
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aknur Turgumbayeva | -- | 2971 | 2024-02-05 16:04:27 | | | |
| 2 | Fanny Huang | Meta information modification | 2971 | 2024-02-07 06:38:15 | | |
Video Upload Options
Since ancient times, various scientists and doctors have utilized different herbs to heal diseases. Due to the rise in drug resistance and the negative effects of chemosynthetic drugs, researchers and the general public around the world have become more interested in medicinal herbs and plant metabolites/extracts. This is due to its non-toxicity and its several health benefits when used to treat diseases in clinical and medical settings. Ocimum basilicum is one such plant, possessing a wide range of bioactive phytochemicals including alkaloids, phenolics, flavonoids, tannins, saponins, reducing sugars, cardiac glycosides, steroids and glycosides, as well as complex pharmacological activities, including anti-inflammatory, antifungal, antibacterial, antioxidant, wound healing and antiviral properties.
1. Introduction

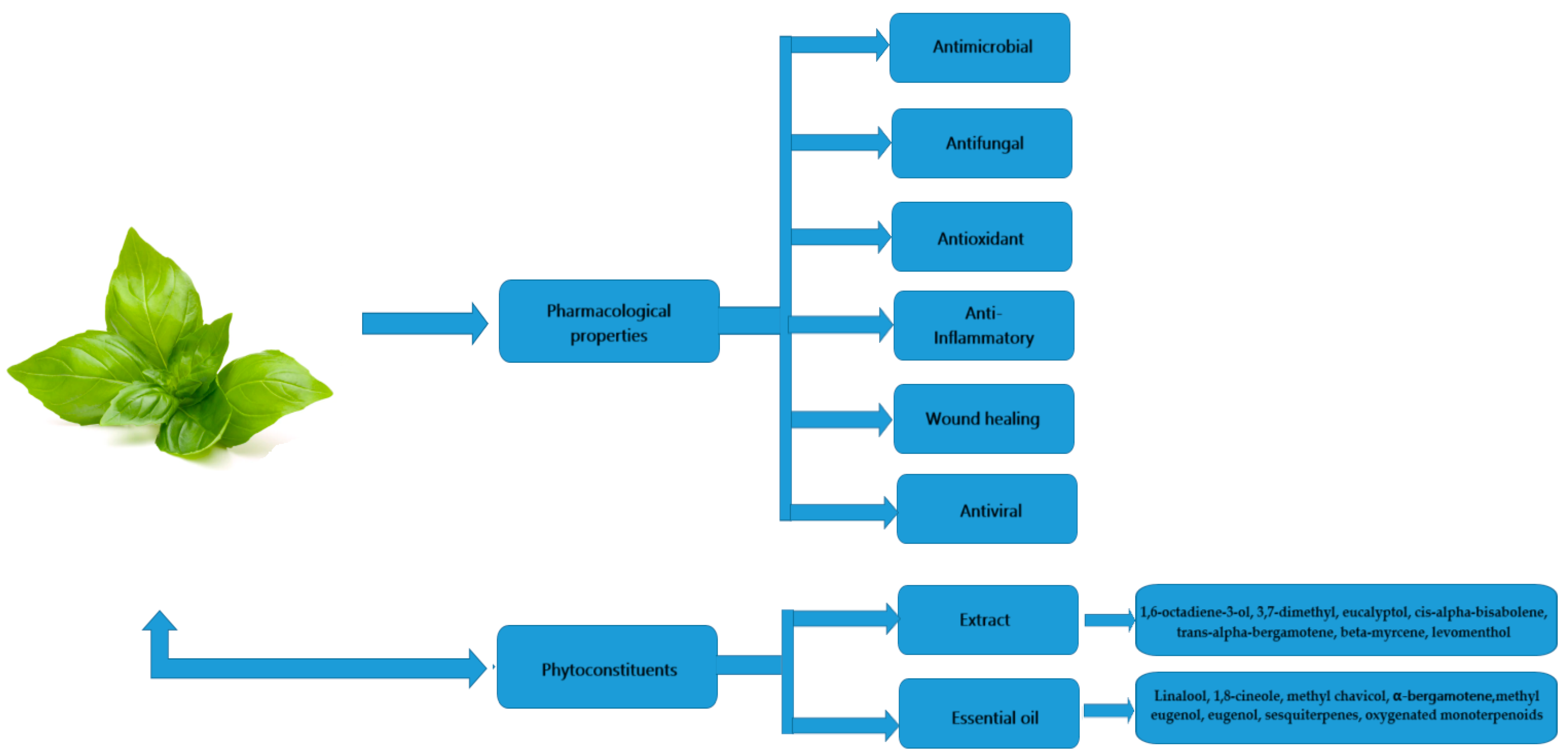
2. Phytoconstituents
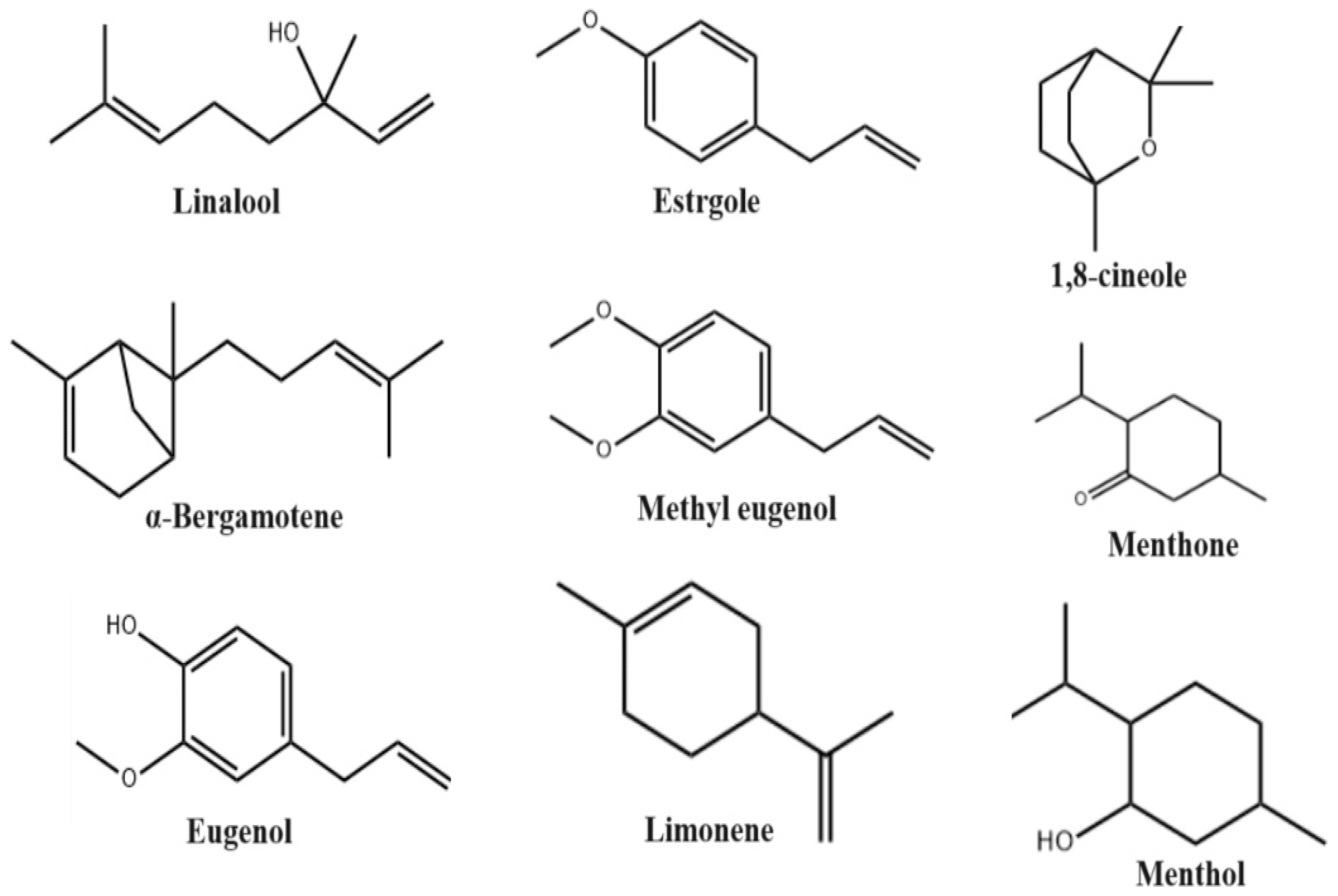
| Extracts | Plant Part | Method | Biological Active Compounds | Pharmacology Activity | Country | Ref. |
|---|---|---|---|---|---|---|
| Essential oil | Leaves, seed, root | GC/MS | Menthone (33.1%), oxygenated monoterpenoids (77.8%), estragole (21.5%), oxygenated monoterpenes (75.3%), isoneomenthol (7.5%), transcaryophyllene (2.2%), menthol (6.1%), limonene (1.5%), pulegone (3.7%), sesquiterpene hydrocarbons (8.8%), trans-β-farnesene (1.1%), germacrene D (1.4%), α-amorphene (1.1%), menthyl acetate (5.6%), α-cadinol (2.9%), methyl eugenol (1%), sesquiterpenoids (12.8%). | Antioxidant, Antimicrobial, Ani-Inflammatory |
