1. Classification of Natural Polysaccharides
Polysaccharides, which belong to the third main class of biopolymers (carbohydrates), are important for the immune system, blood coagulation, fertilization, pathogenesis prevention, and therapeutic effectiveness
[1]. Structure support, energy storage, lubrication, and cell signal transduction are only a few of the biological functions that polysaccharides have an impact on in cells
[2].
Based on their chemical structure, which consists of monosaccharide units joined by glycosidic linkages, polysaccharides—the most prevalent type of carbohydrates in nature—are categorized
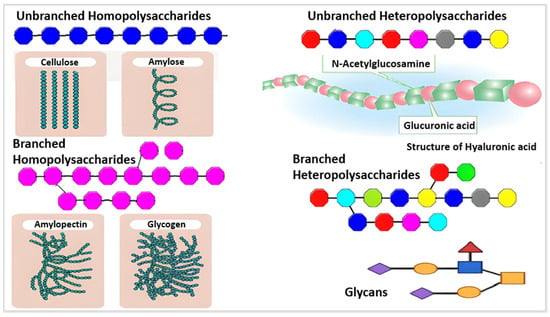
[3]. They may also establish covalent bonds with other structures like lipids, peptides, and amino acids. In contrast to heteropolysaccharides, which are heteroglycans made up of several monosaccharides, homopolysaccharides are homoglycans composed of the same monosaccharide (
Figure 1)
[2].
Figure 1. Different monosaccharides are represented by various colors, including branched and unbranched homopolysaccharides and heteropolysaccharides
[4].
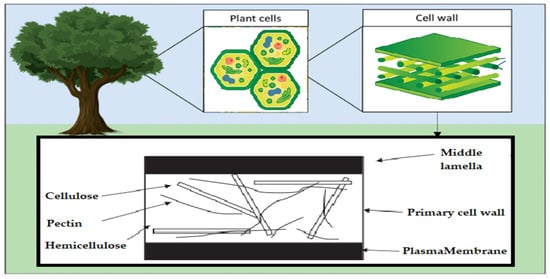
Acetic linkages are used to join homopolymers of glucose or amino sugars. Polysaccharides are the world’s most abundant renewable resource, according to current statistics. Each year, photosynthesis produces several orders of magnitude more carbohydrate than is generated artificially (
Figure 2)
[5].
Figure 2. Schematic of the polysaccharide components of the plant cell wall.
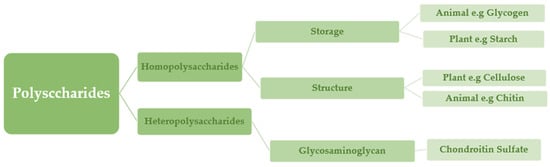
Polysaccharides are classified into various classes based on their structure or function (
Table 1). There are three basic categories: polysaccharides include structural polysaccharides like cellulose and chitin, storage polysaccharides like starch and glycogen, and gel-forming polysaccharides like alginic acid and mucopolysaccharides. Ionic or nonionic (cationic and anionic) polymers can be found as well as branched or straight-chained polymers
[6].
Table 1. List of some polysaccharides from various sources.
| Source |
Polymer |
| Cells walls of plants |
Pectin |
| Seeds and roots |
Galactomannans |
| Seaweeds |
Carragenans, alginates, agar |
| Animal cell walls |
Hyaluronan |
| Shells of aquatic animals |
Chitin |
| Wood |
Cellulose, lignin, hemicellulose |
| Skins and bones of animals and scales of fish |
Gelatin |
| Bacteria |
Xanthan, hyaluronan, gellan |
| Fungi |
Cardlan, scleroglucan, schizophylla |
Natural polysaccharides, such as cellulose (
Figure 3), have stability and physical structure, which adds to their ability to retain food, such as starch
[4]. Various examples of negatively charged polysaccharides are pectin, heparin, hyaluronic acid, and alginate. Chitosan is a polysaccharide that is positively charged, whereas pectin, pectinol, and alginate are polyelectrolyte-based polysaccharides
[2].
Figure 3. Classification of polysaccharides based on physiological properties and the type of monosaccharides utilized as building blocks.
1.1. Cellulose
Since it constitutes the majority of plant cell walls and almost half of the biomass in photosynthetic species, cellulose may be the most common chemical on the planet. Several materials, including cotton, linen, wood, hemp, jute, kenaf, sugar beet cereal straws, and flax, have long been solid sources of cellulose. Other cellulose sources include bacteria (such as
Acetobacter), algae (like
Valonia and
Microdicyon), and marine animals of the
Ascite family
[7].
