Micro-computed tomography (microCT) is a common tool for the visualization of the internal composition of organic tissues. Collagen comprises approximately 25–35% of the whole-body protein content in mammals, and the structure and arrangement of collagen fibers contribute significantly to the integrity of tissues. Collagen type I is also frequently used as a key structural component in tissue-engineered and bioprinted tissues. However, the imaging of collagenous tissues is limited by their inherently low X-ray attenuation, which makes them indistinguishable from most other soft tissues. An imaging contrast agent that selectively alters X-ray attenuation is thus essential to properly visualize collagenous tissue using a standard X-ray tube microCT scanner. The entry provides basic understanding of the physical and chemical aspects of contrast agents that are specific for collagen and some of the key biological factors in selecting these agents for microCT.
1. Introduction
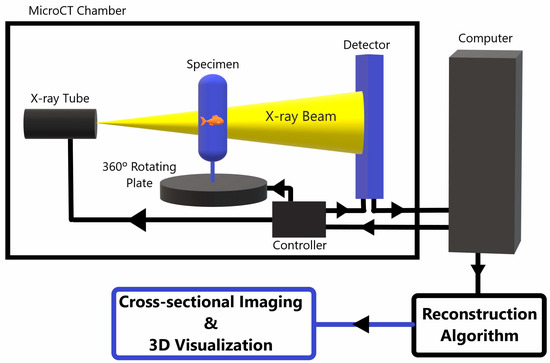
MicroCT can analyze biological tissues at high resolution on a microscopic level. It relies on the use of X-rays for three-dimensional (3D) imaging, and serves as an important tool for analyzing tissue specimens or bioengineered tissue, as it allows for volumetric visualization and provides a key supplement to microscopic optical imaging. Figure 1 shows a basic schematic of a standard table X-ray tube microCT scanner.
Figure 1. A schematic diagram of a standard X-ray tube MicroCT scanner. The specimen is placed between an X-ray source and a detector. The specimen rotates 360° while the X-ray tube and detector stay fixed within the scanner. The detector picks up the 2D X-ray penetration patters at each rotational position and sends the data to a computer, which processes the 2D data from each rotational view to tomographically reconstruct the 2D X-ray data into a 3D microCT image data set. This data can be post-processed and reformatted into various projections for improved visualization of the internal composition of the imaged object [
1,
2,
3,
4].
Like microscopic visualization of histochemical staining, microCT has the potential to analyze biological tissues at high resolution on a microscopic level, with the advantages of three-dimensional tissue imaging, and without the need to physically cut up the specimen. However, there are relatively limited data available regarding the effectiveness of contrast-enhancing agents in microCT [
5]. Most of the contrast agents that have been tested are the same or are similar to agents already used in histological staining or macroscopic contrast-enhanced CT (CE-CT) given their wide commercial availability, relatively low cost, familiarity among researchers and clinicians, and known binding properties. Some have been shown to be quite effective with CE-microCT as well. There are also new contrast agents being developed for microCT based on the combined knowledge of X-ray attenuation, biophysics, and molecular binding properties. All of these will be discussed later in this review.
The ability to visualize collagen microarchitecture requires not only that the collagen fibers themselves be differentiated from surrounding structures (i.e., contrast resolution, as described above), but also be a small enough pixel size (i.e., spatial resolution) to differentiate between two adjacent fibers. Depending on the type and density of tissue being studied, the resolution of collagen fibers requires a voxel size on the scale of 0.5 to 25 μm. Several microCT (and nanoCT) scanners are commercially available which have the means of achieving a sufficiently small voxel size for collagen microarchitecture. Unfortunately, despite the ability of these scanners to achieve a high spatial resolution, without a way to improve contrast resolution or the signal-to-noise ratio, the precise three-dimensional arrangement and orientation of these fibers is difficult to visualize using a standard microCT scanner. However, with the right contrast agent—as combined with sound tissue preparation and scanning techniques—sufficient resolution of individual collagen fibers can be achieved.
2. Visualizing Collagen Tissue Microarchitecture
The 48 contrast agents can be categorized based on their ionization and molecular structure (
Table 1). The categories include ionic iodinated, nonionic iodinated, gadolinium-based, polyoxometalates (POMs), or other metallic compounds [
13]. These characteristics are the most relevant in terms of what gives the contrast agent its ability to bind target molecules and attenuate X-rays.
