
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annio Posar | -- | 5473 | 2024-02-02 17:11:29 | | | |
| 2 | Fanny Huang | Meta information modification | 5473 | 2024-02-05 03:55:59 | | |
Video Upload Options
In the context of childhood epilepsy, the concept of continuous spike–waves during slow sleep (CSWS) includes several childhood-onset heterogeneous conditions that share electroencephalograms (EEGs) characterized by a high frequency of paroxysmal abnormalities during sleep, which have negative effects on the cognitive development and behavior of the child. These negative effects may have the characteristics of a clear regression or of a slowdown in development. Seizures are very often present, but not constantly. The above makes it clear why CSWS have been included in epileptic encephalopathies, in which, by definition, frequent EEG paroxysmal abnormalities have an unfavorable impact on cognitive functions, including socio-communicative skills, causing autistic features, even regardless of the presence of clinically overt seizures.
1. Introduction
2. Continuous Spike–Waves during Slow Sleep
2.1. EEGs and Clinics of Typical and Atypical ESES
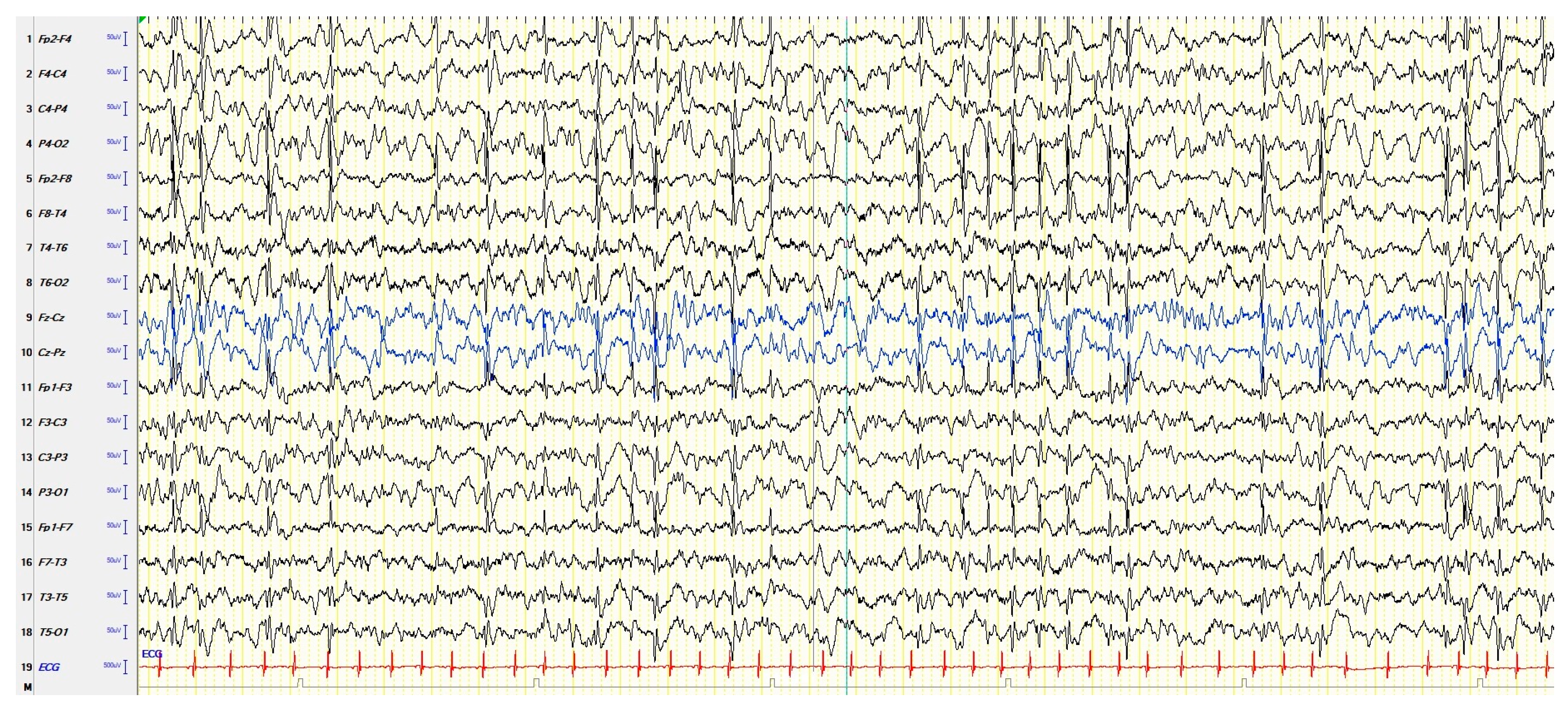
| Clinics | EEG | |
|---|---|---|
| Typical ESES |
Cognitive deterioration. Behavioral disorders, including attention deficit, hyperactivity, aggressiveness, difficulty in social interaction, and (more rarely) psychosis; autistic behavior is also possible. Epileptic seizures, focal or apparently generalized, with a heterogeneous semeiology. Motor signs, including dyspraxia, ataxia, and dystonia. |
Electrical-status epilepticus lasting for ≥85% of NREM (slow) sleep, documented by more than 2 EEG recordings during a period of ≥1 month. |
| Atypical ESES |
Great heterogeneity in the reported clinical features depending on the frequency and localization of the EEG paroxysmal abnormalities: a cognitive deterioration has been reported, but less frequently than typical ESES. Behavioral disorders, in particular ADHD-like symptoms. Epileptic seizures, focal or apparently generalized, with a heterogeneous semeiology. |
Paroxysmal abnormalities lasting for <85% of NREM sleep (usually > 50% < 85%), with a localization more heterogeneous than typical forms: focal, multifocal, unilateral, asymmetric or symmetric bilateral, and diffuse. |
2.2. Predictive Factors of the Evolution into CSWS