Iran | [32] |
| Essential oil | Leaves, seed | GC/MS | Bolloso Napoletano: linalool (47.75%), 1,8-cineole (10.23%), methyl chavicol (20.21%); Foglie di Lattuga: linalool (48.65%), 1,8-cineole (12.59%), methyl chavicol (18.55%). Thai Siam: linalool (36.60%), methyl chavicol (7.50%), (E)-methyl cinnamate (21.90%). | Antioxidant, Anti-Inflammatory, Antiviral |
Poland | [33] |
| Etanolic, Metanolic |
Stem, seed | HPLC, GC/MS | 1,6-octadiene-3-ol, 3,7-dimethyl (29.49%), eucalyptol (3.31%), cis-alpha-bisabolene (1.92%), trans-alpha-bergamotene (5.32%), beta-myrcene (1.11%), levomenthol (1.81%). | Antimicrobial, Antioxidant | South Africa | [34] |
| Etanolic, Metanolic |
Leaves, seed, root | HPLC, GC/MS |
1,8-cineole (10.56%), linalool 48.4%, methyl chavicol 14.3%, α-bergamotene 27%, oxygen monoterpenes (57.42%), β-bisabolol 4.1%, methyl eugenol (10.09%), stragol (55.95%), sesquiterpene hydrocarbons (6.9%). | Antioxidant, Anti-Inflammatory, Antifungal |
Egypt | [35] |
| Essential oil | Leaves | GC/MS | Linalyl acetate (19.1%), linalool (52.1%). Aliphatic compounds (9980–17,929 nanograms per gram fresh weight), including (E)-2-hexenal: 1519–1991, (Z)-3-hexenal (4991–10731), (E)-2-hexen-1-ol: 75–144, (Z)-3-hexen-1-ol: 1436–2219, n-hexanol: 73 –175, 1-octen-3-ol: 1610–2689, (Z)-3-hexenyl acetate: 54–99; eugenol (66,142–131,926). α-pinene: 875–1198, camphene: 153–295, β-pinene: 1780–2771, 2-carene: 42–142, myrcene: 2770–3030, limonene: 712–870, 1,8-cineole: 26,640–52,799, 3-carene: 41–48, linalool: 42,726–65,033, bornyl acetate: 332–1163, camphor: 164–463, tepinen-4-ol: 185–364, eugenol: 945 –1948, α-terpineol: 159–310, α-bergamotene: 202–406 and (E,E)-α farnesene: 32–65), α-humulene: 141–538, caryophyllene: 641–1432. | Wound Healing, Antiviral, Antimicrobial |