Ionic iodinated compounds have been seen to work well as contrast agents for some collagen-containing tissues such as cartilage. However, they provide contrast due to their interaction with negatively charged glycosaminoglycans (GAGs) within the tissues rather than inert, water-insoluble collagen fibers. Most of the articles detailing the use of these compounds commented on the tissue’s GAG content, but had little, if anything, to conclude about collagen [
13,
14,
15]. The most used of these compounds in the reviewed literature is Lugol’s solution (I
3K). Lugol’s primary advantage over other CESAs is its relatively quick diffusion time due to its small size and water solubility [
14]. Balint et al. stained porcine tendons and ligaments with Lugol’s, phosphotungstic acid (PTA, H
3PW
12O
40), and phosphomolybdic acid (PMA, H
3PMo
12O
40). MicroCT showed that Lugol’s solution took <1 day to diffuse to the center of a porcine anterior cruciate ligament, whereas PTA and PMA took approximately 5 days (~1–2 mm/day) [
16]. However, Lugol’s solution was not shown to provide sufficient contrast enhancement to see collagen fibers at a voxel size of 7.5 µm. Although it did diffuse quickly through the collagenous tissue, it did not selectively bind to it, since it stained other constituents of the tissue as well. Therefore, the contrast was only mildly improved even when scanned with a voxel size of 1 µm [
13,
17,
18]. Based on the reviewed articles, Lugol’s solution and other ionic iodinated compounds would not be considered optimal for achieving the micrometer-scale contrast resolution required to study collagen microarchitecture, although it is useful for allowing visualization of tissue organization on a larger scale.
Gadolinium-based agents have become the standard CESA for use in magnetic resonance imaging (MRI), as these agents are paramagnetic and can be detected on MRI. However, the density of Gadolinium-based agents also attenuates X-rays and can serve as a CESA for microCT as well. As with ionic iodinated compounds, gadolinium-based CESAs have been shown to increase contrast resolution with the microCT of proteoglycan-rich tissues, but little to nothing is written in the reviewed literature about the visualization of collagen networks specifically [
13,
19]. Gadolinium-based CESAs were able to diffuse through bovine nasal cartilage disks of 6 mm diameter and 1 mm thick after being soaked for 24 h [
19]. However, the scans were performed at a voxel size of 25 µm, the upper limit of voxel size for resolving collagen fibers. Although no singular studies were found directly comparing PTA and gadolinium-based CESAs, the diffusion rate of gadolinium-based compounds appears to be faster than that of PTA based on a comparison of multiple studies.
POMs are polyatomic ions that typically contain three or more transition metals linked by oxygen atoms to form sophisticated three-dimensional networks. The high atomic number and electron density of these compounds gives them strong X-ray attenuation properties [
20]. The most widely used POMs for biomedical purposes are (PTA) and (PMA). These compounds are used for histochemical staining processes due to their ability to selectively bind fibrin, collagen, and other connective tissue fibers. PTA and PMA have a relatively high negative charge density and a higher volume fraction of metal to molecular weight ratio compared to other CESAs. These properties give PTA and PMA a strong affinity for collagen, which has a positive net charge in low pH [
21,
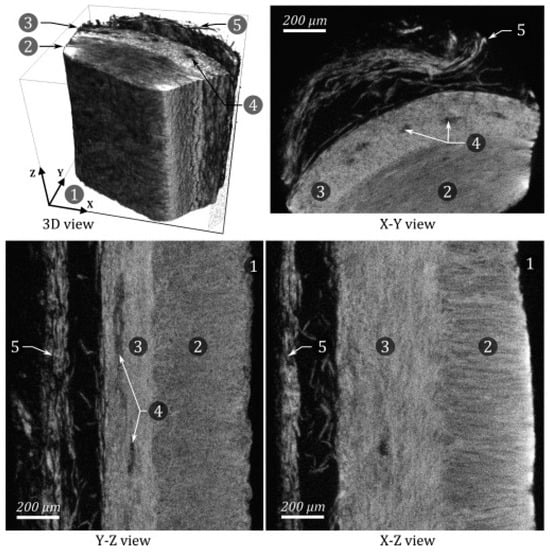
22]. As discussed above, this combination of being able to selectively bind collagen and to allow increased X-ray attenuation once bound is the foundation of an effective CESA. PTA was the most used CESA for staining soft tissues in the reviewed literature due to its ability to significantly improve the image contrast resolution of collagen. Nierenberger et al. reported that PTA allowed enough image contrast to see the three-dimensional arrangement of collagen fibers in the walls of porcine veins, as demonstrated in
Figure 2 (scanned at a voxel size of 1 µm) [
17].
Figure 2. Different MicroCT views of a small sample of PTA-stained porcine vein wall. 1. Lumen of the vein. 2. Media. 3. Adventitia. 4. Vasa vasorum. 5. Surrounding conjunctive tissue. Note how sufficient resolution is achieved to visualize that the collagen fibers in the media lie perpendicular to the fibers that comprise the adventitia. It should be noted that while the direction of the collagen fibers can be inferred from the image, what the image actually shows is a moiré interference pattern caused by the overlapping and close proximity of the near parallel fibers, and not the fibers themselves. Reprinted/adapted with permission from Ref [
17]. Copyright © 2023 Academié des sciences. Published by Elsevier Masson SAS.
