 2- Four times increase in IgG titers in paired tests |
| Congenital CMV infection | Detection of CMV (culture) or CMV-DNA via PCR in the newborn’s saliva, urine, or blood obtained up to 3 weeks of age or in the amniotic fluid [2] |
References
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276.
- Swanson, E.C.; Schleiss, M.R. Congenital Cytomegalovirus Infection: New Prospects for Pevention and Therapy. Pediatr. Clin. N. Am. 2013, 60, 335–349.
- Zammarchi, L.; Tomasoni, L.R.; Luizzi, G.; Simonazzi, G.; Dionisi, C.; Mazzarelli, L.L.; Seidenari, A.; Maruotti, G.M.; Ornaghi, S.; Castelli, F.; et al. Treatment with valacyclovir during pregnancy for prevention of congenital cytomegalovirus infection: A real-life multicenter Italian observacional study. Am. J. Obstet. Gynecol. MFM 2023, 5, 101101.
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034.
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. 2014, 22, 44–48.
- Coppola, T.; Mangold, J.F.; Cantrell, S.; Permar, S.R. Impact of Maternal Immunity on Congenital Cytomegalovirus Birth Prevalence and Infant Outcomes: A Systematic Review. Vaccines 2019, 7, 129.
- Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Aragon, D.C.; Duarte, G.; Fowler, K.B.; Boppana, S.; Britt, W.J. Seroconversion for Cytomegalovirus Infection during Pregnancy and Fetal Infection in a Highly Seropositive Population: “The BraCHS Study”. J. Infect. Dis. 2018, 218, 1200–1204.
- Xie, M.; Tripathi, T.; Holmes, N.E.; Hui, L. Serological screening for cytomegalovirus during pregnacy: A sytematic review of clinical practice guidelines and consensus statements. Prenat. Diagns. 2023, 43, 959–967.
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; KaczanowKa, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659.
- Maltezou, P.G.; Kourlaba, G.; Kourkouni, E.; Luck, S.; Blászquez-Gamero, D.; Ville, Y.; Lilleri, D.; Dimopoulou, D.; Karalexi, M.; Papaevangelou, V. Maternal type of CMV infection and sequelae in infants with congenital CMV: Systematic review and meta-analysis. J. Clin. Virol. 2020, 129, 104518.
- Njue, A.; Coyne, C.; Margulis, A.V.; Wang, D.; Marks, M.A.; Russell, K.; Das, R.; Sinha, A. The role of Congenital Cytomegalovirus Infection in Adverse Birth Outcomes: A Review of the Potencial Machanisms. Viruses 2021, 13, 20.
- Dinsmoor, M.J.; Fette, L.M.; Hughes, B.L.; Rouse, D.J.; Saade, G.R.; Reddy, U.M.; Allard, D.; Mallett, G.; Thom, E.A.; Gyamfi-Bannerman, C.; et al. Amniocentesis to diagnose congenital cytomegalovirus infection following maternal primary infection. Am. J. Obstet. Gynecol. MFM. 2022, 4, 100641.
- Chatzakis, C.; Ville, Y.; Makrydimas, G.; Dinas, K.; Zavlanos, A.; Sotiriadis, A. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am. J. Obstet. Gynecol. 2020, 223, 870–883.e11.
- McCarthy, F.P.; Giles, M.L.; Rowlands, S.; Purcell, K.J.; Jones, C.A. Antenatal interventions for preventing the transmission of cytomegalovirus (CMV) from the mother to fetus during pregnancy and adverse outcomes in the congenitally infected infant. Cochrane Database Syst. Rev. 2011, 3, CD008371.
- Seidel, V.; Feiterna-Sperling, C.; Siedentopf, J.; Hofmann, J.; Henrich, W.; Bührer, C.; Bührer, C.; Weizsäcker, K. Intrauterine therapy of cytomegalovirus infection with valganciclovir: A review of the literature. Med. Microbiol. Immunol. 2017, 206, 347–354.
- Leruez-Ville, M.; Ghout, I.; Bussières, L.; Stirnemann, J.; Magny, J.F.; Couderc, S.; Salomon, L.J.; Guilleminot, T.; Aegerter, P.; Benoist, G.; et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am. J. Obstet. Gynecol. 2016, 215, 462.e1–462.e10.
- Shaha-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valacyclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: A double-blind, placebo-controlled trial. Lancet 2020, 396, 779–785.