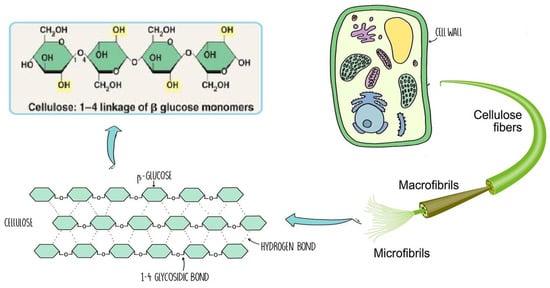
The chemical structure of cellulose is illustrated in
Figure 4 as having three hydroxyl groups per AGU, with the exception of the terminal ends. These hydroxyl groups collaborate to generate intra- and inter-hydroxyl hydrogen bonds, resulting in a supramolecular polymer with crystalline (ordered) sections
[8]. When cellulose molecules are randomly organized, they form amorphous patches that connect the ordered crystalline areas together
[9]. The structure of cellulose has a significant impact on its reactivity. The hydroxyl groups on the surface of the cellulose molecule can create both intramolecular and intermolecular hydrogen bonds
[10]. Many of the physical and chemical features of the polymeric chains are dictated by the hydrogen bonds formed between them, which may increase their linear integrity. Nonetheless, cellulose has several intrinsic disadvantages, including low crease resistance, poor solvent solubility, and a lack of thermoplasticity. To improve the properties of cellulose, controlled chemical or physical surface modification is necessary
[11]. The biosynthesized component of natural cellulose, in general, cannot be processed as a synthetic polymer
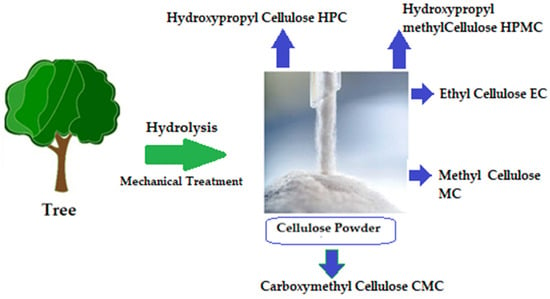
[12]. One proposed solution is to surface-functionalize cellulose molecules with foreign groups. This enables the cellulose surface chemistry to be modified without affecting the drug’s various critical intrinsic properties, including self-assembly, controlled dispersion inside a variety of matrix polymers, and increasing particle–particle and particle–matrix bond strengths. Numerous drug delivery methods use conventional cellulose and its derivatives, including cellulose ethers, cellulose esters, and oxycellulose. Sodium carboxymethyl cellulose (CMC), methylcellulose (MC), ethylcellulose (EC), hydroxypropyl cellulose (HPC), and hydroxypropyl methyl cellulose (HPMC) are among the several cellulose ethers that can be found in nature (
Figure 5)
[13].
Figure 4. Schematic of cellulose chain.
Figure 5. Variety of cellulose ethers derived from different preparation methods
[14].
1.2. Hemicelluloses
Another important component of plant cells is hemicelluloses, which form a matrix around cellulose microfibrils, are composed of a variety of molecules, including xyloglucans, xylans, mannans, and (1–3)-(1–4)-glucans
[15][16][17]. Chemical interactions like hydrogen bonds and van der Waals forces are frequently used to link them to the cellulose microfibrils. Hemicelluloses also function as metabolic reserves and signaling molecules in cells
[18], with a global yearly production of over 60 billion tons, which is the second-most abundant renewable component of lignocellulosic biomass after cellulose
[19][20].
1.3. Pectin
Plant cell walls also contain cellulose, hemicelluloses, and lignin in addition to pectin. It belongs to the same group of polysaccharides as agar and mucopolysaccharides which form gels
[21]. One of the most crucial hydrocolloids for industry is pectin, which is employed in many different food products. Citrus and apple pectin are the main industrial sources of high-methoxyl (>50%) pectin, whereas sunflower pectin is a naturally occurring source of low-methoxyl (50%) pectin
[22].