| Clinical | Early onset of seizures; multiple types of seizures; appearance of new seizures with an increased frequency; seizure semiology including dysarthria or somatosensory auras. |
| EEG | Fronto-centro-temporal focus, with increasing frequency, both in wakefulness and in sleep; pattern EEG of spike–waves. |
2.3. Electrophysiology and Functional Neuroimaging
| Semiautomatic quantification of paroxysmal abnormalities in CSWS is a reliable alternative to the classic quantification based on visual scoring. |
| Global synchronization increase from wakefulness to sleep is strongly correlated with spikes. |
| Associating time-sensitive magnetic source imaging and PET, spike–wave onset is associated with focal hypermetabolism. |
| Magnetoencephalography in non-lesional CSWS children showed dipole clusters located on heterogeneous cortical areas: right Rolandic area, right supramarginal gyrus, left Rolandic area, left supramarginal gyrus, bilateral Rolandic area, and multiple anatomical areas. |
| PET showed hypermetabolism in perisylvian, superior temporal, inferior parietal, and central cortex areas that were related to paroxysmal abnormalities. The diffuse hypometabolism found in regions belonging to the DMN (see prefrontal and posterior cingulate cortices, parahippocampal gyrus and precuneus) could be due to remote inhibition following epileptic activity. |
| EEG-fMRI showed characteristic findings of epileptic encephalopathy: positive changes in BOLD signals in the perisylvian regions, prefrontal cortex, anterior cingulate, and thalamus, while negative changes in BOLD signals were found in the DMN regions. The activation pattern represents a diffusion of epileptic activity. |
| HFOs are hypothesized to be related to alterations in higher brain functions. They seem to be related to the functional disruption of brain networks in CSWS. |
References
- Schmitt, B. Sleep and epilepsy syndromes. Neuropediatrics 2015, 46, 171–180.
- Shbarou, R.; Mikati, M.A. The expanding clinical spectrum of genetic pediatric epileptic encephalopathies. Semin. Pediatr. Neurol. 2016, 23, 134–142.
- Besag, F.M. Epilepsy in patients with autism: Links, risks and treatment challenges. Neuropsychiatr. Dis. Treat. 2017, 14, 1–10.
- Appendino, J.P.; Appendino, J.I. Encefalopatías epilépticas determinadas genéticamente . Medicina 2019, 79 (Suppl. 3), 42–47.