Algeria | [36][37] |
| Essential oil | Leaves | HPLC, GC/MS | Linalool and 1,8-cineole. | Antimicrobial and Antioxidant |
Serbia | [38] |
| Essential oil | Leaves, stem | HPLC, GC/MS | Limonene (30.9%), p-cymene (2.6%), linalool (18.9%), thymol (6.5%), B-phellandrene (15.3%), O-cardinol (2.6%). | Antimicrobial and Antioxidant |
Cameroon | [39] |
| Etanolic, n-hexane |
Leaves, stem | TLC, HPLC | Estragole (>35.71%), trans-α-bergamotene (>0.83%), (E)-β-ocimene (>1.47%), eucalyptol (>0.25%), τ-cadinol (>0.41%). | Antimicrobial and Antioxidant |
Malaysia | [40] |
| Essential oil | Leaves | FT-IR, GC/MS | Eugenol (61.76%), [2-methyl-4-(1))-propyl)phenoxy]silane (2.01%), 2,3-dihydroxypropyl elaidate (5.10%), isopropyl palpitate (11.36%), 2-methoxy-4-(1-propyl)phenol (2.65%), α-cubene (3.85%), vanillin (1.27%), 1-methyl-3-(1-methyl)benzene (1.73%), 1,4-diethylbenzene (1.03%), hexadecanoic acid methyl ester (2.51%). | Wound Healing | Bangladesh | [41] |
| Essential oil | Leaves | HPLC | Methyleugenol (15.5%), patchoulan (6.7%), 2-phenyl-1-hexanol (14.0%), o-nitrocumene (14.0%), 2-methyl- 3,5-dodecadiine (14.0%), 1-(4,5-dimethyl-2-nitrophenyl)-1H-tetraazole (14.0%). | Antimicrobial and Antioxidant |
Nigeria | [42] |
3. Antibacterial Activity
| Tested Microorganism | Ethanolic Extract |
Methanolic Extract |
Aqueous Extract |
Acetone Extract | Linalool | Ref. |
|---|---|---|---|---|---|---|
| Diameter of inhibition zone (mm) | ||||||
| S. aureus | 20.4 ± 1.0 | 26.9 ± 1.2 | 24.1 ± 1.2 | 21.2 ± 1.2 | 26.1 ± 1.1 | [44][45][46] |