- Faure-Bardon, V.; Fourgeaud, J.; Stirnemann, J.; Leruez-Ville, M. Secondary prevention of congenital cytomegalovirus infection with valacyclovir following maternal primary infection in early pregnancy. Ultrasound Obstet. Gynecol. 2021, 58, 576–581.
- Egloff, C.; Sibiude, J.; Vauloup-Fellous, C.; Benachi, A.; Bourthry, E.; Biquard, F.; Hawkins-Villarreal, A.; Houhou-Fidouh, N.; Mandelbrot, L.; Vivanti, A.J.; et al. New data on efficacy of valacyclovir in secondary prevention of maternal-fetal transmission of cytomegalovirus. Ultrasound Obstet. Gynecol. 2023, 61, 59–66.
- D’Antonio, F.; Marinceu, D.; Prasad, S.; Khalil, A. Effectiveness and safety of prenatal valacyclovir for congenital cytomegalovirus infection: Systematic review and mata-analysis. Ultrasound Obstet. Gynecol. 2023, 61, 436–444.
- Amir, J.; Chodick, G.; Pardo, J. Revised Protocol for Secondary, Prevention of Congenital Cytomegalovirus Infection with Valacyclovir Following Infection in Early Pregnancy. Clin. Infect. Dis. 2023, 77, 467–471.
- Chatzakis, C.; Shahar-Nissan, K.; Faure-Bardon, V.; Picone, O.; Hadar, E.; Amir, J.; Egloff, C.; Vivanti, A.; Sotiriadis, A.; Leruez-Ville, M.; et al. The effect of valacyclovir on secondary prevention of congenital cytomegalovirus infection, following primary maternal infection acquired periconceptionally or in the first trimester of pregnancy. Na individual patient data meta-analysis. Am. J. Obstet. Gynecol. 2023. online ahead of print.
- Chatzakis, C.; Sotiriads, A.; Dinas, K.; Ville, Y. Neonatal and long-term outcomes of infants with congenital cytimegalovirus infection and negativa amniocentesis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2023, 61, 158–167.
- Fabbri, E.; Revello, M.G.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Rustico, M.; Nicolini, U.; Ferrazzi, E.; et al. Prognostic markers of symptomatic congenital human cytomegalovirus infection in fetal blood. BJOG 2011, 118, 448–456.
- Kyriakopoulou, A.; Serghiou, S.; Dimopoulou, D.; MSc, I.A.; Psaltopoulou, T.; Dinopoulos, A.; Dinopoulos, A.; Papaevangelou, V. Antenatal Imaging and clinical outcome in congenital CMV infection: A field-wide systematic review and meta-analysis. J. Infect. 2020, 80, 407–418.
- Buca, D.; Di Mascio, D.; Rizzo, G.; Giancotti, A.; D’Amico, A.; Leombroni, M.; Makatsarya, A.; Familiari, A.; Liberati, M.; Nappi, L.; et al. Outcome of fetus with congenital cytomegalovirus infection normal ultrasound at diagnosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 551–559.
- Rybak-Krzyszkowska, M.; Górecka, J.; Huras, H.; Staskiewicz, M.; Kondracka, A.; Stanicze, K.J.; Makatsarya, A.; Familiari, A.; Liberati, M.; Nappi, L.; et al. Ultrasonographic Signs of Cytomegalovirus Infection in the Fetus- A Systematic Review of the Literature. Diagnostics 2023, 13, 2397.
- Calvert, A.; Vandrevala, T.; Parsons, R.; Barber, V.; Book, A.; Book, G.; Carrington, D.; Carrington, D.; Greening, V.; Griffiths, P.; et al. Changing knowledge, atitudes and behaviours towards cytomegalovirus in pregnancy through film-based antenatal education: A feasibility randomised controlled trial of a digital educational intervention. BMC Pregnancy Childbirth 2021, 21, 565.
- Hamilton, S.T.; Zuylen, W.; Shand, A.; Scott, G.M.; Naing, Z.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: A systematic review. Rev. Med. Virol. 2014, 24, 420–433.
- Price, S.M.; Bonilla, E.; Zador, P.; Lecis, D.M.; Kilgo, C.L.; Cannon, M.J. Educating women about cytomegalovirus: Assessment of health education materials through a web-based survey. BMC Women’s Health 2014, 14, 144.
