Cytomegalovirus (CMV) is an enveloped DNA virus that, like other members of the herpes virus family, establishes a lifelong latency period after primary infection and becomes resident in monocytes and granulocytes. For this reason, vertical transmission can occur through primary infection, reactivation of the disease, or even contamination with another strain. CMV infection is spread through contact with contaminated bodily secretions (such as urine, saliva, genital secretions, and breast milk) and generally causes few symptoms in immunocompetent individuals, but can cause serious damage in immunosuppressed individuals, including fetuses. CMV infection is the most common congenital infection worldwide, affecting between 0.7% and 1% of all live births. Approximately 11% of infected newborns are symptomatic at birth, and between 30% and 40% of these are at risk of developing long-term neurological sequelae.
- cytomegalovirus
- pregnancy
- transmission
- serology
- treatment
- contamination
- symptom
- screening
1. Introduction
2. Contamination
CMV contamination occurs through direct contact of the mucous membranes with contaminated body fluids, such as urine, saliva, blood, genital secretions, tears, contaminated breast milk, solid organ transplants, and stem cells [8][9][30][8,9,30]. The major risk factor for maternal infection is contact with children younger than 2 years, who can shed the virus in saliva and urine for up to 24 months [1][8][50][1,8,50]. Another significant route is sexual transmission [1]. There are 3 types of infection: primary, when the mother has previously tested negative for CMV (IgG and IgM) and seroconversion occurs during pregnancy; reactivation of latent virus; and contamination with a new strain in patients with previous contact, the last two of which are considered non-primary [1][2][3][4][1,2,3,4]. All 3 types of infection can lead to vertical transmission [1][2][3][4][8][30][59][1,2,3,4,8,30,59]. An interesting finding from a systematic review of the literature is that although the rate of CMV infection is higher among childcare workers, the rate is not high among healthcare workers. This finding may suggest that extra care is not needed for pregnant women who belong to the latter category [42].3. Symptomatology
CMV infection generally causes minimal or no symptoms in immunocompetent individuals, but can cause serious illness in immunosuppressed individuals (HIV-positive, transplant patients, immunosuppressant users, and fetuses) [1][9][1,9]. In immunosuppressed individuals, viral replication tends to be uncontrolled, which is associated with viremia and dissemination to several organs and can lead to pneumonitis, hepatitis, retinitis, or gastroenteritis [4][9][4,9].4. Screening
In 2023, a systematic review of the literature carried out by Xie et al. [8] on the existence of guidelines and consensuses for CMV screening during pregnancy found that as of June 2022, none of the 13 included studies suggested universal screening. Eight guidelines and 2 consensuses were against universal testing in this population. The UK’s Royal College of Obstetricians and Gynaecologists recommends universal screening for research purposes only, while the Society of Obstetricians and Gynaecologists of Canada accepts universal screening if the IgG avidity test is available. Five guidelines recommend targeted screening only for patients at high risk of infection, i.e., pregnant women who have children up to 3 years of age or who work in daycare centers [8]. However, the guidelines differ on how this testing should be performed, noting 2 types of approaches: the first using IgG, IgM, and IgG avidity testing, and the second using only specific IgG testing. The study by Xie et al. [8] was limited to English language guidelines, and 10 others were excluded due to translation difficulties. According to Fowler et al. [9] in a systematic review of the literature published in 2022, the rate of seroprevalence of IgG immunoglobulin for CMV in women of reproductive age varies between countries and continents, with 45.6–95.7% in Europe, 60.2% in Japan, 58.3–94.5% in Latin America, and 24.6–81% in North America. Seroprevalence increases with age and is higher in developing countries than in developed countries. The same study found a heterogeneous prevalence of IgM immunoglobulin for CMV in women of reproductive age: Europe, 1–4.6%; North America, 2.3–4.5%; Japan, 0.8%; and Latin America, 0–0.7% [9].5. Serologies and Interpretations
CMV testing can be performed by testing for specific antibodies (IgG, IgM, and IgG avidity) or by detecting cytomegalovirus DNA in body fluids (blood, urine, and saliva) [3][56][3,56]. In 2020, Maltezou et al. [10] suggested interpreting combinations of the results of these serologies in the case of fetal infection, as shown in Table 1. Figure 1 shows the flowchart of serology screening of intrauterine CMV infection until 14 weeks of gestation.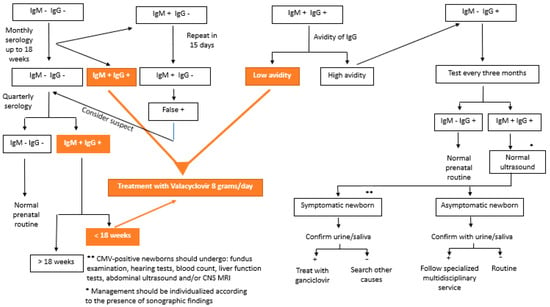
| Type of Infection | Definition |
|---|---|
| Confirmed primary infection | IgG and IgM− previously, showing serum conversion during pregnancy * |
| Presumed primary infection | CMV IgG+, with low avidity ** and IgM+, in the first trimester or CMV IgG and IgM+, with undetermined IgG avidity, with detection of CMV-DNA in at least 1 body fluid (blood, urine, or saliva) during pregnancy |
| False positive | IgM+ and IgG− in paired tests with a difference of at least 2 weeks * |
| Confirmed non-primary infection | CMV IgG+ before pregnancy or CMV IgG+ and IgM− in the 1st trimester |
| Presumed non-primary infection | CMV IgM− and 1- IgG+ before 12 weeks with unknown IgM or 2- Four times increase in IgG titers in paired tests |
| Congenital CMV infection | Detection of CMV (culture) or CMV-DNA via PCR in the newborn’s saliva, urine, or blood obtained up to 3 weeks of age or in the amniotic fluid [2] |