- Specchio, N.; Wirrell, E.C.; Scheffer, I.E.; Nabbout, R.; Riney, K.; Samia, P.; Guerreiro, M.; Gwer, S.; Zuberi, S.M.; Wilmshurst, J.M.; et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1398–1442.
- Van Bogaert, P. Long-term outcome of developmental and epileptic encephalopathies. Rev. Neurol. 2022, 178, 659–665.
- Van Bogaert, P. Epilepsy syndromes of childhood with sleep activation: Insights from functional imaging. Eur. J. Paediatr. Neurol. 2020, 24, 58–60.
- Auvin, S.; Cilio, M.R.; Vezzani, A. Current understanding and neurobiology of epileptic encephalopathies. Neurobiol. Dis. 2016, 92, 72–89.
- Japaridze, N.; Menzel, E.; von Ondarza, G.; Steinmann, E.; Stephani, U. Risk factors of cognitive outcome in patients with atypical benign partial epilepsy/pseudo-Lennox syndrome (ABPE/PLS) and continues spike and wave during sleep (CSWS). Eur. J. Paediatr. Neurol. 2014, 18, 368–375.
- Kennedy, A.; Hill, D. Dementia infantilis with cortical dysrhythmia. Arch. Dis. Child. 1942, 17, 122–129.
- Patry, G.; Lyagoubi, S.; Tassinari, C.A. Subclinical “electrical status epilepticus” induced by sleep in children. A clinical and electroencephalographic study of six cases. Arch. Neurol. 1971, 24, 242–252.
- Tassinari, C.A.; Rubboli, G.; Volpi, L.; Meletti, S.; d’Orsi, G.; Franca, M.; Sabetta, A.R.; Riguzzi, P.; Gardella, E.; Zaniboni, A.; et al. Encephalopathy with electrical status epilepticus during slow sleep or ESES syndrome including the acquired aphasia. Clin. Neurophysiol. 2000, 111 (Suppl. 2), S94–S102.
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685.
- Azcona, G.; Gurtubay, I.G.; Mosquera, A.; Ibanez, B.; Cambra, K.; Aguilera-Albesa, S.; Yoldi-Petri, M.E. Estudio comparativo entre tres sistemas de cuantificacion del indice de punta-onda en pacientes con punta-onda continua del sueño lento . Rev. Neurol. 2017, 65, 439–446.
- Hirsch, E.; Caraballo, R.; Dalla Bernardina, B.; Loddenkemper, T.; Zuberi, S.M. Encephalopathy related to status epilepticus during slow sleep: From concepts to terminology. Epileptic Disord. 2019, 21, S5–S12.
- Jansen, F.E.; Nikanorova, M.; Peltola, M. Current treatment options for encephalopathy related to status epilepticus during slow sleep. Epileptic Disord. 2019, 21, S76–S81.
- Singhal, N.S.; Sullivan, J.E. Continuous spike-wave during slow wave sleep and related conditions. ISRN Neurol. 2014, 2014, 619079.
- RamachandranNair, R. Encephalopathy associated with electrical status epilepticus of sleep (ESES): A practical approach. Indian J. Pediatr. 2020, 87, 1057–1061.
- Sonnek, B.; Döring, J.H.; Mütze, U.; Schubert-Bast, S.; Bast, T.; Balke, D.; Reuner, G.; Schuler, E.; Klabunde-Cherwon, A.; Hoffmann, G.F.; et al. Clinical spectrum and treatment outcome of 95 children with continuous spikes and waves during sleep (CSWS). Eur. J. Paediatr. Neurol. 2021, 30, 121–127.
- Rubboli, G.; Huber, R.; Tononi, G.; Tassinari, C.A. Encephalopathy related to status epilepticus during slow sleep: A link with sleep homeostasis? Epileptic Disord. 2019, 21, S62–S70.
- Rubboli, G.; Gardella, E.; Cantalupo, G.; Tassinari, C.A. Encephalopathy related to status epilepticus during slow sleep (ESES). Pathophysiological insights and nosological considerations. Epilepsy Behav. 2023, 140, 109105.