- Khalil, A.; Sotiriadis, A.; Chaoui, R.; da Silva Costa, F.; D’Antonio, F.; Heath, P.T.; Jones, C.; Malinger, G.; Odibo, A.; Prefumo, F.; et al. ISUOG Practice Guidelines: Role of ultrasound in congenital infection. Ultrasound Obstet. Gynecol. 2020, 56, 128–151.
- Revello, M.G.; Lazzarott, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A Randomized of hyperimmune Globulin to Prevent Congenital Cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326.
- Barber, V.; Calvert, A.; Vandrevala, T.; Star, C.; Khalil, A.; Griffhs, P.; Heath, P.T.; Jones, C.E. Prevention of Acquisition of Cytomegalovirus Infection in Pregnancy through Hygiene-based Behavioral Interventions: A Systematic Review and Gap Analysis. Pediatr. Infect. Dis. J. 2020, 39, 949–954.
- Devlieger, R.; Buxmann, H.; Nigro, G.; Enders, M.; Jückstock, J.; Siklós, P.; Wartenberg-Demand, A.; Schüttrumpf, J.; Schütze, J.; Rippel, N.; et al. Serial Monitoring and Hyperimmunoglobilin versus of Care to Prevent Congenital Cytomegalovirus Infection: A Phase III Randomized Trial. Fetal Diagn. Ther. 2021, 48, 611–623.
- El-Quhayri, A.E.; Ghozy, S.; Abbas, A.S.; Dibas, M.; Dahy, A.; Mahmoud, A.R.; Afifi, A.M.; El-Khazragy, N. Hyperimmunoglobulin therapy for the prevention and treatment of congenital cytomegalovirus: A systematic review and meta-analysis. Expert. Rev. Anti Infect. Ther. 2021, 19, 661–669.
- Fitzpatrick, A.; Cooper, C.; Vasilunas, N.; Ritchie, B. Describing the Impact of Maternal Hyperimmune Globilin and Valacyclovir in Pregnacy: A Systematic Review. Clin. Infect. Dis. 2022, 75, 1467–1480.
- Hughes, B.L.; Clifton, R.G.; Rouse, D.J.; Saade, G.R.; Dinsmoor, M.J.; Reddy, U.M.; Pass, R.; Allard, D.; Mallett, G.; Fette, L.M.; et al. A Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2021, 385, 436–444.
- Benou, S.; Dimitriou, G.; Papaevangelou, V.; Gkentzi, D. Congenital cytomegalovirus infection: Do pregnant women and healthcare providers know enough? A systematic review. J. Matern. Fetal Neonatal Med. 2022, 35, 6566–6575.
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus infection. Semin. Perinatol. 2018, 42, 149–154.
- Diaz-Decaro, J.; Myers, E.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O. A systematic literature review on the humanistic burden of cytomegalovirus. Curr. Med. Opin. 2023, 39, 739–750.
- Hutton, J.; Rowan, P.J. Vertical Transmission and Discordance of Cytomegalovirus in Twin Pregnancies. Front. Cell Infect. Microbiol. 2021, 11, 676988.
- Balegamire, S.J.; McClymont, E.; Croteau, A.; Dodin, P.; Gantt, S.; Besharati, A.A.; Renaud, C.; Mâsse, B.; Boucoiran, I. Prevalence, incidence, and risk factors associated with cytomegalovirus infection in healthcare and childcare worker: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 131.
- Shears, A.; Yan, G.; Mortimer, H.; Cross, E.; Sapuan, S.; Kadambari, S.; Luck, S.; Heath, P.T.; Walter, S.; Fidler, K.J. Vestibular and balance dysfunction in children with congenital CMV: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 630–636.
- Périllaud-Dubois, C.; Belhadi, D.; Laouénan, C.; Mandelbrot, L.; Picone, O.; Vauloup-Fellous, C. Current practices of management of maternal and congenital Cytomegalovirus infection during pregnancy after a maternal primary infection occurring in first trimester of pregnancy: Systematic review. PLoS ONE. 2021, 16, e0261011.
- Hu, X.; Hu, W.; Sun, X.; Chen, L.; Luo, X. Transmission of cytomegalovirus via breast milk in low birth weight and premature infants: A systematic review and meta-analysis. BMC Pediatr. 2021, 21, 520.
- Zhang, L.; Li, Z.; Han, X.; Du, H.; Cao, Y.; Liu, Y.; Wang, W. Association between Congenital Cytomegalovirus Infection and Brain Injury in Neonates: A Meta-analysis of Cohort Studies. Behav. Neurol. 2021, 2021, 9603660.
