Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shinichi Okazumi | -- | 1466 | 2024-02-05 10:21:28 | | | |
| 2 | Mona Zou | -4 word(s) | 1462 | 2024-02-07 08:15:26 | | | | |
| 3 | Mona Zou | + 2 word(s) | 1464 | 2024-02-08 09:25:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Okazumi, S.; Ohira, G.; Hayano, K.; Aoyagi, T.; Imanishi, S.; Matsubara, H. Qualitative Diagnostic Imaging for Decision Making. Encyclopedia. Available online: https://encyclopedia.pub/entry/54749 (accessed on 13 January 2026).
Okazumi S, Ohira G, Hayano K, Aoyagi T, Imanishi S, Matsubara H. Qualitative Diagnostic Imaging for Decision Making. Encyclopedia. Available at: https://encyclopedia.pub/entry/54749. Accessed January 13, 2026.
Okazumi, Shinichi, Gaku Ohira, Koichi Hayano, Tomoyoshi Aoyagi, Shunsuke Imanishi, Hisahiro Matsubara. "Qualitative Diagnostic Imaging for Decision Making" Encyclopedia, https://encyclopedia.pub/entry/54749 (accessed January 13, 2026).
Okazumi, S., Ohira, G., Hayano, K., Aoyagi, T., Imanishi, S., & Matsubara, H. (2024, February 05). Qualitative Diagnostic Imaging for Decision Making. In Encyclopedia. https://encyclopedia.pub/entry/54749
Okazumi, Shinichi, et al. "Qualitative Diagnostic Imaging for Decision Making." Encyclopedia. Web. 05 February, 2024.
Copy Citation
Recently, in the treatment of advanced esophageal cancer, evidence for combined-modality therapy has been obtained, and the usefulness of neoadjuvant therapy and conversion surgery has been reported. Neoadjuvant therapy is known to improve the results of surgery for advanced esophageal cancer. In particular, neoadjuvant chemoradiotherapy (NACRT) showed a better prognosis than chemotherapy. When a histologically effective response is obtained, the presence of a good response has been shown to significantly affect the resection rate and the long-term prognosis; thus, precise evaluation has become more important for determining the treatment strategy.
esophageal cancer
neoadjuvant therapy
perfusion-CT
diffusion-MRI
FDG-PET
1. Introduction
Recently, in the treatment of advanced esophageal cancer, evidence for combined-modality therapy has been obtained, and the usefulness of neoadjuvant therapy [1][2] and conversion surgery has been reported [3][4]. Neoadjuvant therapy is known to improve the results of surgery for advanced esophageal cancer. In particular, neoadjuvant chemoradiotherapy (NACRT) showed a better prognosis than chemotherapy [5][6][7][8]. When a histologically effective response is obtained, the presence of a good response has been shown to significantly affect the resection rate [9] and the long-term prognosis [10][11]; thus, precise evaluation has become more important for determining the treatment strategy.
2. Response Prediction before Neoadjuvant Therapy
A highly detailed prediction of treatment outcome requires evaluation of the physiological or biochemical characteristics of the tumor, which must be considered when selecting the drug delivery system or irradiation conditions. Tumors show angiogenesis with increased VEGF, which can be seen through evaluation of perfusion status, or stromal changes, which can be seen through evaluation of diffusion status [12]. Therefore, qualitative imaging modalities such as perfusion-CT or diffusion-MRI can show the dynamics of blood flow or diffusion of the tumor and surrounding tissue, which are considered useful for the prediction [13][14].
Perfusion analysis: It was thought that the high blood flow presented advantages with respect to pharmacokinetics or oxygenation to obtain a good pathological response. Perfusion-CT is a method that shows tumor tissue hemodynamics [15][16] . The tumor blood flow after CRT and the ◿blood flow could be evaluated using this modality. And the predictive ability of this method has also been reported. The quantity of tumor blood flow before treatment reflects the degree of response. Hayano et al. [17], Makari [18], and Li [19] reported that higher tumor blood flow could be the index for predicting responders. On the other hand, DCE-MRI also provides perfusion images. In the analysis of DCE-MRI, the Ktrans value is used as an index that reflects tissue permeability and blood flow. Lei et al. [20] and Ye et al. [21] reported that the pre-treatment Ktrans value was significantly higher in complete responders and could be a response predictor [22].