1.4. Starch
Pure starch is an odorless white powder. The polysaccharide is composed of the molecules of amylose and amylopectin. Each has a varied percentage depending on the source and kind of starch, although amylose and amylopectin generally account for 20–25% and 75–80% of total starch, respectively
[23]. Starch, which is present in fruits, seeds, and roots in the form of grains in leaves, tubers, stem core, and rhizomes, is the most significant polysaccharide for storing energy in plants
[24][25][26]. Similar to potatoes, rice, wheat, maize, and cassava, it constitutes the majority of the human diet’s carbohydrate intake
[27].
1.5. Glycogen
Another type of carbohydrate that stores energy is called glycogen. It is similar to other storage polysaccharides like amylopectin
[6] but is more compacted and branched. In general, the cytoplasm of animal cells contains glycogen, which is a type of glucose. Although glycogen is essential for physiological metabolism, it has no industrial use and is only included here for completeness
[28].
1.6. Chitin
Chitin is a linear polysaccharide obtained from animal that is extremely hydrophobic and includes acetyl and amino groups inside its unit. It is insoluble in water and conventional organic solvents, but it is soluble in specialized solvents such as dimethylacetamide with 5% lithium chloride and chloroalcohols in aqueous mineral acid solutions. Another frequent structural polysaccharide, it may be found in fungi’s mycelia and spores, as well as the exoskeletons of insects, crustaceans, and other invertebrates
[29][30].
1.7. Hyaluronic Acid
Hyaluronic acid is one type of glycoprotein, known as mucopolysaccharides or mucins. In comparison to other polysaccharides like heparin, it is one among the few polysaccharides discovered in vertebrate tissue and is more frequently found in developing embryos. These are polysaccharides that have been covalently bonded to proteins. Proteoglycans are one of the others. Hyaluronic acid, frequently known as hyaluronan, is a straight-chain polysaccharide with a molecular mass of about 7 × 10
6 g/mol that is frequently tightly linked to proteins by a hydrogen bond such those of water
[31]. As a result, extraction and isolation are difficult tasks. It has certain biological applications, such as being used in surgical applications with alginate to improve wound healing
[32][33].
1.8. Alginate
The seaweed alginate is a long-chain hydrophilic polymer that gives flexibility and strength to the cell walls of the algae. Since 600 B.C., it has been used as food.
However, it was not until 1896 that Akrefting was able to effectively extract pure alginate from seaweed. For the first time, alginate was used as a stabilizing component in ice cream by the company Kelco in 1929, and it became a commercially accessible product
[30]. Alginate is a safe and natural food ingredient. Alginate has excellent biological properties compared to other seaweed polysaccharides, including ion cross-linking, pH sensitivity, biocompatibility, and biodegradability, which have been extensively used in the food and nutraceutical industries. Alginate is the only polysaccharide with carboxyl groups naturally present in each component residue
[34].
2. Biological Applications of Natural Polymers
2.1. Anti-Inflammatory Activity
Natural polysaccharides are frequently utilized in nanotechnology for the treatment of inflammatory diseases
[35], and their anti-inflammation properties have been investigated
[36]. For example, TCM polysaccharides mainly block chemotactic and adhesion factor expression as well as the activity of key enzymes involved in the inflammatory process
[37]. While other polysaccharides inhibit inflammatory-related mediators like cytokines (IL-1b, IL-6, TNF-), NO (nitric oxide), and NO (nitric oxide), and reduce inflammatory cell infiltration, sulfated polysaccharides derived from algae have an anti-inflammatory effect by preventing leukocyte migration to the sites of inflammation
[38]. Due to their potential therapeutic benefits, nutritional supplements derived from plants and animals have been intensively explored in the development of new drugs
[39]. Natural products, their derivatives, and biomacromolecules constitute around 50 percent of the current drugs on the market
[40]. These supplements are widely recommended because of their variety of chemical components and lack of side effects compared to synthetic varieties.
Certain plants include natural substances that may have the ability to act as NSAID-like anti-inflammatory agents and be used as osteoarthritis drugs. Curcumin, capsaicin, berberine, sinomenine, rapamycin, white willow bark, and other natural substances all exhibit proven pharmacological effects
[41].