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries with Universal Screening: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e2120736.
- Vos, B.; Noll, D.; Whittingham, J.; Pigeon, M.; Bagatto, M.; Fitzpatrick, E.M. Cytomegalovirus-A Risk Factor for Childhood Hearing Loss: A Systematic Review. Ear Hear. 2021, 42, 1447–1461.
- D’Amico, A.; Buca, D.; Rizzo, G.; Khalil, A.; Silvi, C.; Makatsariya, A.; Nappi, L.; Liberati, M.; D’Antonio, F. Outcome of fetal echogenic bowel: A systematic review and meta-analysis. Prenat. Diagn. 2021, 41, 391–399.
- Romero Starke, K.; Kofahl, M.; Freiberg, A.; Schubert, M.; Groß, M.L.; Schmauder, S.; Hegewald, J.; Kämpf, D.; Stranzinger, J.; Nienhaus, A.; et al. The risk of cytomegalovirus infection in daycare workers: A systematic review and meta-analysis. Int. Arch. Occup. Environ. Health 2020, 93, 11–28.
- Riga, M.; Korres, G.; Chouridis, P.; Naxakis, S.; Danielides, V. Congenital cytomegalovirus infection inducing non-congenital sensorineural hearing loss during childhood; a systematic review. Int. J. Pediatr. Otorhinolaryngol. 2018, 115, 156–164.
- Zheng, Q.Y.; Huynh, K.T.; van Zuylen, W.J.; Craig, M.E.; Rawlinson, W.D. Cytomegalovirus infection in day care centres: A systematic review and meta-analysis of prevalence of infection in children. Rev. Med. Virol. 2019, 29, e2011.
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982.
- Schlesinger, Y.; Halle, D.; Eidelman, A.I.; Reich, D.; Dayan, D.; Rudensky, B.; Raveh, D.; Branski, D.; Kaplan, M.; Shefer, V.; et al. Urine polymerase chain reaction as a screening tool for the detection of congenital cytomegalovirus infection. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F371–F374.
- Dogan, Y.; Yuksel, A.; Kalelioglu, I.H.; Has, R.; Tatli, B.; Yildirim, A. Intracranial ultrasound abnormalities and fetal cytomegalovirus infection: Report of 8 cases and review of the literature. Fetal Diagn. Ther. 2011, 30, 141–149.
- Leruez-Ville, M.; Ville, Y. Fetal cytomegalovirus infection. Best. Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 97–107.
- Rawlinson, W.D.; Hamilton, S.T.; van Zuylen, W.J. Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Curr. Opin. Infect. Dis. 2016, 29, 615–624.
- Gunkel, J.; van der Knoop, B.J.; Nijman, J.; de Vries, L.S.; Manten, G.T.R.; Nikkels, P.G.J.; Murk, J.L.; de Vries, J.I.P.; Wolfs, T.F.W. Congenital Cytomegalovirus Infection in the Absence of Maternal Cytomegalovirus-IgM Antibodies. Fetal Diagn. Ther. 2017, 42, 144–149.
- Simonazzi, G.; Curti, A.; Cervi, F.; Gabrielli, L.; Contoli, M.; Capretti, M.G.; Rizzo, N.; Guerra, B.; Farina, A.; Lazzarotto, T. Perinatal Outcomes of Non-Primary Maternal Cytomegalovirus Infection: A 15-Year Experience. Fetal Diagn. Ther. 2018, 43, 138–142.
- Gabrani, C.; Mitsikas, D.; Giannakou, K.; Lamnisos, D. Congenital Cytomegalovirus Infection and Ophthalmological Disorders: A Systematic Review. J. Pediatr. Ophthalmol. Strabismus 2023, 60, 86–94.
- Ross, S.A.; Pati, P.; Jensen, T.L.; Goll, J.B.; Gelber, C.E.; Singh, A.; McNeal, M.; Boppana, S.B.; Bernstein, D.I. Cytomegalovirus Genetic Diversity Following Primary Infection. J. Infect. Dis. 2020, 221, 715–720.
- Choodinatha, H.K.; Jeon, M.R.; Choi, B.Y.; Lee, K.N.; Kim, H.J.; Park, J.Y. Cytomegalovirus infection during pregnancy. Obstet. Gynecol. Sci. 2023, 66, 463–476.