| P. multocida | 24.4 ± 1.1 | 25.3 ± 1.1 | 23.2 ± 1.4 | 22.2 ± 1.3 | 24.0 ± 1.0 | [43] |
| B. subtilis | 13.2 ± 0.8 | 19.5 ± 1.1 | 13.5 ± 0.8 | 11.4 ± 0.6 | 16.2 ± 1.0 | [44] |
| E. coli | 13.6 ± 0.8 | 22.3 ± 1.0 | 18.4 ± 1.0 | 16.1 ± 1.0 | 18.0 ± 0.9 | [44] |
| M. mucedo | 19.4 ± 1.1 | 21.4 ± 1.0 | 17.7 ± 1.3 | 15.2 ± 0.7 | 11.7 ± 0.7 | [43] |
| A. niger | 21.6 ± 1.2 | 23.3 ± 0.8 | 20.4 ± 1.2 | 18.4 ± 1.2 | 18.7 ± 0.7 | [43] |
| F. solani | 13.6 ± 0.8 | 11.2 ± 0.6 | 9.7 ± 0.6 | 11.1 ± 0.9 | 9.7 ± 0.6 | [43] |
| R. solani | 17.2 ± 1.0 | 17.6 ± 1.0 | 16.6 ± 1.0 | 14.3 ± 1.1 | 13.6 ± 0.8 | [43] |
| B. theobromae | 13.5 ± 0.8 | 17.3 ± 0.8 | 14.3 ± 0.8 | 12.3 ± 0.7 | 10.3 ± 0.6 | [43] |
| Minimum inhibitory concentration (mg/mL) | ||||||
| S. aureus | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 1.4 ± 0.0 | 0.3 ± 0.0 | [44][45][46] |
| P. multocida | 1.5 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.0 | 0.4 ± 0.0 | [43] |
| B. subtilis | 0.06 ± 0.1 | 0.03 ± 0.1 | 2.0 ± 0.1 | 2.6 ± 0.1 | 0.9 ± 0.0 | [44] |
| E. coli | 2.2 ± 0.1 | 4.5 ± 0.2 | 2.7 ± 0.1 | 3.2 ± 0.2 | 1.0 ± 0.1 | [44] |
| M. mucedo | 2.0 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.1 | 0.9 ± 0.0 | [43] |
| A. niger | 3.0 ± 0.2 | 5.0 ± 0.3 | 2.9 ± 0.2 | 4.3 ± 0.2 | 1.5 ± 0.1 | [43] |
| F. solani | 2.7 ± 0.1 | 4.9 ± 0.2 | 3.2 ± 0.2 | 3.6 ± 0.2 | 1.6 ± 0.1 | [43] |
| R. solani | 2.3 ± 0.1 | 4.6 ± 0.2 | 2.9 ± 0.2 | 4.1 ± 0.2 | 1.1 ± 0.0 | [43] |
| B. theobromae | 3.8 ± 0.2 | 5.1 ± 0.3 | 4.6 ± 0.2 | 4.9 ± 0.3 | 1.9 ± 0.1 | [43] |
4. Antifungal Activity
References
- Salehi, B.; Ata, A.; Anil Kumar, V.N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551.