Diffusion analysis: Tumors are considered to show trends in their diffusion status due to their microvessels and stromal characteristics [23]. Diffusion-MRI is a method to calculate the diffusion dynamics of tissue through imaging. According to the treatment effect, the apparent diffusion coefficient (ADC) is a quantitative measure of the state of diffusion. ADC value was reported to be inversely correlated to the tissue VEGF expression [23][24] or tumor stromal density [23]. Therefore, it could reflect the drug delivery or radiation circumstance and was expected to be a predictive marker of treatment response [24]. It has been reported in several articles that the diffusion of the tumor before treatment correlates with the degree of response to CRT [21][24][25][26]. Aoyagi et al. [25] described that the susceptibility to treatment could be predicted using this method, with ADC > 1.1 identifying responders, but no consensus was reached regarding the concept and index value for the prediction of response. Li et al. [27] speculated that tumors with high necrosis might have higher ADC values and that they would be associated with poor therapy outcomes. Hirata et al. reported that a high ADC value was related to a lower response [28].
3. Response Evaluation Reflecting the Degree of Pathological Regression after Treatment
In neoadjuvant therapy, when a histologically effective response was obtained, the prognosis after surgery was significantly better [29][30]. Therefore, it becomes essential to assess the degree of response after treatment accurately to determine whether to proceed with surgical resection. Tumor perfusion, diffusion, glucose metabolism, and tissue heterogeneity and their relationships have been found to change according to the histologic response to therapy.
Perfusion analysis: It has been reported that, on perfusion CT, the tumor part shows a significant blood flow decrease with treatment, and the images reflect the histologic response. Significant tumor blood flow decreases were seen in responders to neoadjuvant chemoradiotherapy compared with non-responders (Figure 1), and responders were diagnosed using this index of tumor blood flow decrease. Hayano et al. [31] reported an index of a 15% decrease in tumor blood flow after treatment, which reflected histological response, and Stefanovic et al. reported [32] a post-CRT blood flow < 30 (mL/min/100 g) as a response.
Diffusion analysis: A consensus was seen in several reports that tumor diffusion had been shown to have a tendency to increase after treatment according to the degree of the histologic response, and ◿ADC (pre–post) was a good marker which reflected histologic response [14][33][34][35][36]. In the researchers' results obtained via diffusion-weighted imaging with 1.5T-MRI for advanced esophageal cancer during the treatment of CRT, the responder group showed a significant earlier increase in the ADC value at the irradiation dose of 20 Gy than the non-responder group, and it seemed that an evaluation of the early treatment effect would be possible. Moreover, the possibility of its evaluation in the early treatment course was also recommended in several articles [33][35][37]. Imanishi et al. [33] reported that responders to CRT showed an ADC increase of 15% at 20 Gy and 40% at 40 Gy. Guo et al. [26], Vollenbrock et al. [38], and Borggreve [37] reported that the optimal timing for early prediction of the effect was 2 weeks after the beginning of treatment. Vollenbrock et al. [38] described that the ADC increase with treatment effect was due to the early effect of radiotherapy on the tumor microenvironment inducing tumor necrosis with a loss of cell membrane, resulting in an increase in the ADC.
Glucose metabolism analysis: FDG-PET is a technique for imaging the state of glucose metabolism, which reflects tumor proliferative activity, and the degree of glucose integration is assessed with the standardized uptake value (SUV) calculated from an image [39]. Evaluations of the mean uptake of the tumor and of total uptake are possible, and the histologic responses have been reported to correlate with the mean uptake [40][41]. As indices to identify responders, Higuchi et al. [42] reported post-treatment SUV < 2.5, and Swisher et al. [40] reported SUV < 3.1. Furthermore, through evaluation in the early treatment period, a prediction of the final effect was also considered possible. Otto et al. [43] assumed that a 35% decrease in accumulation two weeks after the start of chemotherapy for esophageal adenocarcinoma was a predictor of response. This occurred before the morphologic decrease in the tumor, which was thought to be due to decreased glucose metabolism prior to its morphologic decrease. In addition, the prognostic value of metastatic lymph node uptake after treatment, as well as the main tumor, was reported [44], and Yasuda et al. reported the prognostic value of residual lymph node uptake after treatment [45][46].