2.2. Hypoglycemic and Hypocholesterolemic Activities
It is possible to treat hyperglycemia, hyperlipidemia, hyperinsulinemia, and insulin resistance with ganoderma atrum polysaccharide, and it can also assist in avoiding kidney damage in type 2 diabetics. Since the 1980s, polysaccharides have been extensively researched for their hypoglycemic and hypocholesterolemic effects in clinical trials. To increase the therapeutic efficacy of loaded proteins and increase their stability, natural polysaccharides can be employed as nanocarriers. Orally administered insulin-loaded dextran–chitosan nanoparticulate polyelectrolyte complex had a better bioavailability and a more prolonged hypoglycemic impact
[2]. Additional examples of polysaccharides having hypoglycemic and hypocholesterolemic properties include chitosan, kefiran, and sulfated polysaccharides from Bullacta exarate
[42][43][44].
According to a number of studies,
α-glucosidase and
α-amylase are crucial for the insulin regulation. As a result, it is believed that inhibiting these enzymes is a good choice for treating diabetes. Inhibiting these enzymes can, in fact, limit carbohydrate digestion of and delay the absorption of glucose
[45]. In this context, acarbose and plant extracts (which may include numerous biologically active substances) have been described as hypoglycemic drugs that exhibit α-glucosidase inhibitory action and can reduce the blood glucose level (BGL)
[46].
Concerning actions that decrease cholesterol, it is generally recognized that dietary intake and cholesterol production, absorption, and secretion frequently have an impact on plasma cholesterol levels, especially LDL-c. In fact, several studies have reported evidence that different dietary proteins and their hydrolysates can improve blood lipid profiles; for instance, diets with soy
[47], milk proteins
[48], and fish proteins
[49].
2.3. Anticoagulant Activity
Sulfated polysaccharides called unfractionated and low molecular weight heparins are employed as anticoagulants; however, they have side effects include bleeding and thrombocytopenia
[50]. Among the several polysaccharide properties, anticoagulant action has received much attention. Polysaccharides, particularly sulfated polysaccharides, have been shown to exhibit biological effects such as anti-tumor, antioxidant, and anticoagulant characteristics
[42]. The anticoagulant action of these sulfated polysaccharides is significantly influenced by the high sulfate concentration
[51]. Natural polysaccharides from a variety of marine sources, including corals, marine fungi, microalgae, and shellfish (shrimp, crab, squilla, lobster, and crayfish)
[52], may be regarded as anticoagulant agents
[53].
2.4. Antiviral Activity
The ability of sulfated polysaccharides from seaweeds to block the replication of enveloped viruses such as the herpes simplex virus (HSV), human immunodeficiency virus (HIV), human cytomegalovirus, dengue virus, and respiratory syncytial virus has been known since the 1950s
[38]. Sulfated exopolysaccharides, which have a crucial biological role as antiviral agents, can be produced by a variety of microalgae species
[51]. Chinese traditional medicine-derived polysaccharides have been utilized as antiviral medications for a long time because they can boost and strengthen the immune system by stimulating macrophagocytes to enhance their phagocytic activity and trigger the release of IFN- and antibodies
[37]. Biochemical characteristics of microalgal polysaccharides include antioxidant, antibacterial, and antiviral action
[54].
3. Application of Natural Polymers in Food
The fact that polymers can be branched and that individual monomers might have distinct characteristics, like charge (in the case of polyelectrolytes) and hydrophobicity (in the case of amphipathic polymers), adds to the complexity. Proteins and polysaccharides are the main types of food polymers. Several polysaccharides, including starch, cellulose, chitosan, galactomannans, carrageenans, alginates, agars, inulins, pectins, xanthans, and gums, are abundant in nature and important sources of nutrition. They are also frequently employed as necessary bulk food. Furthermore, polysaccharides might be utilized as significant food additives because of their remarkable and occasionally exceptional properties which include thickening, stabilizing, gelling, and emulsifying agents. Polysaccharides (i.e., starches, celluloses), polyamides (proteins), and minor amounts of polynucleotides (DNA, RNA) are the three principal types of naturally occurring polymers that make up the majority of meals
[55].
Proteins are linear complex heteropolyelectrolytes that have a distinctive sequence of up to 20 different amino acid monomers with a variety of charge, hydrophobicity, and aromatic structural configurations. In live cells and animals, protein, which has an average degree of polymerization of about 103, performs a variety of biological functions. Meals typically contain some form of protein, which also serves as a source of amino acids for our own metabolism. They have the ability to produce and stabilize emulsions, foams, and gels, as well as enhance viscosity and water holding capacity. Despite their use, proteins are more expensive than other natural biopolymers, particularly starches and celluloses, which restricts their use in technology. Some of the key components include gelatin, milk proteins, egg proteins, and plant proteins
[56].