- Tesfahuneygn, G.; Gebreegziabher, G. Medicinal Plants Used in Traditional Medicine by Ethiopians: A Review Article. J. Resp. Med. Lung Dis. 2019, 4, 1040.
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal Immunomodulators—A Remedial Panacea for Designing and Developing Effective Drugs and Medicines: Current Scenario and Future Prospects. Curr. Drug Metab. 2018, 19, 264–301.
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug Metab. 2018, 19, 236–263.
- Dhama, K.; Tiwari, R.; Chakraborty, S.; Saminathan, M.; Kumar, A.; Karthik, K. Evidence Based Antibacterial Potentials of Medicinal Plants and Herbs Countering Bacterial Pathogens Especially in the Era of Emerging Drug Resistance: An Integrated Update. Int. J. Pharmacol. 2014, 10, 1–43.
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738.
- Mahima; Rahal, A.; Deb, R.; Latheef, S.K.; Samad, H.A.; Tiwari, R.; Verma, A.K.; Kumar, A.; Dhama, K. Immunomodulatory and Therapeutic Potentials of Herbal, Traditional/indigenous and Ethnoveterinary Medicines. Pak. J. Biol. Sci. 2012, 15, 754–774.
- Dhama, K.; Tiwari, R.; Khan, R.U.; Chakraborty, S.; Gopi, M.; Karthik, K.; Saminathan, M.; Desingu, P.A.; Sunkara, L.T. Growth Promoters and Novel Feed Additives Improving Poultry Production and Health, Bioactive Principles and Beneficial Applications: The Trends and Advances—A Review. Int. J. Pharmacol. 2014, 10, 129–159.
- Thakur, P.; Chawla, R.; Chakotiya, A.S.; Tanwar, A.; Goel, R.; Narula, A.; Arora, R.; Sharma, R.K. Camellia Sinensis Ameliorates the Efficacy of Last Line Antibiotics against Carbapenem Resistant Escherichia Coli. Phytother. Res. 2016, 30, 314–322.
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021.
- Islam, M.T.; Bardaweel, S.K.; Mubarak, M.S.; Koch, W.; Gaweł-Beben, K.; Antosiewicz, B.; Sharifi-Rad, J. Immunomodulatory Effects of Diterpenes and Their Derivatives through Nlrp3 Inflammasome Pathway: A Review. Front. Immunol. 2020, 11, 572136.
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264.
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent. Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58.
- Abdoly, M.; Farnam, A.; Fathiazad, F.; Khaki, A.; Ibrahimi, A.; Khaki, A.A.; Ibrahimi, O.A. Antidepressant-like Activities of Ocimum Basilicum (Sweet Basil) in the Forced Swimming Test of Rats Exposed to Electromagnetic Field (EMF). Afr. J. Pharm. Pharmacol. 2012, 6, 211–215.
- Hozayen, W.G.; El-Desouky, M.A.; Soliman, H.A.; Ahmed, R.R.; Khaliefa, A.K. Antiosteoporotic Effect of Petroselinumcrispum, Ocimum Basilicum and Cichoriumintybus L. In Glucocorticoid Induced Osteoporosis in Rats. BMC Complement. Altern. Med. 2016, 16, 165.