Heterogeneity analysis: Tumor heterogeneity has been reported to reflect malignancy or prognosis, and it has been studied through texture analysis using CT. It was reported that tumor texture showed a tendency to homogenize, decrease entropy, and increase uniformity after treatment [47][48][49][50]. When planning for conversion surgery, an evaluation of the heterogeneity of the effect on the tumor is needed to diagnose downstaging. If T4 status could be diagnosed as resolved, conversion surgery with R0 resection could be possible. For such a diagnostic aim, tissue-selective reconstruction of enhanced CT was applied to show the fibrous changes in the tumor due to treatment. In the researchers' study, contrast-enhanced CT (100 mL/body/30 s of contrast medium) was performed using multi-detector CT, and a depiction of the layer of connective tissue or adipose tissue between a tumor and adjacent organs was enabled through the contrast-enhanced CT image reconstruction method, which emphasizes the CT values of the tissue (Figure 1). It was found to be useful for the diagnosis of T4 cases. Furthermore, after treatment, the decrease in the CT level of the tumor has been found to correspond to the histologic response. In T4-diagnosed cases before treatment, the presence of a fibrotic change in the tumor and the emergence of a fibrous layer between the tumor and the adjacent organs after treatment have been reported to be evidence of downstaging that would permit conversion surgery with R0 resection [51].
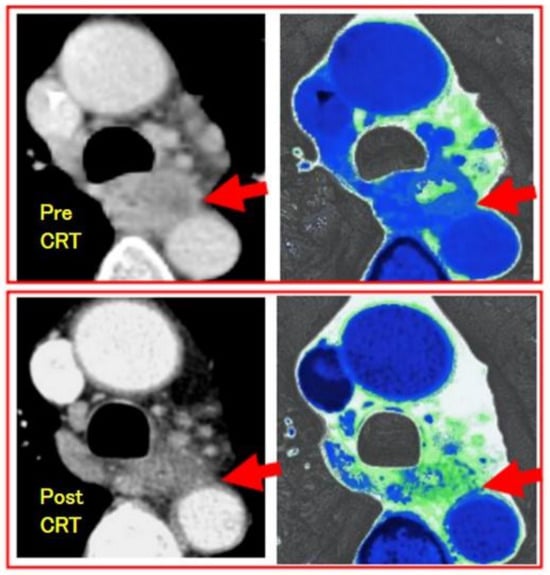
Figure 1. Downstaging evaluation using fibrous-tissue-enhanced CT reconstruction image of the case of esophageal squamous cell carcinoma after CRT. Contrast-enhanced CT was performed using multi-detector CT (GE light speed ultra). Arrows show the tumor. The upper is pre-CRT T4 image, which shows no interstitial plane between the tumor and aorta. The lower is post-CRT image, which shows fibrous plane as green and the case was diagnosed as downstaging and curatively resected with surgery.
References
- Kumar, T.; Pai, E.; Singh, R.; Francis, N.J.; Pandey, M. Neoadjuvant strategies in resectable carcinoma esophagus: A meta-analysis of randomized trials. World J. Surg. Oncol. 2020, 18, 59.
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. Clin. Oncol. 2018, 36, 2796–2803.
- Terada, M.; Hara, H.; Daiko, H.; Mizusawa, J.; Kadota, T.; Hori, K. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510, TRIANgLE). Jpn. J. Clin. Oncol. 2019, 49, 1055–1060.
- Miyata, H.; Sugimura, K.; Motoori, M.; Omori, T.; Yamamoto, K.; Yanagimoto, Y. Clinical Implications of Conversion Surgery After Induction Therapy for T4b Thoracic Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2019, 26, 4737–4743.
- Von Döbeln, G.A.; Klevebro, F.; Jacobsen, B.; Johannessen, O.; Nielsen, H.; Johnsen, G. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: Long-term results of a randomized clinical trial. Dis. Esophagus 2018, 32, doy078.
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692.
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P. Ten-Year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The Randomized controlled CROSS Trial. J. Clin. Oncol. 2021, 39, 1995–2004.
- Tang, H.; Wang, H.; Fang, Y.; Zhu, J.Y.; Yin, J.; Shen, Y.X. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: A prospective multicenter randomized clinical trial. Ann. Oncol. 2023, 34, 163–172.