- Samanta, D.; Al Khalili, Y. Electrical status epilepticus in sleep. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Nath, A.; Whitworth, E.; Bretz, D.; Davila-Williams, D.; McIntosh, L. Electrical status epilepticus in sleep (ESES) in an elderly adult: A case report. Cureus 2022, 14, e26372.
- Rubboli, G.; Tassinari, C.A. Linking epilepsy, sleep disruption and cognitive impairment in encephalopathy related to status epilepticus during slow sleep (ESES). Epileptic Disord. 2019, 21, S1–S2.
- Camfield, P.; Camfield, C. Regression in children with epilepsy. Neurosci. Biobehav. Rev. 2019, 96, 210–218.
- Dorris, L.; O’Regan, M.; Wilson, M.; Zuberi, S.M. Progressive intellectual impairment in children with encephalopathy related to status epilepticus during slow sleep. Epileptic Disord. 2019, 21, S88–S96.
- Jacob, J. Cortical interneuron dysfunction in epilepsy associated with autism spectrum disorders. Epilepsia 2016, 57, 182–193.
- Besag, F.; Aldenkamp, A.; Caplan, R.; Dunn, D.W.; Gobbi, G.; Sillanpää, M. Psychiatric and behavioural disorders in children with epilepsy (ILAE Task Force Report): Epilepsy and autism. Epileptic Disord. 2016, 18 (Suppl. 1), S16–S23.
- Besag, F.; Gobbi, G.; Aldenkamp, A.; Caplan, R.; Dunn, D.W.; Sillanpää, M. Psychiatric and behavioural disorders in children with epilepsy (ILAE Task Force Report): Behavioural and psychiatric disorders associated with epilepsy syndromes. Epileptic Disord. 2016, 18 (Suppl. 1), S37–S48.
- Gong, P.; Xue, J.; Qian, P.; Yang, H.; Liu, X.; Zhang, Y.; Jiang, Y.; Yang, Z. Epileptic negative myoclonus restricted to lower limbs in benign childhood focal epilepsy with vertex spikes. Eur. J. Neurol. 2019, 26, 1318–1326.
- Gong, P.; Xue, J.; Qian, P.; Yang, H.P.; Zhang, Y.H.; Jiang, Y.W.; Yang, Z.X. Electroclinical characteristics of epilepsy children with midline epileptiform discharges related epileptic negative myoclonus as the first symptom. Chin. J. Pediatr. 2019, 57, 943–949.
- Aoun, M.A.; Eisermann, M.; Chemaly, N.; Losito, E.; Desguerre, I.; Nabbout, R.; Kaminska, A. Jerking during absences: Video-EEG and polygraphy of epileptic myoclonus associated with two paediatric epilepsy syndromes. Epileptic Disord. 2021, 23, 191–200.
- Kalscheur, E.J.; Farias-Moeller, R.; Koop, J. Role of neuropsychology in identification of CSWS in a school-aged child with a remote neurological insult. Epilepsy Behav. Rep. 2021, 18, 100514.
- Maltoni, L.; Posar, A.; Parmeggiani, A. Long-term follow-up of cognitive functions in patients with continuous spike-waves during sleep (CSWS). Epilepsy Behav. 2016, 60, 211–217.
- Carvalho, D.; Mendonça, C.; Carvalho, J.; Martins, A.; Leal, A. High incidence of early thalamic lesions in the continuous spike-wave related with slow sleep (CSWS). Epilepsy Behav. 2023, 138, 109031.
- Bennett-Back, O.; Uliel-Siboni, S.; Kramer, U. The yield of video-EEG telemetry evaluation for non-surgical candidate children. Eur. J. Paediatr. Neurol. 2016, 20, 848–854.
- Nagyova, R.; Horsburgh, G.; Robertson, A.; Zuberi, S.M. The clinical utility of ambulatory EEG in childhood. Seizure 2019, 64, 45–49.
- Tassinari, C.A.; Rubboli, G. Encephalopathy related to status epilepticus during slow sleep: Current concepts and future directions. Epileptic Disord. 2019, 21, S82–S87.