- Rubab, S.; Hussain, I.; Khan, B.A.; Unar, A.A.; Abbas, K.A.; Khich, Z.H.; Khan, M.; Khanum, S.; Khan, K.U.H. Biomedical Description of Ocimum basilicum L. J. Islam. Int. Med. Colleg. 2017, 12, 59–67.
- Shehata, A.M.; Nosir, W. Response of Sweet Basil Plants (Ocimum Basilicum, L.) Grown under Salinity Stress to Spraying Seaweed Extract. Future J. Biol. 2019, 2, 16–28.
- Brar, B.; Duhan, J.S.; Rakha, P. Antidepressant Activity of Various Extract from Seed of Ocimum Basilicum Linn. Int. J. Sci. Res. 2015, 4, 41–43.
- Ahmad, C.M.; Naz, S.B.; Sharif, A.; Akram, M.; Saeed, M.A. Biological and Pharmacological Properties of the Sweet Basil (Ocimum Basilicum). Br. J. Pharm. Res. 2015, 7, 330–339.
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The Potential Effects of Ocimum Basilicum on Health: A Review of Pharmacological and Toxicological Studies. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 679–692.
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev. Int. 2023, 39, 119–147.
- Da-Silva, F.; Santos, R.H.S.; Diniz, E.R.; Barbosa, L.C.A.; Casali, V.W.D.; De-Lima, R.R. Content and composition of basil essential oil at two different hours in the day and two seasons. Rev. Bras. De Plants Med. 2003, 6, 33–38.
- Sajjadi, S. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru 2006, 14, 128–130.
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylskmi, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995.
- Benedec, D.; Vlase, L.; Hanganu, D.; Oniga, I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Dig. J. Nanomater. Biostructures 2012, 7, 1263–1270.
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656.
- Kwee, E.; Niemeyer, E. Variations in phenolic compositions and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050.
- Güez, C.; de Souza, R.; Fischer, P.; de Moura Leão, M.; Duarte, J.; Boligon, A.; Athayde, M.; Zuravski, L.; de Oliveira, L.; Machado, M. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and antiinflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 2017, 53, e15098.
- Marzouk, A. Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. Z. Fur Naturforschung C 2009, 64, 201–209.
- Zhan, Y.; An, X.; Wang, S.; Sun, M.; Zhou, H. Basil polysaccharides: A review on extraction, bioactivities and pharmacological applications. Bioorg Med. Chem. 2020, 28, 115179.
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of Ocimum basilicum—A review. Int. J. Pharm. Res. 2021, 13, 2997–3013.
- Hassanpouraghdam, B.; Hassani, A.; Shalamzari, S. Menthone and estragole-rich essential oil of cultivated Ocimum basilicum L. from northwest Iran. Chemija 2010, 21, 59–62.
- Wesolowska, A.; Kosecka, D.; Jadczak, D. Essential oil composition of three sweet basil (Ocimum basilicum L.) cultivars. Herba Pol. 2012, 58, 5–16.
- Falowo, A.; Mukumbo, F.; Idamokoro, E.; Afolayan, A.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from Boran and Nguni cattle. Hindawi Intern. J. Food Sci. 2019, 2019, 2628747.
- Elsherbiny, E.; El-Khateeb, A.; Azzaz, N. Chemical composition and fungicidal effects of Ocimum basilicum essential oil on Bipolaris and Cochliobolus species. J. Agr. Sci. Tech. 2016, 18, 1143–1152.
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Flamini, G.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. &Reut. from the Algerian Saharan Atlas. BMC Compl. Altern. Med. 2019, 19, 146.
- Loughrin, J.; Kasperbauer, M. Aroma content of fresh basil (Ocimum basilicum L.) leaves is affected by light reflected from colored mulches. J. Agric. Food Chem. 2003, 51, 2272–2276.
- Beatovi, D.; Krstic-Miloševi, D.; Trifunovic, S.; Šiljegovic, G.; Glamoclija, J.; Ristic, M.; Jelacic, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75.
- Belong, P.; Ntonga, P.; Fils, E.; Dadji, G.; Tamesse, J. Chemical composition and residue activities of Ocimum canum Sims and Ocimum basilicum L. essential oils on adult female Anopheles funestus ss. J. Anim. Plant Sci. 2013, 19, 2854–2863.