- Nusrath, S.; Thammineedi, S.R.; Vijaya Narsimha Raju, K.V.; Patnaik, S.C.; Pawar, S.; Santa, A. Short-term Outcomes in Patients with Carcinoma of the Esophagus and Gastroesophageal Junction Receiving Neoadjuvant Chemotherapy or Chemoradiation before Surgery. A Prospective Study. Rambam Maimonides Med. J. 2019, 10, e0002.
- Thomas, M.; Borggreve, A.S.; van Rossum, P.S.N.; Perneel, C.; Moons, J.; Van Daele, E.; van Hillegersberg, R. Radiation dose and pathological response in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery: A multi-institutional analysis. Acta Oncol. 2019, 58, 1358–1365.
- Andreollo, N.A.; Beraldo, G.C.; Alves, I.P.F.; Tercioti-Junior, V.; Ferrer, J.A.P.; Coelho-Neto, J.S. Pathologic complete response (YPT0 YPN0) after chemo therapy and radiotherapy neoadjuvant followed by esophagectomy in the squamous cell carcinoma of the esophagus. Arq. Bras. Cir. Dig. 2018, 31, e1405.
- Aoyagi, T.; Shuto, K.; Okazumi, O.; Hayano, K.; Satoh, A.; Saitoh, H. Apparent Diffusion Coefficient Correlation With Oesophageal Tumour Stroma and Angiogenesis. Eur. Radiol. 2012, 22, 1172–1177.
- Tamandl, D.; Fueger, B.; Haug, A.; Schmid, R.; Stift, J.; Schoppmann, S.F. Diagnostic Algorithm That Combines Quantitative 18F-FDG PET Parameters and Contrast-Enhanced CT Improves Posttherapeutic Locoregional Restaging and Prognostication of Survival in Patients With Esophageal Cancer. Clin. Nucl. Med. 2019, 44, e13–e21.
- Borggreve, A.S.; Goense, L.; van Rossum, P.S.N.; Heethuis, S.E.; van Hillegersberg, R.; Lagendijk, J.J.W. Preoperative Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients With Esophageal Cancer Using 18F-FDG PET/CT and DW-MRI: A Prospective Multicenter Study. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 998–1009.
- Miles, K.A.; Hayball, M.; Dixon, A.K. Colour perfusion imaging: A new application of computed tomography. Lancet 1991, 337, 643–645.
- Chen, T.W.; Yang, Z.G.; Wang, Q.L.; Li, Y.; Qian, L.L.; Chen, H. Whole tumour quantitative measurement of first-pass perfusion of oesophageal squamous cell carcinoma using 64-row multidetector computed tomography: Correlation with microvessel density. J. Eur. J. Radiol. 2011, 79, 218–223.
- Hayano, K.; Okazumi, S.; Shuto, K.; Matsubara, H.; Shimada, H.; Nabeya, Y. Perfusion CT can predict the response to chemoradiation therapy and survival in esophageal squamouscell carcinoma: Initial clinical results. Oncol. Rep. 2007, 18, 901–908.
- Makari, Y.; Yasuda, T.; Doki, Y.; Miyata, H.; Fujiwara, Y.; Takiguchi, S. Correlation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancers. Surg. Oncol. 2007, 96, 220–229.
- Li, M.H.; Shang, D.P.; Chen, C.; Xu, L.; Huang, Y.; Kong, L. Perfusion computed tomography in predicting treatment response of advanced esophageal squamous cell carcinomas. Asian Pac. J. Cancer Prev. 2015, 16, 797–802.
- Lei, J.; Han, Q.; Zhu, S.; Shi, D.; Dou, S.; Su, Z. Assessment of esophageal carcinoma undergoing concurrent chemoradiotherapy with quantitative dynamic contrast-enhanced magnetic resonance imaging. Oncol. Lett. 2015, 10, 3607–3612.
- Ye, Z.M.; Dai, S.J.; Yan, F.Q.; Wang, L.; Fang, J.; Fu, Z.F. DCE-MRI-Derived Volume Transfer Constant (K(trans)) and DWI Apparent Diffusion Coefficient as Predictive Markers of Short- and Long-Term Efficacy of Chemoradiotherapy in Patients With Esophageal Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533034618765254.
- Sun, N.N.; Ge, X.L.; Liu, X.S.; Xu, L.L. Histogram analysis of DCE-MRI for chemoradiotherapy response evaluation in locally advanced esophageal squamous cell carcinoma. Radiol. Med. 2020, 125, 165–176.