The significant downsides of PTA and PMA are that they are highly acidic, causing protein denaturation and tissue breakdown, and that they diffuse through tissues relatively slowly, as noted above, which makes them difficult to use in larger specimens. Multiple studies have shown that tissues stained with PTA and PMA deform and degrade significantly over the course of just several days, with as much as 20% inhomogeneous shrinkage [
16]. However, a study by Missbach-Guentner et al. reported little to no tissue distortion by PTA in the staining of murine kidneys when using their sample preparation method [
23]. This could be due to the differing effects of PTA on kidneys vs. the more fibrous tissues tested in other articles, or to Missbach-Guentner et al.’s more gradual, stepwise approach to dehydrating the tissue specimens before adding the CESA, followed by a gradual rehydration process before scanning [
23,
24]. More investigation is needed to understand these differences. Other methods for stabilizing tissue with hydrogel during preparation for staining solvent conditions have been discussed by Wong et. al. through using the low-molecular-weight Lugol’s (EtOH) stain which is equally applicable to POMs agents [
25].
Other lesser-known POMs have also been tested with microCT. De Clercq et al., and Kerckhofs performed microCT studies using different isomers and metal substitutions of Wells–Dawson POMs (WD-POMs) at voxel sizes of 7 µm and 2 µm respectively [
26,
27,
28]. Unlike the other CESAs discussed, these molecules are not commercially available, and must be synthesized before use [
29,
30]. The WD POMs can be dissolved in a phosphate buffer solution at or close to physiological pH, thus significantly reducing tissue destruction compared with PTA and PMA. Of the WD POMs tested, Monolacunary WD POM (Mono-WD POM), Parent-WD POM, and Hafnium-substituted WD POM (Hf-WD POM) offer about the same contrast resolution enhancement compared to PTA in the staining of kidney and long bone tissue [
26].
Mono-WD POM and Hf-WD POM were shown to have the fastest diffusion rates compared to all other POMs. Parent WD-POM and PTA failed to diffuse all the way to the core of a murine kidney after 4 days, while the other POMs used in the study were able to do so [
26]. Mono-WD POM is an intermediate in the synthesis of Hf-WD POM, so its synthesis is more cost-effective and easier. With this in mind, Mono-WD POM appears to be one of the most promising CESAs found in the literature for staining soft tissues for CE-MicroCT if it can be synthesized. Its contrast enhancement and diffusion rate were improved even further when LiCl was added to the staining solution [
26]. Kerckhofs et al. confirmed Hf-POM and PTA have a strong binding affinity for collagen I, II, and fibrin using Raman spectroscopy, which showed an average drop of 28% in the peak intensity ratio between the POM peak and protein peak before and after rinsing of the tissue samples [
28]. While Mono-WD POM was not tested for its binding affinity for collagen specifically, given its similar molecular structure and characteristics, it may have a similar affinity. Thus, further study is warranted.
Many other metallic compounds have also been tested as microCT CESAs for collagenous soft tissues [
12,
13,
31]. Pawels et al. tested the binding affinity of various CESAs for connective tissue vs. adipose vs. muscle, as well as their diffusion rates and whether the CESAs would remain fixed in the tissue samples over time and after rinsing. Pauwels et al. performed their scans at voxel sizes ranging from 23 to 40 µm, in the upper range of voxel size needed to resolve collagen fibers. Therefore, more data using a smaller voxel size would be helpful to draw a more definitive conclusion on their ability to highlight collagen specifically, but they found binding properties which show promising results. Their comparison showed that iron(III) chloride and sodium tungstate stained connective tissue more than muscle. Ammonium molybdate, mercury(II) chloride, sodium tungstate, lead nitrate, barium nitrate, and barium chlorate provided the best visualization of tendons under microCT. Individual muscle fascicles were best visualized with PMA, PTA, and mercury(II) chloride. The only CESAs that remained fixed in the tissue samples after leaving the stained samples in water for 4 days were mercury(II) chloride, PTA, PMA, and ammonium orthomolybdate. Based on these conclusions, mercury(II) chloride should also be considered an option, as it is relatively inexpensive compared to the POMs and may provide sufficient resolution under the right preparation and scanning conditions. Unfortunately, like PTA and PMA, it diffuses relatively slowly through tissues, is highly toxic, and generates hazardous waste, which can be difficult and expensive to dispose. Sodium tungstate is another potential option, as tungsten has a high binding affinity for collagen and diffuses more easily through tissues than many of the other CESAs mentioned above.