- Belousova, E.D.; Ermakov, A. Épilepticheskaia éntsefalopatiia s prodolzhennoĭ spaĭk-vol-no-voĭ aktivnost’iu vo sne: Obzor literatury . Zh. Nevrol. Psikhiatr. Im. S.S. Korsakova 2014, 114, 52–58.
- Altunel, A.; Altunel, E.Ö.; Sever, A. Response to adrenocorticotropic in attention deficit hyperactivity disorder-like symptoms in electrical status epilepticus in sleep syndrome is related to electroencephalographic improvement: A retrospective study. Epilepsy Behav. 2017, 74, 161–166.
- van Iterson, L.; Vrij, S.; Sie, L.T.L.; Augustijn, P.B.; Rooze, A.C.S.; Jansen, F.E. Acquired visual agnosia as an uncommon presentation of epileptic encephalopathy in a 6-year-old boy with CSWS. Epilepsy Behav. Rep. 2021, 16, 100465.
- Kuki, I.; Kawawaki, H.; Okazaki, S.; Ikeda, H.; Tomiwa, K. Epileptic encephalopathy with continuous spikes and waves in the occipito-temporal region during slow-wave sleep in two patients with acquired Kanji dysgraphia. Epileptic Disord. 2014, 16, 540–545.
- Tassinari, C.A.; Cantalupo, G.; Rubboli, G. Focal ESES as a selective focal brain dysfunction: A challenge for clinicians, an opportunity for cognitive neuroscientists. Epileptic Disord. 2015, 17, 345–347.
- Pavlidis, E.; Møller, R.S.; Nikanorova, M.; Kölmel, M.S.; Stendevad, P.; Beniczky, S.; Tassinari, C.A.; Rubboli, G.; Gardella, E. Idiopathic encephalopathy related to status epilepticus during slow sleep (ESES) as a “pure” model of epileptic encephalopathy. An electroclinical, genetic, and follow-up study. Epilepsy Behav. 2019, 97, 244–252.
- Mohammadi, M.; Kowkabi, S.; Asadi-Pooya, A.A.; Malamiri, R.A.; Badv, R.S. Hemi-ESES associated with agenesis of the corpus callosum and normal cognition. Epilepsy Behav. Case Rep. 2019, 11, 96–98.
- Caraballo, R.H.; Veggiotti, P.; Kaltenmeier, M.C.; Piazza, E.; Gamboni, B.; Lopez Avaria, M.F.; Noli, D.; Adi, J.; Cersosimo, R. Encephalopathy with status epilepticus during sleep or continuous spikes and waves during slow sleep syndrome: A multicenter, long-term follow-up study of 117 patients. Epilepsy Res. 2013, 105, 164–173.
- Gencpinar, P.; Dundar, N.O.; Tekgul, H. Electrical status epilepticus in sleep (ESES)/continuous spikes and waves during slow sleep (CSWS) syndrome in children: An electroclinical evaluation according to the EEG patterns. Epilepsy Behav. 2016, 61, 107–111.
- Shiraishi, H.; Haginoya, K.; Nakagawa, E.; Saitoh, S.; Kaneko, Y.; Nakasato, N.; Chan, D.; Otsubo, H. Magnetoencephalography localizing spike sources of atypical benign partial epilepsy. Brain Dev. 2014, 36, 21–27.
- Nonclercq, A.; Foulon, M.; Verheulpen, D.; De Cock, C.; Buzatu, M.; Mathys, P.; Van Bogaert, P. Cluster-based spike detection algorithm adapts to interpatient and intrapatient variation in spike morphology. J. Neurosci. Methods 2012, 210, 259–265.
- Yu, Y.; Chen, Y.; Li, Y.; Gao, Z.; Gai, Z.; Zhou, Y. SQNN: A spike-wave index quantification neural network with a pre-labeling algorithm for epileptiform activity identification and quantification in children. J. Neural Eng. 2022, 19, 016040.