- Saaban, K.F.; Ang, C.H.; Khor, S.M.; Chuah, C.H. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran J. Chem. Chem. Eng. 2019, 38, 139–152.
- Dev, N.; Das, A.; Hossain, M.; Rahman, S. Chemical compositions of different extracts of Ocimum basilicum leaves. J. Sci. Res. 2011, 3, 197–206.
- Ololade, Z.; Fakankun, O.; Alao, F.; Udi, O. Ocimum basilicum var purpureum floral essential oil: Phytochemicals phenolic content antioxidant free radical scavenging and antimicrobial potentials. Glob. J. Sci. Front. Res. B 2014, 14, 31–38.
- Adigüzel, A.; Güllüce, M.; Şengül, M.; Öğütcü, H.; Şahin, F.; Karaman, I. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk. J. Biol. 2005, 29, 155–160.
- Ababutain, I.M. Antimicrobial Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Saudi Arabian Ocimum basilicum Leaves Extracts. J. Pure Appl. Microbiol. 2019, 13, 61.
- Backiam, A.D.S.; Duraisamy, S.; Karuppaiya, P.; Balakrishnan, S.; Sathyan, A.; Kumarasamy, A.; Raju, A. Analysis of the main bioactive compounds from Ocimum basilicum for their antimicrobial and antioxidant activity. Biotechnol. Appl. Biochem. 2023, 70, 2038–2051.
- Yibeltal, G.; Yusuf, Z.; Desta, M. Physicochemical properties, antioxidant and antimicrobial activities of Ethiopian sweet basil (Ocimum basilicum L.) leaf and flower oil extracts. Recent. Adv. Anti-Infect. Drug Discov. Former. Recent. Pat. Anti-Infect. Drug Discov. 2022, 17, 131–138.
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569.
- Shafique, M.; Khan, S.J.; Khan, N.H. Study of antioxidant and antimicrobial activity of sweet basil (Ocimum basilicum) essential oil. Pharmacologyonline 2011, 1, 105–111.
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151.
- Sahu, A.; Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Kar, D.; Kuanar, A. Antioxidant and antimicrobial activities of Ocimum basilicum var. thyrsiflora against some oral microbes. Multidiscip. Sci. J. 2024, 6, 2024026.
- Tarayrah, H.; Akkawi, M.; Yaghmour, R. Investigations of the Palestinian medicinal plant basil (Ocimum basilicum): Antioxidant, antimicrobial activities, and their phase behavior. Pharm. Pharmacol. Int. J. 2022, 10, 97–104.
- Abbasi, F.; Pournaghi, P. Investigating the Anti-Depressant and Anxiolytic Effects of the Rosa canina L. Fruit in Syrian Rats Treated with Bisphenol A. J. Ardabil Univ. Med. Sci. 2022, 22, 154–167.
- Kocić-Tanackov, S.; Dimić, G.; Lević, J.; Tanackov, I.; Tuco, D. Antifungal activities of basil (Ocimum basilicum L.) extract on Fusarium species. Afr. J. Biotechnol. 2011, 10, 10188–10195.
- Ahmad, K.; talha Khalil, A.; Somayya, R. Antifungal, phytotoxic and hemagglutination activity of methanolic extracts of Ocimum basilicum. J. Tradit. Chin. Med. 2016, 36, 794–798.
- Jacob, J.K.S.; Carlos, R.C.A.; Divina, C.C. Phytochemical composition, antibacterial and antifungal activities of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2016, 7, 84–90.
- Piyo, A.; Udomsilp, J.; Khang-Khun, P.; Thobunluepop, P. Antifungal activity of essential oils from basil (Ocimum basilicum Linn.) and sweet fennel (Ocimum gratissimum Linn.): Alternative strategies to control pathogenic fungi in organic rice. Asian J. Food Agro-Ind. 2009, S2–S9. Available online: https://www.thaiscience.info/Journals/Article/AFAI/10850172.pdf (accessed on 8 January 2024).
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Molecules 2018, 23, 1116.