Table 1. Comparison of contrast-enhancing staining agents used on collagen-containing soft tissues.
CESA
|
Proven to Show Collagen Fiber Arrangement?
|
Tissue Distortion
|
Diffusion Time
|
Comments
|
Anionic Iodinated
|
|
|
|
|
| Ioxaglate (C24H2II6N5O8), Iothalamate (C11H8I3N2O4) [13,15] |
No |
No data |
Slow |
Studied using a voxel size of 12 µm. Large quantities required, improve contrast in GAG-rich tissues (cartilage). |
| Lugol’s (I3K) [1,12,13,16,17,32,33] |
Limited |
Yes |
Fast |
Studied using a voxel size of 1 to 7.5 µm. Increases attenuation of collagen. However, it also stains other constituents of tissue, only mildly increasing contrast resolution. |
Cationic Iodinated
|
|
|
|
|
| CA4+, CA1+, CA2+ [13,14] |
No |
Requires further study |
Fast through GAG-rich or anionic tissues |
Studied using a voxel size of 30 µm. Higher positive charge correlates with a higher attenuation. |
Nonionic Iodinated
|
|
|
|
|
| Itopride (C18H24I3N3O8), Iodixanol (C35H44I6N6O15), Iomeprol (C17H22I3N3O8) [13,14] |
No |
No data |
Slow |
Studied using a voxel size of 30 µm. Pharmaceutical radiocontrast that partitions due to MW or hydration. |
Gadolinium
|
|
|
|
|
| Gadopentetate (dimeglumine, C28H54GdN5O20), Gadoteridol (C17H29GdN4O7), Gd3+ [13,19] |
Requires further study |
Requires further study |
Faster than PTA |
Studied using a voxel size of 25 µm. Effectiveness as a CESA directly correlates with proteoglycan content of tissue. |
POMs
|
|
|
|
|
PTA (H3PW12O40) [12,13,16,17,21,23,24,26,28,34,35,36,37,38,39]
PMA (H3PMo12O40) [12,13,16,17] |
Yes |
Yes |
Slow |
Studied using voxel size of 1 to 40 µm. PTA is a known histochemical staining agent for binding collagen. |
Zr-POM ((Et2NH2)10[Zr(Pw11O39)2]·7H2O) [27]
1:2 Hafnium (IV) substituted WD POM (K16[Hf(a2-P2W17O61)2]·19H2O) [26,28]
Parent WD POM (a-/b-K6P2W18O62·14/19H2O) [26]
Monolacunary WD POM (a2-K10P2W17O61·20H2O) [26]
Trilacunary WD POM (Na12[a-P2W15O56]·24H2O) [26] |
Yes |
No |
Slow to Fast |
Studied using voxel size of 2 to 7 µm. High binding affinity for collagen. Hf-WD POM, parent WD POM, and Mono-WD POM shown to provide sufficient contrast for visualizing collagen fibers. Hf-WD POM and Mono-WD POM have faster diffusion rates than PTA.
Not available commercially, must be synthesized in the lab. Of these options, Mono-WD POM is more cost-effective to synthesize |
Other Metallic Compounds [12,13]
|
Comments
|
FeCl3
(NH4)2MoO4
HgCl2
Na2WO4
Ba(ClO3)2
Ba (NO3)2
Pb (NO3)2 |
Studied using a voxel size ranging from 23 µm to 40 µm.
Iron (III) chloride and sodium tungstate stained connective tissue more than muscle.
Ammonium molybdate, (mercury (II) chloride, sodium tungstate, lead nitrate, barium nitrate, and barium chlorate provided the best visualization of tendons under microCT.
Ammonium molybdate shown to be effective at visualizing tendinous tissue under microCT.
Mercury(II) chloride is good for visualizing individual muscle fascicles. However, it is highly toxic and generates hazardous waste.
Mercury(II) chloride and ammonium orthomolybdate remained fixed in tissue over time. |
AgNO4
BaCl2, BaSO4 [31]
Cs2CO3, CsCl, CsNO3
Cu(NO3)2, CuSO4
FeCl3, FeSO4
KBr, KIO3, KMnO4
La(NO3)3
Na2MoO4
Pb(C2H3O2)2, C6H8O7Pb
VOSO4
OsO4 |
Studied using a voxel size ranging from 23 µm to 40 µm except for barium sulfate, which was studied using a voxel size of 2.93 µm [31].
All these compounds were not shown to be effective for visualizing collagen microarchitecture with microCT.
OsO4 is one of the first CESAs used for microCT. Binds well to adipose, but is highly toxic [12]. |