- Sánchez Fernández, I.; Peters, J.M.; Hadjiloizou, S.; Prabhu, S.P.; Zarowski, M.; Stannard, K.M.; Takeoka, M.; Rotenberg, A.; Kothare, S.V.; Loddenkemper, T. Clinical staging and electroencephalographic evolution of continuous spikes and waves during sleep. Epilepsia 2012, 53, 1185–1195.
- Cantalupo, G.; Pavlidis, E.; Beniczky, S.; Avanzini, P.; Gardella, E.; Larsson, P.G. Quantitative EEG analysis in encephalopathy related to status epilepticus during slow sleep. Epileptic Disord. 2019, 21, S31–S40.
- Sánchez Fernández, I.; Chapman, K.E.; Peters, J.M.; Kothare, S.V.; Nordli, D.R., Jr.; Jensen, F.E.; Berg, A.T.; Loddenkemper, T. The tower of Babel: Survey on concepts and terminology in electrical status epilepticus in sleep and continuous spikes and waves during sleep in North America. Epilepsia 2013, 54, 741–750.
- Carvalho, D.; Mendes, T.; Dias, A.I.; Leal, A. Interictal spike quantification in continuous spike-wave of sleep (CSWS): Clinical usefulness of a wearable EEG device. Epilepsy Behav. 2020, 104, 106902.
- Poothrikovil, R.P.; Koul, R.L.; Mani, R.; Al Futaisi, A. Evolution of Ohtahara syndrome to continuous spikes and waves during slow sleep in an infant. Neurodiagn. J. 2012, 52, 261–274.
- Oguni, H.; Hirano, Y.; Nagata, S. Encephalopathy related to status epilepticus during slow sleep (ESES) as atypical evolution of Panayiotopoulos syndrome: An EEG and neuropsychological study. Epileptic Disord. 2020, 22, 67–72.
- Nava, E.; Mori, A.C.; Striano, P.; Ramantani, G. Atypical presentation of sunflower epilepsy featuring an EEG pattern of continuous spike waves during slow-wave sleep. Epileptic Disord. 2021, 23, 927–932.
- Sánchez Fernández, I.; Chapman, K.E.; Peters, J.M.; Harini, C.; Rotenberg, A.; Loddenkemper, T. Continuous spikes and waves during sleep: Electroclinical presentation and suggestions for management. Epilepsy Res. Treat. 2013, 2013, 583531.
- Arzimanoglou, A.; Cross, H.J. Cognitive impairment and behavioral disorders in encephalopathy related to status epilepticus during slow sleep: Diagnostic assessment and outcome. Epileptic Disord. 2019, 21, S71–S75.
- Margari, L.; Buttiglione, M.; Legrottaglie, A.R.; Presicci, A.; Craig, F.; Curatolo, P. Neuropsychiatric impairment in children with continuous spikes and waves during slow sleep: A long-term follow-up study. Epilepsy Behav. 2012, 25, 558–562.
- Curnow, S.R.; Vogrin, S.J.; Barton, S.; Bailey, C.A.; Harvey, A.S. Focal cortical hypermetabolism in atypical benign rolandic epilepsy. Epilepsy Res. 2020, 161, 106288.
- Bölsterli Heinzle, B.K.; Fattinger, S.; Kurth, S.; Lebourgeois, M.K.; Ringli, M.; Bast, T.; Critelli, H.; Schmitt, B.; Huber, R. Spike wave location and density disturb sleep slow waves in patients with CSWS (continuous spike waves during sleep). Epilepsia 2014, 55, 584–591.
- Bölsterli, B.K.; Gardella, E.; Pavlidis, E.; Wehrle, F.M.; Tassinari, C.A.; Huber, R.; Rubboli, G. Remission of encephalopathy with status epilepticus (ESES) during sleep renormalizes regulation of slow wave sleep. Epilepsia 2017, 58, 1892–1901.
- Oser, N.; Hubacher, M.; Nageleisen-Weiss, A.; van Mierlo, P.; Huber, R.; Weber, P.; Bölsterli, B.K.; Datta, A.N. 6-year course of sleep homeostasis in a case with epilepsy-aphasia spectrum disorder. Epilepsy Behav. Rep. 2021, 16, 100488.