Heteropolymers of sugars and their derivatives are known as polysaccharides. Many are polyelectrolytes that can be linear or branched. Polysaccharides generally have a degree of polymerization between 103 to 104. ”Nutritional” functions, according to biological processes, serve as energy storage for metabolism (in particular starch in plants and glycogen in animals) and “building material” (such as cellulose in plants and chitin in fungus cell walls and the exoskeletons of arthropods like crustaceans). The latter category, known as structural polysaccharides, is composed mostly of mixed and very complex structures and occurs in several forms (
Table 2)
[57].
Table 2. Sources of commercially important hydrocolloids used in the food industry
[57].
| Botanical |
Plants |
Starch, pectin, cellulose |
| Trees |
Cellulose |
| Tree gum exudates |
Gum arabic (acasia), gum tragacanth, karaya |
| Seeds |
Guar gum, tara gum, locust bean gum, |
| Tubers |
Konjac mannan (glucomannan), potato starch |
| Algal |
Red seaweed |
Agar, carrageenan |
| Brown seaweed |
Alginate |
| Microbial |
|
Xanthan gum, dextran, gellan gum, cellulose |
| Animal |
|
Gelatin, caseinate, whey protein, chitosan |
Starch and protein are both essential dietary components that are rich in macronutrients. This section will concentrate on natural polymers as food additives and components in food formulations having a specialized purpose beyond basic nutritional value.
These specific applications of natural polymers in food may be divided into two basic categories:
-
Stabilizing food microstructures through gelling, thickening, emulsion, and foaming as well as using processing aids including cryoprotectants to increase freeze–thaw stability, drying aids, and encapsulant material.
-
Additional physiological and biological functionality, such as those provided by functional foods with specific health claims including lowering blood cholesterol levels, raising satiety, enhancing bioavailability, and inhibiting microbial growth
[58].
Regulatory Aspects
Natural polymers that are classified as either additives or ingredients, with the bulk falling under the additive category. Gelatin, on the other hand, is categorized as an ingredient. Ingredients are listed by name in the product’s list of ingredients, whereas additives must be identified by a distinctive number (the E-number in the European Union and the INS number abroad) and are optionally identified by name. In the US, additives are governed by the JECFA (Joint/WHO Expert Committee on Food Additives) and the European Commission. The legislation is used to protect consumer health and to ensure ethical behavior in the food sector
[59].
The goal of additives regulation is to establish acceptable ingestion limits and purity criteria for diverse compounds. An acceptable daily intake (ADI) and an international number (INS, International Number System) or an E-number are assigned to the additive when it has been approved. The INS/E-number validates its acceptability. The European Food and Safety Agency (EFSA) is in charge of monitoring health claims made for natural polymers used as additives in the EU. The EFSA assesses whether or not the scientific evidence currently available for the health claim is sufficient. In the EU, only EFSA-approved health claims may be advertised on food packaging. Insufficient research (RCTs are preferred), inadequate investigations, or insufficient test subjects used in the studies result in the majority of applications being refused. Many natural polymers, however, passed EFSA evaluation and had their health claims approved (
Table 3)
[60].
Table 3. EFSA-approved health claims for products made with natural polymers.
| Claim |
Hydrocolloid |
| Maintenance of normal blood cholesterol concentrations |
Beta-glucan, konjacmannan glucomannan, pectins, guar gum |
| Maintenance or achievement of a normal body weight |
Konjacmannan glucomannan |
| Reduction in postprandial glycemic responses |
Beta-glucan, pectins |
4. Pharmaceutical Applications of Natural Polymers
Natural polymers are used for a wide range of applications in both the polymer and pharmaceutical industries. Because the pharmaceutical industry is so extensive, there is a continual need to consider different applications. As a result, knowing how natural polymers are utilized in the pharmaceutical sector would logically benefit the polymer business in recognizing the broader uses of these polymers and adding the essential requirements to fulfil the end applications (e.g., give varied functions). The pharmaceutical industry’s polymer applications are largely focused on medication delivery systems. The following section of this chapter discusses drug entrance sites into the body and gives an outline of the potential applications of natural polymers.