- Hayano, K.; Ohira, G.; Hirata, A.; Aoyagi, T.; Imanishi, S.; Tochigi, T. Imaging biomarkers for the treatment of esophageal cancer. World J. Gastroenterol. 2019, 25, 3021–3029.
- Cong, Q.; Li, G.; Wang, Y.; Zhang, S.; Zhang, H. DW-MRI for esophageal squamous cell carcinoma, correlations between ADC values with histologic differentiation and VEGF expression: A retrospective study. Oncol. Lett. 2019, 17, 2770–2776.
- Aoyagi, T.; Shuto, K.; Okazumi, S.; Shimada, H.; Kazama, T.; Matsubara, H. Apparent Diffusion Coefficient Values Measured by Diffusion-Weighted Imaging Predict Chemoradiotherapeutic Effect for Advanced Esophageal Cancer. Dig. Surg. 2011, 28, 252–257.
- Guo, L.; Zhang, L.; Zhao, J. CT scan and magnetic resonance diffusion-weighted imaging in the diagnosis and treatment of esophageal cancer. Oncol. Lett. 2018, 16, 7117–7122.
- Li, S.P.; Padhani, A.R. Tumor response assessments with diffusion and perfusion MRI. J. Magn. Reson. Imaging 2012, 35, 745–763.
- Hirata, A.; Hayano, K.; Ohira, G.; Imanishi, S.; Hanaoka, T.; Murakami, K. Volumetric histogram analysis of apparent diffusion coefficient for predicting pathological complete response and survival in esophageal cancer patients treated with chemoradiotherapy. Am. J. Surg. 2020, 219, 1024–1029.
- Chao, Y.K.; Chuang, W.Y.; Chang, H.K.; Tseng, C.K.; Yeh, C.J.; Liu, Y.H. Prognosis of patients with esophageal squamous cell carcinoma who achieve major histopathological response after neoadjuvant chemoradiotherapy. Eur. J. Surg. Oncol. 2017, 43, 234–239.
- Noble, F.; Nolan, L.; Bateman, A.C.; Byrne, J.P.; Kelly, J.J.; Bailey, I.S. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J. Gastroenterol. 2013, 19, 9282–9293.
- Hayano, K.; Shuto, K.; Satoh, A.; Aoyagi, T.; Narushima, K.; Gunji, H. Tumor blood flow change measured by CT perfusion during chemoradiation therapy (CRT) for monitoring response and predicting survival in patients with esophageal cancer. Esophagus 2014, 11, 72–79.
- Djuric-Stefanovic, A.; Micev, M.; Stojanovic-Rundic, S.; Pesko, P.; Saranovic, D. Absolute CT perfusion parameter values after the neoadjuvant chemoradiotherapy of the squamous cell esophageal carcinoma correlate with the histopathologic tumor regression grade. Eur. J. Radiol. 2015, 84, 2477–2484.
- Imanishi, S.; Shuto, K.; Aoyagi, T.; Kono, T.; Saito, H.; Matsubara, H. Diffusion-weighted Magnetic Resonance Imaging for Predicting and Detecting the Early Response to Chemoradiotherapy of Advanced Esophageal Squamous Cell Carcinoma. Dig. Surg. 2013, 30, 240–248.
- Cheng, B.; Yu, J. Predictive value of diffusion-weighted MR imaging in early response to chemoradiotherapy of esophageal cancer: A meta-analysis. Dis. Esophagus 2018, 32, doy065.
- Heethuis, S.E.; Goense, L.; van Rossum, P.S.N.; Borggreve, A.S.; Mook, S.; Voncken, F.E.M. DW-MRI and DCE-MRI are of complementary value in predicting pathologic response to neoadjuvant chemoradiotherapy for esophageal cancer. Acta Oncol. 2018, 57, 1201–1208.
- Li, Q.W.; Qiu, B.; Wang, B.; Wang, D.L.; Yin, S.H.; Yang, H. Prediction of pathologic responders to neoadjuvant chemoradiotherapy by diffusion-weighted magnetic resonance imaging in locally advanced esophageal squamous cell carcinoma: A prospective study. Dis. Esophagus 2017, 31, dox121.