- Bölsterli Heinzle, B.K.; Bast, T.; Critelli, H.; Huber, R.; Schmitt, B. Age-dependency of location of epileptic foci in “continuous spike-and-waves during sleep”: A parallel to the posterior-anterior trajectory of slow wave activity. Neuropediatrics 2017, 48, 36–41.
- Filippini, M.; Arzimanoglou, A.; Gobbi, G. Neuropsychological approaches to epileptic encephalopathies. Epilepsia 2013, 54 (Suppl. 8), 38–44.
- Halász, P.; Szűcs, A. Sleep and epilepsy link by plasticity. Front. Neurol. 2020, 11, 911.
- Ng, R.; Hodges, E. Neurocognitive profiles of pediatric patients with ESES, generalized epilepsy, or focal epilepsy. Epilepsy Res. 2020, 167, 106351.
- Panda, P.K.; Natarajan, V.; Sharawat, I.K. Neurocognitive profile of children with ESES, generalized, and focal epilepsy: Is there any difference? Epilepsy Res. 2021, 172, 106451.
- Pesántez-Ríos, G.; Martínez-Bermejo, A.; Arcas, J.; Merino-Andreu, M.; Ugalde-Canitrot, A. Las evoluciones atipicas de la epilepsia rolandica son complicaciones predecibles . Rev. Neurol. 2015, 61, 106–113.
- Porat Rein, A.; Kramer, U.; Mitelpunkt, A. Development of ontology for self-limited epilepsy with centrotemporal spikes and application of data mining algorithms to identify new subtypes. Isr. Med. Assoc. J. 2019, 21, 503.
- Porat Rein, A.; Kramer, U.; Hausman Kedem, M.; Fattal-Valevski, A.; Mitelpunkt, A. Early prediction of encephalopathic transformation in children with benign epilepsy with centro-temporal spikes. Brain Dev. 2021, 43, 268–279.
- Desprairies, C.; Dozières-Puyravel, B.; Ilea, A.; Bellavoine, V.; Nasser, H.; Delanöe, C.; Auvin, S. Early identification of epileptic encephalopathy with continuous spikes-and-waves during sleep: A case-control study. Eur. J. Paediatr. Neurol. 2018, 22, 837–844.
- Caraballo, R.; Pavlidis, E.; Nikanorova, M.; Loddenkemper, T. Encephalopathy with continuous spike-waves during slow-wave sleep: Evolution and prognosis. Epileptic Disord. 2019, 21, S15–S21.
- Aeby, A.; Santalucia, R.; Van Hecke, A.; Nebbioso, A.; Vermeiren, J.; Deconinck, N.; De Tiège, X.; Van Bogaert, P. A qualitative awake EEG score for the diagnosis of continuous spike and waves during sleep (CSWS) syndrome in self-limited focal epilepsy (SFE): A case-control study. Seizure 2021, 84, 34–39.
- Peltola, M.E.; Palmu, K.; Liukkonen, E.; Gaily, E.; Vanhatalo, S. Semiautomatic quantification of spiking in patients with continuous spikes and waves in sleep: Sensitivity to settings and correspondence to visual assessment. Clin. Neurophysiol. 2012, 123, 1284–1290.
- Peltola, M.E.; Sairanen, V.; Gaily, E.; Vanhatalo, S. Measuring spike strength in patients with continuous spikes and waves during sleep: Comparison of methods for prospective use as a clinical index. Clin. Neurophysiol. 2014, 125, 1639–1646.
- Ouyang, G.; Wang, Y.; Yang, Z.; Li, X. Global synchronization of multichannel EEG in patients with electrical status epilepticus in sleep. Clin. EEG Neurosci. 2015, 46, 357–363.
- Balaram, N.; Jose, J.; Gafoor, A.V.; Balachandran, S. Classification of electrical status epilepticus in sleep based on EEG patterns and spatiotemporal mapping of spikes. Epileptic Disord. 2022, 24, 1060–1072.
- De Tiège, X.; Trotta, N.; Op de Beeck, M.; Bourguignon, M.; Marty, B.; Wens, V.; Nonclercq, A.; Goldman, S.; Van Bogaert, P. Neurophysiological activity underlying altered brain metabolism in epileptic encephalopathies with CSWS. Epilepsy Res. 2013, 105, 316–325.