4.1. Transdermal Drug Delivery Devices
Polymers are commonly used in transdermal medicine administration procedures. They function as crucial packaging components, coatings, penetration enhancers, ease of handling drug device handling, structural support for the device in the form of a backing layer, and control drug release rate control. They are frequently used in transdermal patches because of their distinctive properties. The development of transdermal drug delivery device components from more readily accessible natural polymers becomes essential as petrochemical-based resources for the manufacturing of synthetic polymers grow more expensive and scarcer. Following a description of the various components of the transdermal drug delivery system, the usage of natural polymers in each element, the matrix, adhesive layer, rate-controlling membrane, backing layer, release liner, and penetration improver will be discussed. Polymers are used more than any other material in transdermal drug delivery (TDD) because they contain features that are significant in the drug delivery process
[61]. They can help with pharmaceutical release control from carrier formulations
[62]. Silicones, polyvinyl alcohol, chitosan, polyacrylates, and polyesters including PLGA, PELA, and PLA, and cellulose derivatives are among the polymers often used in TDD.
Natural polymers are the ideal choice in TDD because they are easily available, inexpensive, potentially biodegradable, and biocompatible. They may also be treated to various chemical and surface modifications to match the requirements of the TDD system. A TDD system combines one or more polymers with an embedded medication for regulated and sustained drug delivery into or through the skin
[63]. High analytical product yields demand for the chemical inertness and purity of polymers utilized in TDD systems. Additionally, it must have physical properties that are sufficient for the intended use. The material must be suitable for processing and must not degrade. In addition, because a TDD patch system would be in touch with a person’s skin for a prolonged period of time, biodegradability and safety are paramount properties to include in the design
[64].
4.2. Natural Polymers in Transdermal Drug Delivery
Polymers have been used in transdermal medicine delivery since the 1980s. The drug is to be absorbed into the skin from a matrix of cross-linked linear polymer chains seen in the majority of transdermal patches
[63]. Transdermal drug delivery polymers include silicones, chitosan, polyvinyl alcohol, polyvinylparrolidone, polyacrylates, and cellulose derivatives. Both natural and synthetic polymers have been used as matrices, gelling agents, emulsifiers, penetration enhancers, and adhesives in transdermal delivery systems
[65]; for example, use as in demonstrated successful transdermal testosterone delivery to lab rats using a silicone elastomer synthetic polymer matrix. Another group studied the use of pectin hydrogels for insulin transdermal delivery. Diabetes-prone rats with type 2 diabetes mellitus were administered insulin-loaded pectin hydrogels
[66]. Diabetes-prone rats with type 2 diabetes mellitus were administered insulin-loaded pectin hydrogels. The outcomes demonstrated that the transdermal patch effectively and pharmacologically administered insulin across the skin in a dose-dependent manner;
[67][68] studied the use of natural polymers such as rubber latex as the adhesive for the backing layer in nicotine patches. As a result, more research into the potential of natural polymers for transdermal medication administration is required.
Natural polymers derived from plants and animals are emerging as a preferable option to synthetic polymers in the development of TDD systems, because they are biocompatible, biodegradable, and degrade into non-toxic monomers, as well as being more easily accessible synthetic polymers are increasingly being employed in the development of TDD systems
[15][69]. Synthetic polymers derived from petroleum and synthetically altered polypeptides are known to have limited therapeutic uses due to their toxicity and slow biodegradation rates
[61][70][71]. Natural-derived polymeric polysaccharides outperformed traditional carriers like polylactic acid (PLA), poly (lactic-co-glycolic acid) (PLGA), and polyvinyl pyrrolidone (PVP) in terms of drug delivery due to their high-water retention, lack of toxicity, good biocompatibility, biodegradability, and several other important biological properties. For example, sodium alginate could be used for drug encapsulation by cross-linking with metal ions to prepare nanoparticles and thereby improve skin penetration of drugs
[72]; chitosan (CS) has significant slow-release properties for drug delivery
[73]; hyaluronic acid (HA) can increase skin hydration and involve cell signaling to promote tissue regeneration and wound healing
[74], etc.