- Borggreve, A.S.; Heethuis, S.E.; Boekhoff, M.R.; Goense, L.; van Rossum, P.S.N.; Brosens, L.A.A. Optimal timing for prediction of pathologic complete response to neoadjuvant chemoradiotherapy with diffusion-weighted MRI in patients with esophageal cancer. J. Eur. Radiol. 2020, 30, 1896–1907.
- Vollenbrock, S.E.; Voncken, F.E.M.; Bartels, L.W.; Beets-Tan, R.G.H.; Bartels-Rutten, A. Diffusion-weighted MRI with ADC mapping for response prediction and assessment of oesophageal cancer: A systematic review. Radiother. Oncol. 2020, 142, 17–26.
- Odawara, S.; Kitajima, K.; Katsuura, T.; Kurahashi, Y.; Shinohara, H.; Yamaka, K. Tumor Response to Neoadjuvant Chemotherapy in Patients With Esophageal Cancer Assessed With CT and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1. Eur. J. Radiol. 2018, 101, 65–71.
- Swisher, S.G.; Maish, M.; Erasmus, J.J.; Correa, A.M.; Komaki, R.; Macapinlac, H. Utility of PET, CT, and EUS to Identify Pathologic Responders in Esophageal Cancer. Ann. Thorac. Surg. 2004, 78, 1152–1160.
- Arnett, A.H.; Merrell, K.W.; Macintosh, E.M.; James, S.E.; Nathan, M.A.; Shen, K.R. Utility of 18F-FDG PET for Predicting Histopathologic Response in Esophageal Carcinoma following Chemoradiation. J. Thorac. Oncol. 2016, 12, 121–128.
- Higuchi, I.; Yasuda, T.; Yano, M.; Doki, Y.; Miyata, H.; Tatsumi, M. Lack of fludeoxyglucose F 18 uptake in posttreatment positron emission tomography as a significant predictor of survival after subsequent surgery in multimodality treatment for patients with locally advanced esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2008, 136, 205–212.
- Ott, K.; Weber, W.A.; Lordick, F.; Becker, K.; Busch, R.; Herrmann, K.; Wieder, H. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J. Clin. Oncol. 2006, 24, 4692–4698.
- Izumi, D.; Yoshida, N.; Watanabe, M.; Shiraishi, S.; Ishimoto, T.; Kosumi, K. Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J. Gastroenterol. 2016, 51, 788–795.
- Yasuda, T.; Yano, M.; Miyata, H.; Yamasaki, M.; Higuchi, I.; Takiguchi, S. Systemic control and evaluation of the response to neoadjuvant chemotherapy in resectable thoracic esophageal squamous cell carcinoma with 18F-fluorodeoxyglucose positron emission tomography-positive lymph nodes. Surg. Today 2015, 45, 335–345.
- Hamai, Y.; Hihara, J.; Emi, M.; Ibuki, Y.; Murakami, Y.; Nishibuchi, I. Clinical Significance of 18F-Fluorodeoxyglucose-Positron Emission Tomography-Positive Lymph Nodes to Outcomes of Trimodal Therapy for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2019, 26, 1869–1878.
- Yip, C.; Davnall, F.; Kozarski, R.; Landau, D.B.; Cook, G.J.; Ross, P. Assessment of changes in tumor heterogeneity following neoadjuvant chemotherapy in primary esophageal cancer. Dis. Esophagus 2015, 28, 172–179.
- Ganeshan, B.; Skogen, K.; Pressney, I.; Coutroubis, D.; Miles, K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: Preliminary evidence of an association with tumour metabolism, stage, and survival. Clin. Radiol. 2012, 67, 157–164.
- Yip, C.; Landau, D.; Kozarski, R.; Ganeshan, B.; Thomas, R.; Michaelidou, A. Primary esophageal cancer: Heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 2014, 270, 141–148.
- Liu, S.; Zheng, H.; Pan, X.; Chen, L.; Shi, M.; Guan, Y. Texture analysis of CT imaging for assessment of esophageal squamous cancer aggressiveness. J. Thorac. Dis. 2017, 9, 4724–4732.
- Okazumi, S.; Shimada, H.; Matsubara, H. Estimation of downstaging after chmoradiotherapy for T4 esophageal cancer by qualitative response evaluation using rendered MD-CT and the outcome of curative resection. Dis. Esophagus 2018, 31 (Suppl. S1), 140–141.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
343
Revisions:
3 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