- Japaridze, N.; Muthuraman, M.; Dierck, C.; von Spiczak, S.; Boor, R.; Mideksa, K.G.; Anwar, R.A.; Deuschl, G.; Stephani, U.; Siniatchkin, M. Neuronal networks in epileptic encephalopathies with CSWS. Epilepsia 2016, 57, 1245–1255.
- Magara, S.; Komatsubara, T.; Hojo, M.; Kobayashi, Y.; Yoshino, M.; Saitoh, A.; Tohyama, J. The association of epileptic focus estimated by magnetoencephalography with cognitive function in non-lesional epilepsy with continuous spikes and waves during slow wave sleep (ECSWS) children. Brain Dev. 2019, 41, 163–172.
- Li, Y.; Li, Y.; Sun, J.; Niu, K.; Wang, P.; Xu, Y.; Wang, Y.; Chen, Q.; Zhang, K.; Wang, X. Relationship between brain activity, cognitive function, and sleep spiking activation in new-onset self-limited epilepsy with centrotemporal spikes. Front. Neurol. 2022, 13, 956838.
- Moeller, F.; Moehring, J.; Ick, I.; Steinmann, E.; Wolff, S.; Jansen, O.; Boor, R.; Stephani, U.; Siniatchkin, M. EEG-fMRI in atypical benign partial epilepsy. Epilepsia 2013, 54, e103–e108.
- Siniatchkin, M.; Van Bogaert, P. Pathophysiology of encephalopathy related to continuous spike and waves during sleep: The contribution of neuroimaging. Epileptic Disord. 2019, 21, S48–S53.
- Toda, Y.; Kobayashi, K.; Hayashi, Y.; Inoue, T.; Oka, M.; Ohtsuka, Y. Effects of intravenous diazepam on high-frequency oscillations in EEGs with CSWS. Brain Dev. 2013, 35, 540–547.
- Ohuchi, Y.; Akiyama, T.; Matsuhashi, M.; Kobayashi, K. High-frequency oscillations in a spectrum of pediatric epilepsies characterized by sleep-activated spikes in scalp EEG. Clin. Neurophysiol. 2019, 130, 1971–1980.
- Cao, D.; Chen, Y.; Liao, J.; Nariai, H.; Li, L.; Zhu, Y.; Zhao, X.; Hu, Y.; Wen, F.; Zhai, Q. Scalp EEG high frequency oscillations as a biomarker of treatment response in epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS). Seizure 2019, 71, 151–157.
- Gong, P.; Yang, Z.X.; Xue, J.; Qian, P.; Yang, H.P.; Liu, X.Y.; Bian, K.G. Application of scalp-recorded high-frequency oscillations in epileptic encephalopathy with continuous spike-and-wave during sleep. J. Peking Univ. Health Sci. 2018, 50, 213–220.
- Gong, P.; Xue, J.; Qian, P.; Yang, H.; Liu, X.; Cai, L.; Bian, K.; Yang, Z. Scalp-recorded high-frequency oscillations in childhood epileptic encephalopathy with continuous spike-and-wave during sleep with different etiologies. Brain Dev. 2018, 40, 299–310.
- Giacomini, T.; Luria, G.; D’Amario, V.; Croci, C.; Cataldi, M.; Piai, M.; Nobile, G.; Bruni, O.; Consales, A.; Mancardi, M.M.; et al. On the role of REM sleep microstructure in suppressing interictal spikes in electrical status epilepticus during sleep. Clin. Neurophysiol. 2022, 136, 62–68.
- Filippini, M.; Boni, A.; Giannotta, M.; Pini, A.; Russo, A.; Musti, M.A.; Guerra, A.; Lassonde, M.; Gobbi, G. Comparing cortical auditory processing in children with typical and atypical benign epilepsy with centrotemporal spikes: Electrophysiologic evidence of the role of non-rapid eye movement sleep abnormalities. Epilepsia 2015, 56, 726–734.