4.3. Natural Polymers in Topical Delivery Systems
Mucoadhesive microspheres, liposomes, solutions, gels, and mucoadhesive hydrogels are commonly used to deliver drugs through the nose. Starch, chitosan, alginate, dextran, hyaluronic acid, and gelatin are examples of natural polymers utilized for nasal medication administration
[75]. The interaction of the drug formulation with the mucin surface is known as mucoadhesion. By increasing the contact duration between the drug formulation and nasal mucosa layers in the cavity, the idea behind mucoadhesion is to enable sustained drug administration in nasal membranes
[76]. Mucoadhesion reduces the chance of complete mucociliary clearance while promoting drug absorption
[77]. Starch is a biodegradable polysaccharide that may easily be converted into microspheres
[78]. Examples of starch-loaded intranasal medications in the development stage include insulin
[76], morphine
[79], inactivated influenza
[80], and salbutamol
[81]. Chitosan, a naturally occurring polysaccharide with mucoadhesive characteristics, exhibits excellent adhesion to nasal epithelial cells and the overlying mucus layer
[82].
Compared to chitosan, PLA, and carboxymethyl cellulose, alginate is a divalent cation-induced rapid gelation, natural polysaccharide with higher mucoadhesion strength
[77]. Usually, mucoadhesive polymers are combined with alginate gel to increase the vehicle’s strength and drug-loading efficiency
[83].
Polysaccharides have recently gained a great deal of interest in the area of tissue engineering
[84], cosmetics, and wound healing
[85]. They are also extensively applied as drug carriers, building blocks for drug delivery, bioactive materials, and excipients to increase drug delivery
[86]. Natural polysaccharides are a potential candidate for numerous uses, including the delivery of medications and vaccinations, due to their versatility in structuring and modification to achieve specific goals. Microorganism-derived polysaccharides including scleroglucan, gellan gum, and xanthan gum have all been intensively researched for utilization in drug delivery
[87]. Drugs can be incorporated into bioadhesive polysaccharide nanoparticle carriers to improve their absorption
[9]. Pectin, guar gum, amylose, inulin, dextran, chitosan, and chondroitin sulfate are other naturally occurring polysaccharides that have been studied for their potential to be employed as pharmaceutical excipients and for colon-specific drug release
[88]. In addition, chitin and chitosan are low-immunogenic and tissue compatible polysaccharides that have demonstrated effectiveness in the delivery of drugs and vaccines
[89], as well as the transport of pharmaceuticals to the colon as prodrugs or coating tablets
[90]. Chitosan is one of the biopolymers that has been the most widely used as a drug and vaccine delivery system in many preparations (
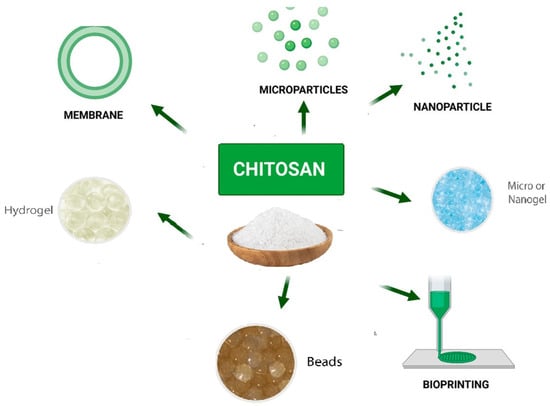
Figure 6)
[91]. This is due to the wide variety of antigen that could be encapsulated under mild conditions and without using organic solvents, which avoids the degradation and denaturation of the antigen during processing or after loading
[92].
Figure 6. Chitosan-based drug delivery systems
[93].
4.4. Natural Polymer Implants
Natural polymer uses in implants are particularly appealing for the same reasons that every other biomedical application has been (
Table 4)
[94]. Because these materials will be embedded in the body for extended periods of time, they must be biocompatible and functional for the entire time that they will be there until they are removed or degraded into the body and release non-toxic, biocompatible degradation products that can be safely eliminated from the body.
Table 4. Examples of natural polymers used in implants.
| Polymer |
API/Implant |
References |
| Chitosan |
Flurbiprofen |
[95] |
| Timolol maleaic |
[95] |
| Thymol |
[96] |
| Quercetin |
[96] |
| Dexamethason |
[95] |
| Vancomycin |
[97] |
| Hyaluronic acid/chitosan multilayer coating |
Chitosan Imidazole/siRNA nanoplex |
[98] |
| Chitosan/carbonnanotube |
Titanium implant |
[99] |
| Collagen/cellulose |
Juca extract |
[100] |
| Agarose |
Thymolol |
[96] |
| Quercetin |
| Poly (Lactic acid) |
Ibuprofen |
[100] |
| Acetylsalicylic acid |